Factors Influencing the Adoption of Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Painful Bone Metastases in Europe, A Group Concept Mapping Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Settings
2.2. GCM
2.3. Participant Selection of the GCM Study
2.4. Data Collection and Analysis
2.4.1. Phase I—Brainstorming
2.4.2. Phase II—Sorting and Rating
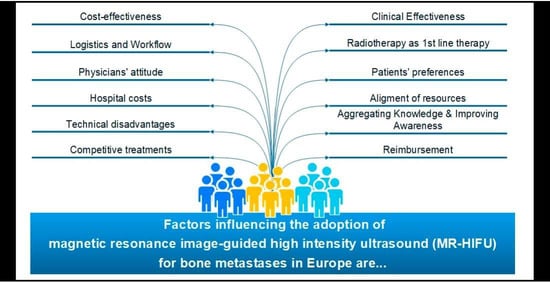
3. Results
3.1. Participants
3.2. Collected Statements
3.3. Concept Maps
Importance and Changeability of Statements and Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paice, J.A.; Ferrell, B. The management of cancer pain. CA Cancer J. Clin. 2011, 61, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. Bone cancer pain: From mechanism to therapy. Curr. Opin. Support. Palliat. Care 2014, 8, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; van der Linden, Y.M.; Roos, D.; Hartsell, W.F.; Hoskin, P.; Wu, J.S.; Brundage, M.D.; Nabid, A.; Tissing-Tan, C.J.; Oei, B.; et al. Single versus multiple fractions of repeat radiation for painful bone metastases: A randomised, controlled, non-inferiority trial. Lancet Oncol. 2014, 15, 164–171. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef]
- van der Linden, Y.M.; Lok, J.J.; Steenland, E.; Martijn, H.; van Houwelingen, H.; Marijnen, C.A.; Leer, J.W. Single fraction radiotherapy is efficacious: A further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Baal, J.D.; Chen, W.C.; Baal, U.; Wagle, S.; Baal, J.H.; Link, T.M.; Bucknor, M.D. Efficacy and safety of magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: A systematic review and meta-analysis. Skelet. Radiol. 2021, 50, 2459–2469. [Google Scholar] [CrossRef]
- Han, X.; Huang, R.; Meng, T.; Yin, H.; Song, D. The Roles of Magnetic Resonance-Guided Focused Ultrasound in Pain Relief in Patients With Bone Metastases: A Systemic Review and Meta-Analysis. Front. Oncol. 2021, 11, 617295. [Google Scholar] [CrossRef]
- Huisman, M.; ter Haar, G.; Napoli, A.; Hananel, A.; Ghanouni, P.; Lovey, G.; Nijenhuis, R.J.; van den Bosch, M.A.; Rieke, V.; Majumdar, S.; et al. International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions. Int. J. Hyperth. 2015, 31, 251–259. [Google Scholar] [CrossRef]
- Scipione, R.; Anzidei, M.; Bazzocchi, A.; Gagliardo, C.; Catalano, C.; Napoli, A. HIFU for Bone Metastases and other Musculoskeletal Applications. Semin. Interv. Radiol. 2018, 35, 261–267. [Google Scholar] [CrossRef]
- Simões Corrêa Galendi, J.; Yeo, S.Y.; Simic, D.; Grüll, H.; Stock, S.; Müller, D. A time-driven activity-based costing approach of magnetic resonance-guided high-intensity focused ultrasound for cancer-induced bone pain. Int, J. Hyperthermia 2022, 39, 173–180. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Ghanouni, P.; Kanaev, S.V.; Iozeffi, D.; Gianfelice, D.; Fennessy, F.M.; Kuten, A.; Meyer, J.E.; LeBlang, S.D.; Roberts, A.; et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: Phase III trial results. J. Natl. Cancer Inst. 2014, 106, dju082. [Google Scholar] [CrossRef]
- Urquhart, R.; Kendell, C.; Geldenhuys, L.; Ross, A.; Rajaraman, M.; Folkes, A.; Madden, L.L.; Sullivan, V.; Rayson, D.; Porter, G.A. The role of scientific evidence in decisions to adopt complex innovations in cancer care settings: A multiple case study in Nova Scotia, Canada. Implement. Sci. 2019, 14, 14. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Procter, R.; Shaw, S. Analysing the role of complexity in explaining the fortunes of technology programmes: Empirical application of the NASSS framework. BMC Med. 2018, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- O’Cathain, A.; Thomas, K.J.; Drabble, S.J.; Rudolph, A.; Hewison, J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013, 3, e002889. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.S.; Kirchner, J. Implementation science: What is it and why should I care? Psychiatry Res. 2020, 283, 112376. [Google Scholar] [CrossRef]
- Trochim, W.M.K. An introduction to concept mapping for planning and evaluation. Eval. Program. Plan. 1989, 12, 1–16. [Google Scholar] [CrossRef]
- Foley, J.; Eames, M.; Snell, J.; Hananel, A.; Kassell, N.; Aubry, J.-F. Image-guided focused ultrasound: State of the technology and the challenges that lie ahead. Imaging Med. 2013, 5, 1190–1203. [Google Scholar] [CrossRef]
- Slotman, D.J.; Bartels, M.M.; Ferrer, C.J.; Bos, C.; Bartels, L.W.; Boomsma, M.F.; Phernambucq, E.C.; Nijholt, I.M.; Morganti, A.G.; Siepe, G.; et al. Focused Ultrasound and RadioTHERapy for Non-Invasive Palliative Pain Treatment in Patients with Bone Metastasis: A Study Protocol for the Three Armed Randomized Controlled FURTHER-trial. PREPRINT Available Res. Sq. 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosas, S.R. Group concept mapping methodology: Toward an epistemology of group conceptualization, complexity, and emergence. Qual. Quant. 2017, 51, 1403–1416. [Google Scholar] [CrossRef]
- Trochim, W.; Kane, M. Concept mapping: An introduction to structured conceptualization in health care. Int. J. Qual. Health Care 2005, 17, 187–191. [Google Scholar] [CrossRef]
- Valerio, M.A.; Rodriguez, N.; Winkler, P.; Lopez, J.; Dennison, M.; Liang, Y.; Turner, B.J. Comparing two sampling methods to engage hard-to-reach communities in research priority setting. BMC Med. Res. Methodol. 2016, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.A.; Cox, T.F. Multidimensional Scaling. In Handbook of Data Visualization; Chen, C.-h., Härdle, W., Unwin, A., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2008; 14p. [Google Scholar]
- Scheller-Kreinsen, D.; Quentin, W.; Busse, R. DRG-based hospital payment systems and technological innovation in 12 European countries. Value Health 2011, 14, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Bak, K.; Dobrow, M.J.; Hodgson, D.; Whitton, A. Factors affecting the implementation of complex and evolving technologies: Multiple case study of intensity-modulated radiation therapy (IMRT) in Ontario, Canada. BMC Health Serv. Res. 2011, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E. Diffusion of Innovations; New York Free Press: New York, NY, USA, 1995; pp. 261–274. [Google Scholar] [CrossRef]
- Clark, D.; Dean, G.; Bolton, S.; Beeson, B. Bench to bedside: The technology adoption pathway in healthcare. Health Technol. 2020, 10, 537–545. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond Adoption: A New Framework for Theorizing and Evaluating Nonadoption, Abandonment, and Challenges to the Scale-Up, Spread, and Sustainability of Health and Care Technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Robert, G.; Macfarlane, F.; Bate, P.; Kyriakidou, O. Diffusion of innovations in service organizations: Systematic review and RECOMMENDATIONS. Milbank Q. 2004, 82, 581–629. [Google Scholar] [CrossRef] [PubMed]
- Dreger, M.; Eckhardt, H.; Felgner, S.; Ermann, H.; Lantzsch, H.; Rombey, T.; Busse, R.; Henschke, C.; Panteli, D. Correction to: Implementation of innovative medical technologies in German inpatient care: Patterns of utilization and evidence development. Implement. Sci. 2022, 17, 8. [Google Scholar] [CrossRef]
- Cook, K.; Bergeron, K. Using Group concept mapping to engage a hard-to-reach population in research: Young adults with life-limiting conditions. Int. J. Qual. Methods 2019, 18, 1609406919891315. [Google Scholar] [CrossRef]
- Rising, K.L.; LaNoue, M.; Gentsch, A.T.; Doty, A.M.B.; Cunningham, A.; Carr, B.G.; Hollander, J.E.; Latimer, L.; Loebell, L.; Weingarten, G.; et al. The power of the group: Comparison of interviews and group concept mapping for identifying patient-important outcomes of care. BMC Med. Res. Methodol. 2019, 19, 7. [Google Scholar] [CrossRef]
- Wu, M.-J.; Zhao, K.; Fils-Aime, F. Response rates of online surveys in published research: A meta-analysis. Comput. Hum. Behav. Rep. 2022, 7, 100206. [Google Scholar] [CrossRef]





| Phase I | Phase II | All Phases | |||
|---|---|---|---|---|---|
| Brainstorming | Sorting | Rating Importance | Rating Changeability | All Tasks | |
| Participants | 28 | 31 | 33 | 29 | 45 |
| Member of the FURTHER consortium? | |||||
| yes | 24 (86%) | 24 (71%) | 23 (70%) | 19 (66%) | 32 (71%) |
| no | 4 (14%) | 10 (29%) | 10 (30%) | 10 (34%) | 13 (28%) |
| Per country | |||||
| Germany | 6 (21%) | 7 (21%) | 7 (21%) | 6 (19%) | 9 (20%) |
| Finland | 4 (14%) | 6 (18%) | 6 (18%) | 6 (19%) | 7 (16%) |
| Italy | 5 (18%) | 11 (32%) | 11 (33%) | 10 (34%) | 12 (27%) |
| Netherlands | 11 (39%) | 10 (29%) | 9 (27%) | 7 (24%) | 15 (33%) |
| Other | 2 (7%) | 0 | 0 | 0 | 2 (4%) |
| Expertise in relation to the MR-HIFU provision | |||||
| Patient | 1 (4%) | 0 | 0 | 0 | 1 (4%) |
| Expertise on performing HIFU treatment | 9 (32%) | 10 (29%) | 10 (30%) | 7 (24%) | 14 (34%) |
| Expertise on other medical specialties | 9 (32%) | 14 (41%) | 14 (42%) | 14 (48%) | 19 (42%) |
| Expertise on the HIFU technology | 7 (25%) | 8 (24%) | 7 (21%) | 6 (21%) | 9 (20 %) |
| Expertise on the Value Proposition/ Financial aspects | 2 (7%) | 2 (6%) | 2 (6%) | 2 (7%) | 2 (4%) |
| Self-perceived knowledge on MR-HIFU latest evidence | |||||
| Excellent | 8 (29%) | 6 (18%) | 5 (15%) | 4 (14%) | 9 (20%) |
| Good | 12 (43%) | 16 (47%) | 16 (48%) | 14 (48%) | 19 (42%) |
| Regular | 5 (18%) | 6 (18%) | 6 (18%) | 5 (17%) | 9 (20%) |
| Low | 2 (7%) | 6 (18%) | 6 (18%) | 6 (21%) | 7 (16%) |
| Cluster | Statements | ||
|---|---|---|---|
| ID Number | Caption | ID Number | Representative Statement (ID) |
| 1 | Competitive treatments | 6 | Availability of ultrasound-guided HIFU as a competitive treatment alternative |
| 2 | Physicians’ attitude | 13 | Unfamiliarity among referring physicians with MR-HIFU as a treatment option |
| 3 | Alignment of resources | 31 | Frequency of time slots at the MRI dedicated for HIFU |
| 4 | Logistics and workflow | 46 | Lack of an established patient workflow (from HIFU-indication to release of the patient) |
| 5 | Technical disadvantages | 7 | MR-HIFU is a lengthy procedure |
| 6 | Radiotherapy as first-line therapy | 25 | HIFU is less flexible with respect to different anatomical regions compared to radiotherapy |
| 7 | Aggregating knowledge and Improving awareness | 12 | Clear position of MR-HIFU in clinical guidelines |
| 8 | Clinical effectiveness | 34 | Clinical evidence from randomized clinical trials on the effectiveness of MR-HIFU |
| 9 | Patients’ preferences | 19 | Enthusiasm for the non-invasive treatment |
| 10 | Reimbursement | 10 | Reimbursement of MR-HIFU as inpatient procedure |
| 11 | Cost-effectiveness and | 40 | Evidence on cost-effectiveness in relation to standard of care |
| 12 | Hospital Costs | 48 | Costs of initial setup (purchase of equipment, installation, etc.) |
| Cluster ID | Cluster | Average Perceived Importance | Coherence of Perception between Countries a |
|---|---|---|---|
| 8 | Clinical effectiveness | 2.99 | 0.14 |
| 6 | Radiotherapy as first-line therapy | 2.89 | 0.03 |
| 9 | Patients’ preferences | 2.73 | 0.03 |
| 3 | Alignment of resources | 2.65 | 0.08 |
| 7 | Aggregating knowledge and improving awareness | 2.62 | 0.00 |
| 10 | Reimbursement | 2.56 | 0.34 |
| 11 | Cost-effectiveness | 2.55 | 0.02 |
| 4 | Logistics and workflow | 2.53 | 0.02 |
| 2 | Physicians’ attitude | 2.45 | 0.02 |
| 12 | Hospital costs | 2.45 | 0.05 |
| 5 | Technical disadvantages | 2.24 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões Corrêa Galendi, J.; Siefen, A.-C.; Moretti, D.M.; Yeo, S.Y.; Grüll, H.; Bratke, G.; Morganti, A.G.; Bazzocchi, A.; Gasperini, C.; De Felice, F.; et al. Factors Influencing the Adoption of Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Painful Bone Metastases in Europe, A Group Concept Mapping Study. Int. J. Environ. Res. Public Health 2023, 20, 1084. https://doi.org/10.3390/ijerph20021084
Simões Corrêa Galendi J, Siefen A-C, Moretti DM, Yeo SY, Grüll H, Bratke G, Morganti AG, Bazzocchi A, Gasperini C, De Felice F, et al. Factors Influencing the Adoption of Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Painful Bone Metastases in Europe, A Group Concept Mapping Study. International Journal of Environmental Research and Public Health. 2023; 20(2):1084. https://doi.org/10.3390/ijerph20021084
Chicago/Turabian StyleSimões Corrêa Galendi, Julia, Ann-Cathrine Siefen, Debora M. Moretti, Sin Yuin Yeo, Holger Grüll, Grischa Bratke, Alessio Giuseppe Morganti, Alberto Bazzocchi, Chiara Gasperini, Francesca De Felice, and et al. 2023. "Factors Influencing the Adoption of Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Painful Bone Metastases in Europe, A Group Concept Mapping Study" International Journal of Environmental Research and Public Health 20, no. 2: 1084. https://doi.org/10.3390/ijerph20021084
APA StyleSimões Corrêa Galendi, J., Siefen, A.-C., Moretti, D. M., Yeo, S. Y., Grüll, H., Bratke, G., Morganti, A. G., Bazzocchi, A., Gasperini, C., De Felice, F., Blanco Sequeiros, R., Huhtala, M., Nijholt, I. M., Boomsma, M. F., Bos, C., Verkooijen, H. M., Müller, D., & Stock, S. (2023). Factors Influencing the Adoption of Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Painful Bone Metastases in Europe, A Group Concept Mapping Study. International Journal of Environmental Research and Public Health, 20(2), 1084. https://doi.org/10.3390/ijerph20021084