First Data on PAE Levels in Surface Water in Lakes of the Eastern Coast of Baikal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
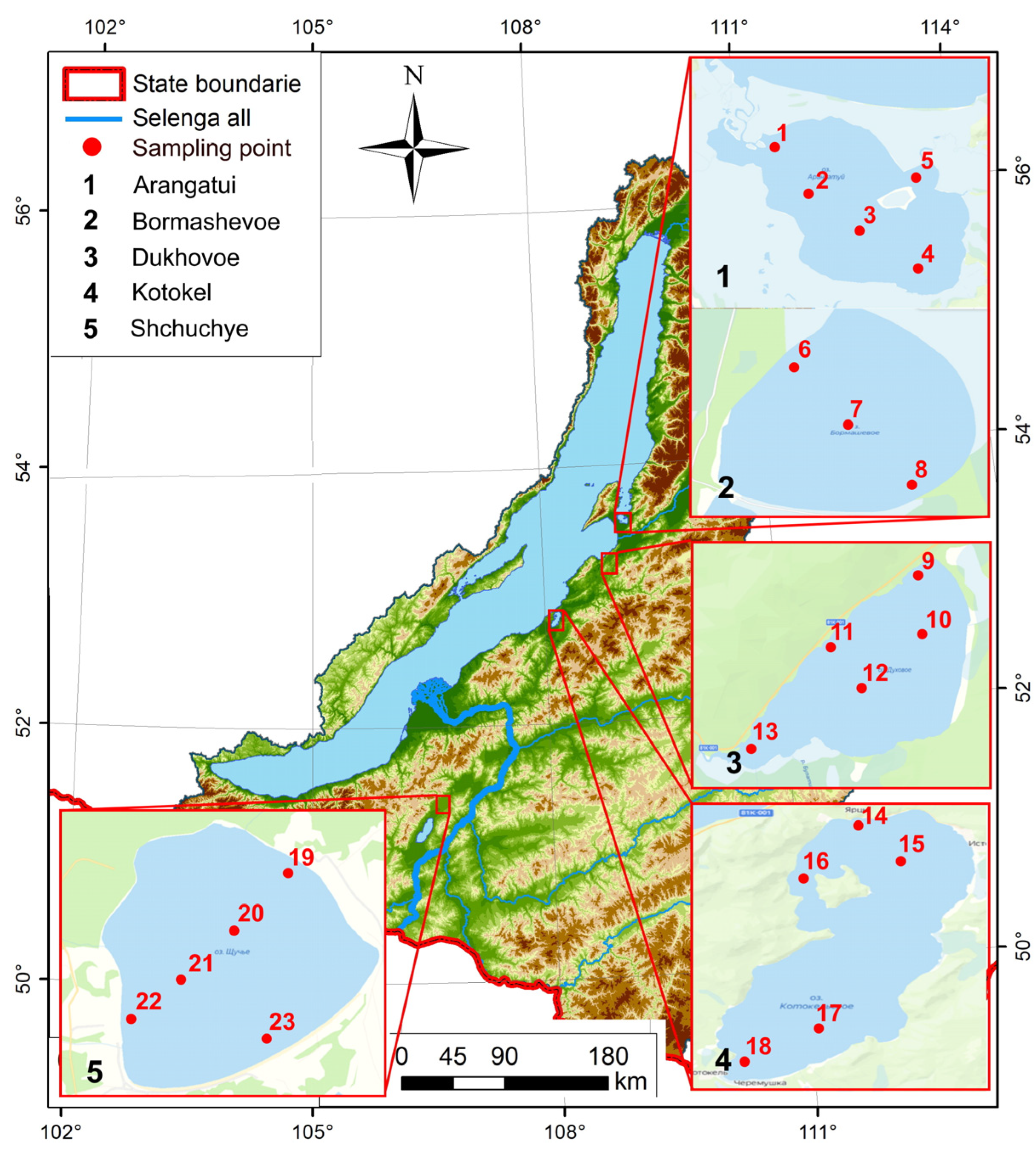
2.2. Study Area and Sampling
2.3. Sample Pretreatment
2.4. GC–MS Analysis
2.5. Quality Assurance and Quality Control
2.6. Analysis of Water Quality Parameters
2.7. Ecological Risk Assessment
2.7.1. Human Health Risk
2.7.2. Freshwater Risk Assessment
2.8. Data Analysis
3. Results
3.1. Water Quality
3.2. Compositional Profiles and Concentrations of PAEs
3.3. Ecological Risk Assessment
Drinking Water Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyche, J.L. 1 Phthalates. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 829–856. [Google Scholar]
- Gómez-Hens, A.; Aguilar-Caballos, M.P. Social and economic interest in the control of phthalic acid esters. TrAC Trends Anal. Chem. 2003, 22, 847–857. [Google Scholar] [CrossRef]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.-I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Assessment of the State-of-the-Science of Endocrine Disruptors. Available online: https://apps.who.int/iris/handle/10665/67357 (accessed on 28 November 2022).
- Tan, S.; Wang, D.; Chi, Z.; Li, W.; Shan, Y. Study on the interaction between typical phthalic acid esters (PAEs) and human haemoglobin (hHb) by molecular docking. Environ. Toxicol. Pharmacol. 2017, 53, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Al-Rajudi, T.; Al-Qudaihi, G.; Manogaran, P. Evaluating the potential genotoxicity of phthalates esters (PAEs) in perfumes using in vitro assays. Environ. Sci. Pollut. Res. 2017, 24, 23903–23914. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Jönsson, B.A.G.; Lindh, C.H.; Jensen, T.K.; Hjollund, N.H.; Vested, A.; Bonde, J.P. Association between Pregnancy Loss and Urinary Phthalate Levels around the Time of Conception. Environ. Health Perspect. 2012, 120, 458–463. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. List of Classifications. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 28 November 2022).
- Yu, L.; Li, H.-X.; Guo, J.-Y.; Huang, Y.-Q.; Wang, H.; Talukder, M.; Li, J.-L. Di (2-ethyl hexyl) phthalate (DEHP)-induced spleen toxicity in quail (Coturnix japonica) via disturbing Nrf2-mediated defense response. Environ. Pollut. 2019, 251, 984–989. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef]
- Baram, G.I.; Azarova, I.N.; Gorshkov, A.G.; Vereshchagin, A.L.; Lang, B.; Kiryukhina, E.D. Determination of bis(2-ethylhexyl) phthalate in water by high-performance liquid chromatography with direct on-column preconcentration. J. Anal. Chem. 2000, 55, 750–754. [Google Scholar] [CrossRef]
- Azarova, I.N. HPLC Method for Determination of di(2-ethylhexyl) Phthalate to Study its Behavior in the Lake Baikal Ecosystem. Ph.D. Thesis, Institute of Physical Chemistry RAS (Moscow), Irkutsk, Russia, 2003. [Google Scholar]
- Ilyina, O.V.; Kolobov, M.Y.; Ilyunsky, V.V. Plastic pollution of the coastal surface water in the middle and southern Baikal. Water Resour. 2021, 48, 56–64. [Google Scholar] [CrossRef]
- Gorshkov, A.G.; Babenko, T.A.; Kustova, O.V.; Izosimova, O.N.; Shishlyannikov, S.M. Priority phthalates in the pelagic and coastal zone of Lake Baikal. Chem. Sustain. Dev. 2017, 25, 375–383. [Google Scholar] [CrossRef]
- Semenov, A.A.; Enikeev, A.G.; Babenko, T.A.; Shafikova, T.N.; Gorshkov, A.G. Phthalates—A strange delusion of ecologists. Theor. Appl. Ecol. 2021, 1, 16–21. [Google Scholar] [CrossRef]
- Babu, B.; Wu, J.-T. Production of phthalate esters by nuisance freshwater algae and cyanobacteria. Sci. Total Environ. 2010, 408, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; et al. Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci. Rep. 2016, 6, 19791. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Lee, J.-E.; Choe, W.; Kim, T.; Lee, J.-Y.; Kho, Y.; Choi, K.; Zoh, K.-D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef]
- U.S. EPA Office of Emergency and Remedial Response. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). EPA/540/1-89/002. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (accessed on 28 November 2022).
- U.S. EPA. Regional Screening Level (RSL)–Generic Tables. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 28 November 2022).
- Wu, B.; Zhang, Y.; Zhang, X.; Cheng, S. Health risk from exposure of organic pollutants through drinking water consumption in Nanjing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 46–50. [Google Scholar] [CrossRef]
- Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances. Part II. Available online: https://op.europa.eu/en/publication-detail/-/publication/9aebb292-39c5-4b9c-b4cb-97fb02d9bea2/language-en/format-PDF/source-276274071 (accessed on 28 November 2022).
- Selvaraj, K.K.; Sundaramoorthy, G.; Ravichandran, P.K.; Girijan, G.K.; Sampath, S.; Ramaswamy, B.R. Phthalate esters in water and sediments of the Kaveri River, India: Environmental levels and ecotoxicological evaluations. Environ. Geochem. Health 2015, 37, 83–96. [Google Scholar] [CrossRef]
- Shanmugam, G.; Sampath, S.; Selvaraj, K.K.; Larsson, D.G.J.; Ramaswamy, B.R. Non-steroidal anti-inflammatory drugs in Indian rivers. Environ. Sci. Pollut. Res. 2014, 21, 921–931. [Google Scholar] [CrossRef]
- Li, X.; Yin, P.; Zhao, L. Phthalate esters in water and surface sediments of the Pearl River Estuary: Distribution, ecological, and human health risks. Environ. Sci. Pollut. Res. 2016, 23, 19341–19349. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. ECOTOX Knowledgebase. Available online: http://cfpub.epa.gov/ecotox (accessed on 28 November 2022).
- Cristale, J.; García Vázquez, A.; Barata, C.; Lacorte, S. Priority and emerging flame retardants in rivers: Occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ. Int. 2013, 59, 232–243. [Google Scholar] [CrossRef]
- Adams, W.J.; Biddinger, G.R.; Robillard, K.A.; Gorsuch, J.W. A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Environ. Toxicol. Chem. 1995, 14, 1569–1574. [Google Scholar] [CrossRef]
- Ortiz-Zarragoitia, M.; Trant, J.M.; Cajaravillet, M.P. Effects of dibutylphthalate and ethynylestradiol on liver peroxisomes, reproduction, and development of zebrafish (Danio rerio). Environ. Toxicol. Chem. 2006, 25, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.E.; Adams, W.J.; Biddinger, G.R.; Robillard, K.A.; Gorsuch, J.W. Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 1995, 14, 1967–1976. [Google Scholar] [CrossRef]
- Gledhill, W.E.; Kaley, R.G.; Adams, W.J.; Hicks, O.; Michael, P.R.; Saeger, V.W.; Leblanc, G.A. An environmental safety assessment of butyl benzyl phthalate. Environ. Sci. Technol. 1980, 14, 301–305. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Yang, Z.; Cai, Z.; Mizuno, T.; Tsuno, H.; Zhu, W.; Zhang, X. Toxicity of seven phthalate esters to embryonic development of the abalone Haliotis diversicolor supertexta. Ecotoxicology 2009, 18, 293–303. [Google Scholar] [CrossRef]
- He, W.; Qin, N.; Kong, X.; Liu, W.; He, Q.; Ouyang, H.; Yang, C.; Jiang, Y.; Wang, Q.; Yang, B.; et al. Spatio-temporal distributions and the ecological and health risks of phthalate esters (PAEs) in the surface water of a large, shallow Chinese lake. Sci. Total Environ. 2013, 461, 672–680. [Google Scholar] [CrossRef]
- Ubugunov, L.L.; Pronin, N.M. (Eds.) Lake Kotokel’skoe: Natural Conditions, Biota, Ecology; Buryat Scientific Center SB RAS Publishing: Ulan-Ude, Russia, 2013; p. 320. [Google Scholar]
- Angakhaeva, N.A.; Plyusnin, A.M.; Ukraintsev, A.U.; Chernyavskii, M.K.; Peryazeva, E.G.; Zhambalova, D.I. Hydrogeochemical features of Lake Kotokel. Earth Sci. Subsoil Use 2021, 44, 106–115. [Google Scholar] [CrossRef]
- Khakhinov, V.V.; Namsaraev, B.B.; Ul’zetueva, I.D.; Barkhutova, D.D.; Abidueva, E.Y.; Banzaraktsaeva, T.G. Hydrochemical and microbiological characteristics of the Gusino-Ubukunskaya group of water bodies. Water Resour. 2005, 32, 73–78. [Google Scholar] [CrossRef]
- Maltsev, A.E.; Bogush, A.A.; Leonova, G.A. Peculiarities of the chemical composition of pore water in the Holocene sapropel section of Dukhovoe Lake (Southern Baikal). Chem. Sustain. Dev. 2014, 22, 517–534. [Google Scholar]
- Tsyrenova, D.D.; Garankina, V.P.; Dagurova, O.P.; Dambaev, V.B. Conditions for the cyanobacteria development in the lakes of Baikal coastal zone. Buryat State Univ. Bull. (Chem. Phys.) 2016, 4, 11–16. [Google Scholar] [CrossRef]
- Peryazeva, E.G.; Plyusnin, A.M.; Garmaeva, S.Z.; Budaev, R.T.; Zhambalova, D.I. Peculiarities of the formation of the chemical composition of water in lakes on the eastern shore of Lake Baikal. Geo. Nat. Res. 2016, 5, 49–59. [Google Scholar] [CrossRef]
- SanPiN 1.2.3685-21 Hygienic Standards and Requirements to Ensure Safety and (or) Harmful for Human Environmental Factors. Available online: https://faolex.fao.org/docs/pdf/taj183394.pdf (accessed on 30 November 2022).
- List of Fishery Regulations: Maximum Allowable Concentrations (MAC) and Safe Reference Levels of Impact (SRLI) for Harmful Substances in Water Bodies of Fishery Significance. Approved by VNIRO Order No. 96, dated 28.04.99.
- Plyusnin, A.M.; Ukraintsev, A.V.; Chernyavskii, M.K.; Peryazeva, E.G.; Angakhaeva, N.A. Factors and processes of salt lake formation on the shore of the baikal. Water Resour. 2021, 48, 194–206. [Google Scholar] [CrossRef]
- Maksimova, L.A.; Shafikova, T.N. Endogenous phthalates as a potent regulator of interspecific relationships in biocenoses. Proc. Universities. Appl. Chem. Biotechnol. 2022, 12, 424–437. [Google Scholar] [CrossRef]
- Chen, C.Y. Biosynthesis of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) from red alga—Bangia atropurpurea. Water Res. 2004, 38, 1014–1018. [Google Scholar] [CrossRef]
- Babu, B.; Wu, J.-T. Biodegradation of phthalate esters by cyanobacteria. J. Phycol. 2010, 46, 1106–1113. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Mostafa, S.W.; Adel, A.A.; Ghanem, K.M. Production of di-(2-ethylhexyl) phthalate by Bacillus subtilis AD35: Isolation, purification, characterization and biological activities. Microb. Pathog. 2018, 124, 89–100. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Wen, Z.; Shang, Y.; Wang, X.; Fang, C.; Song, K. Evaluating the spatial distribution and source of phthalate esters in the surface water of Xingkai Lake, China during summer. J. Great Lakes Res. 2021, 47, 437–446. [Google Scholar] [CrossRef]
- Namikoshi, M.; Fujiwara, T.; Nishikawa, T.; Ukai, K. Natural Abundance 14C Content of Dibutyl Phthalate (DBP) from Three Marine Algae. Mar. Drugs 2006, 4, 290–297. [Google Scholar] [CrossRef]
- Babenko, T.A.; Shishlyannikov, S.M.; Gorshkov, A.G. Phytoplankton as a biogenic source of di-(2-ethylhexyl) phthalate in the water ecosystem of Lake Baikal. In Proceedings of the International Conference «Freshwater Eco-systems—Key Problems», Irkutsk, Russia, 10–14 September 2018; p. 96. [Google Scholar]
- Lertsirisopon, R.; Soda, S.; Sei, K.; Ike, M. Abiotic degradation of four phthalic acid esters in aqueous phase under natural sunlight irradiation. J. Environ. Sci. 2009, 21, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Occurrence and Air−Sea Exchange of Phthalates in the Arctic. Environ. Sci. Technol. 2007, 41, 4555–4560. [Google Scholar] [CrossRef]
- Lenoir, A.; Boulay, R.; Dejean, A.; Touchard, A.; Cuvillier-Hot, V. Phthalate pollution in an Amazonian rainforest. Environ. Sci. Pollut. Res. 2016, 23, 16865–16872. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Jia, H.-Y.; Wang, Y.; Li, T.; Wang, L.; Li, Q.-Q.; Yang, M.-M.; Yue, J.-J.; Yi, X.-L.; Guo, L.-Q. Characterization of PM2.5-bound phthalic acid esters (PAEs) at regional background site in northern China: Long-range transport and risk assessment. Sci. Total Environ. 2019, 659, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zuev, V.Y.; Antonovich, V.V.; Belan, B.D.; Zhbanov, E.F.; Mikushev, M.K.; Panchenko, M.V.; Podanev, A.V.; Tolmachev, G.N.; Shcherbatova, A.V. The phenomenon of air circulation along the Baikal Lake depression contour. Dokl. Akad. Nauk 1992, 325, 1146–1150. [Google Scholar]
- Argunchintseva, A.V.; Vologzhina, S.Z. Pollution of atmospheric air in the Baikal depression. Bull. Irkutsk State Univ. 2011, 4, 28–41. [Google Scholar]
- Arshinov, M.Y.; Belan, B.D.; Davydov, D.K.; Kovalevsky, V.K.; Plotnikov, A.P.; Pokrovsky, E.V.; Sklyadneva, T.K.; Tolmachev, G.N. Automatic post for monitoring of minor gas components of atmospheric air. Meteorol. Hydrol. 1999, 3, 110–118. [Google Scholar]
- Arshinova, V.G.; Arshinov, M.Y.; Belan, B.D.; Belan, S.B.; Ivlev, G.A.; Rasskazchikova, T.M.; Simonenkov, D.V.; Fofonov, A.V. Spatial distribution of small air impurities in the Lake Baikal depression. Atmos. Ocean. Opt. 2012, 25, 613–620. [Google Scholar]
- The Russian Market of Plasticizers will Expand by 2% during 2018. Available online: http://www.rupec.ru/news/39464 (accessed on 28 November 2022).
- Gao, X.; Li, J.; Wang, X.; Zhou, J.; Fan, B.; Li, W.; Liu, Z. Exposure and ecological risk of phthalate esters in the Taihu Lake basin, China. Ecotoxicol. Environ. Saf. 2019, 171, 564–570. [Google Scholar] [CrossRef]
- Luo, X.; Shu, S.; Feng, H.; Zou, H.; Zhang, Y. Seasonal distribution and ecological risks of phthalic acid esters in surface water of Taihu Lake, China. Sci. Total Environ. 2021, 768, 144517. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hong, H.; Liu, X.; Guan, W.; Meng, L.; Ye, Y.; Ma, Y. Graphene-dispersive solid-phase extraction of phthalate acid esters from environmental water. Sci. Total Environ. 2013, 444, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, B.-T.; Teng, Y. Distribution of phthalate acid esters in lakes of Beijing and its relationship with anthropogenic activities. Sci. Total Environ. 2014, 476–477, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nantaba, F.; Palm, W.-U.; Wasswa, J.; Bouwman, H.; Kylin, H.; Kümmerer, K. Temporal dynamics and ecotoxicological risk assessment of personal care products, phthalate ester plasticizers, and organophosphorus flame retardants in water from Lake Victoria, Uganda. Chemosphere 2021, 262, 127716. [Google Scholar] [CrossRef]
- Le, T.M.; Nguyen, H.M.N.; Nguyen, V.K.; Nguyen, A.V.; Vu, N.D.; Yen, N.T.H.; Hoang, A.Q.; Minh, T.B.; Kannan, K.; Tran, T.M. Profiles of phthalic acid esters (PAEs) in bottled water, tap water, lake water, and wastewater samples collected from Hanoi, Vietnam. Sci. Total Environ. 2021, 788, 147831. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Liu, H.; Wan, G.; Zhang, S. The occurrence and ecological risk assessment of phthalate esters (PAEs) in urban aquatic environments of China. Ecotoxicology 2015, 24, 967–984. [Google Scholar] [CrossRef]


| PAE | Hydrobiont | Species | Toxicity, µg/L | Assessment Factor, AF | PNEC (Water), µg/L | Reference |
|---|---|---|---|---|---|---|
| DMP | Algae | Pseudokirchneriella subcapitata | EC50 = 142,000 | 1000 | 142 | [32] |
| Cladoceran | Daphnia magna | EC50 = 33,000 | 1000 | 33 | ||
| Fish | Lepomis macrochirus | EC50 = 50,000 | 1000 | 50 | ||
| DEP | Algae | Pseudokirchneriella subcapitata | EC50 = 16,000 | 1000 | 16 | |
| Cladoceran | Daphnia magna | EC50 = 86,000 | 1000 | 86 | ||
| Fish | Lepomis macrochirus | NOEC = 1650 | 100 | 16,5 | ||
| DBP | Algae | Pseudokirchneriella subcapitata | EC50 = 400 | 1000 | 142 | |
| Cladoceran | Daphnia magna | EC50 = 33,000 | 1000 | 33 | ||
| Fish | Danio rerio (Zebra danio) | NOEC = 100 | 100 | 1 | [33] | |
| BBP | Algae | Pseudokirchneriella subcapitata | NOEC = 30 | 100 | 0.3 | [34] |
| Cladoceran | Daphnia magna | EC50 = 3700 | 1000 | 3.7 | [35] | |
| Fish | Lepomis macrochirus | EC50 = 1700 | 1000 | 17 | [32] | |
| DEHP | Algae | Pseudokirchneriella subcapitata | EC50 = 100 | 1000 | 0.1 | |
| Cladoceran | Daphnia magna | EC50 = 77 | 1000 | 0.77 | [34] | |
| Fish | Lepomis macrochirus | EC50 = 200 | 1000 | 0.2 | [32] | |
| DnOP | Algae | Pseudokirchneriella subcapitata | EC50 = 100 | 1000 | 0.1 | |
| Cladoceran | Molluscs Haliotis diversicolor | NOEC = 17.9 | 100 | 0.179 | [36] | |
| Fish | Channel Catfish | EC50 = 700 | 1000 | 0.7 | [37] |
| PAE | MAC [44] | Lakes | |||||
|---|---|---|---|---|---|---|---|
| Arangatui | Bormashevoe | Dukhovoe | Kotokel | Shchuchye | |||
| DMP | min-max 1 mean | 300 | nd 2–0.23 0.12 | nd–0.52 0.30 | 0.13–0.94 0.51 | 0.03–1.17 0.37 | nd–0.09 0.02 |
| DEP | min-max mean | 3000 | 0.21–2.07 1.04 | 0.01–0.48 0.32 | 0.13–0.61 0.43 | 0.21–0.70 0.46 | 0.15–0.42 0.26 |
| DBP | min-max mean | 200 | nd–8.76 4.29 | 0.95–14.02 5.45 | 2.53–14.73 11.23 | nd–25.50 15.05 | nd–1.62 0.34 |
| BBP | min-max mean | – | nd | nd | nd | nd | nd–0.15 0.07 |
| DEHP | min-max mean | 8 | 2.50–18.48 7.41 | 2.83–20.81 14.45 | nd–1.49 0.49 | nd–4.98 1.25 | nd–4.35 1.48 |
| DnOP | min-max mean | 1600 | nd–0.10 0.03 | nd–0.08 0.03 | nd–0.04 0.01 | nd–0.51 0.21 | nd–0.46 0.13 |
| ∑PAEs | 12.9 | 20.6 | 12.7 | 17.3 | 2.3 | ||
| Lake | PAE | Level of Exposure (AE), µg/kg/Day | RfD, µg/kg/Day [23] | HQ |
|---|---|---|---|---|
| Arangatui | DBP | 0.2942 | 100 | 0.00294 |
| DEHP | 0.2541 | 20 | 0.01270 | |
| DEP | 0.0357 | 800 | 0.00004 | |
| BBP | - | 200 | - | |
| DnOP | 0.0010 | 10 | 0.00010 | |
| HI | 0.01578 | |||
| Bormashevoe | DBP | 0.1869 | 100 | 0.00187 |
| DEHP | 0.4954 | 20 | 0.02477 | |
| DEP | 0.0110 | 800 | 0.00001 | |
| BBP | - | 200 | - | |
| DnOP | 0.0010 | 10 | 0.00010 | |
| HI | 0.02675 | |||
| Dukhovoe | DBP | 0.3850 | 100 | 0.00385 |
| DEHP | 0.0168 | 20 | 0.00084 | |
| DEP | 0.0147 | 800 | 0.00002 | |
| BBP | - | 200 | - | |
| DnOP | 0.0003 | 10 | 0.00003 | |
| HI | 0.00474 | |||
| Kotokel | DBP | 0.5160 | 100 | 0.00516 |
| DEHP | 0.0429 | 20 | 0.00214 | |
| DEP | 0.0158 | 800 | 0.00002 | |
| BBP | - | 200 | - | |
| DnOP | 0.0072 | 10 | 0.00804 | |
| HI | 0.00313 | |||
| Shchuchye | DBP | 0.0117 | 100 | 0.00012 |
| DEHP | 0.0507 | 20 | 0.00254 | |
| DEP | 0.0089 | 800 | 0.00001 | |
| BBP | 0.0024 | 200 | 0.00001 | |
| DnOP | 0.0045 | 10 | 0.00045 | |
| HI | 0.00313 | |||
| Lake | DMP | DEP | DBP | DEHP | BBP | DnOP | Reference |
|---|---|---|---|---|---|---|---|
| Taihu (China) | nd–1.32 | 0.08–4.79 | nd–2.54 | nd–1.41 | 0.08–4.72 | 0.07–0.590 | [63] |
| Taihu (China) | [64] | ||||||
| Dry Season | nd–0.16 | nd-0.15 | nd–0.48 | nd–1.47 | nd | nd | |
| Normal Season | nd–0.80 | nd–0.12 | nd–0.19 | nd–3.31 | nd–1.31 | nd–0.65 | |
| Wet season | nd–0.11 | nd–0.14 | 0.02–2.88 | nd–2.65 | nd–0.68 | nd | |
| Chao Hu (China) | 0.015–3.670 | 0.006–0.283 | 0.070–17.529 | nd–0.576 | nd-0.107 | nd–0.045 | [37] |
| Dong Hu (China) | — | nd | 9 | 14 | — | — | [65] |
| Da Ming Hu (China) | — | nd | 51 | 8 | — | — | [65] |
| Kunming (China) | — | nd | 17 | nd | — | — | [65] |
| Shichahai (China) | 0.047–0.143 | 0.006–0.013 | 0.009–0.157 | 0.140–0.519 | nd–0.512 | 0.015–0.022 | [66] |
| Lakes in Summer Palace (China) | 0.039–0.082 | nd-0.011 | 0.058–0.515 | 0.139–0.393 | nd–0.021 | 0.016–0.024 | [66] |
| Baikal (Russia) | — | — | 0.35–0.89 | 0.06–0.32 | — | — | [16] |
| Small Xingkai (China) | 0.001–0.011 | 0.002–0.007 | 0.104–0.530 | 0.123–3.247 | 0.001–0.005 | nd–0.04 | [51] |
| Large Xingkai (China) | 0.003–0.026 | 0.003–0.018 | 0.109–0.520 | 0.223–3.456 | nd-0.002 | nd–0.007 | |
| Victoria (Uganda) | 0.006–0.400 | 0.038–1.100 | 0.35–16.0 | 0.21–23.0 | — | — | [67] |
| Asan (Korea) | nd–0.18 | nd–0.05 | nd–0.34 | nd–1.34 | nd | nd–0.02 | [21] |
| Thien Quang, Ba Mau, Hoan Kiem, Yen So, Truc Bach, and West Lake (Vietnam) | 0.115–2.950 | 0.639–14.00 | 0.783–34.00 | 1.040–48.70 | 0.182–21.100 | nd–7.310 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazarsadueva, S.V.; Taraskin, V.V.; Budaeva, O.D.; Nikitina, E.P.; Zhigzhitzhapova, S.V.; Shiretorova, V.G.; Bazarzhapov, T.Z.; Radnaeva, L.D. First Data on PAE Levels in Surface Water in Lakes of the Eastern Coast of Baikal. Int. J. Environ. Res. Public Health 2023, 20, 1173. https://doi.org/10.3390/ijerph20021173
Bazarsadueva SV, Taraskin VV, Budaeva OD, Nikitina EP, Zhigzhitzhapova SV, Shiretorova VG, Bazarzhapov TZ, Radnaeva LD. First Data on PAE Levels in Surface Water in Lakes of the Eastern Coast of Baikal. International Journal of Environmental Research and Public Health. 2023; 20(2):1173. https://doi.org/10.3390/ijerph20021173
Chicago/Turabian StyleBazarsadueva, Selmeg V., Vasilii V. Taraskin, Olga D. Budaeva, Elena P. Nikitina, Svetlana V. Zhigzhitzhapova, Valentina G. Shiretorova, Tcogto Zh. Bazarzhapov, and Larisa D. Radnaeva. 2023. "First Data on PAE Levels in Surface Water in Lakes of the Eastern Coast of Baikal" International Journal of Environmental Research and Public Health 20, no. 2: 1173. https://doi.org/10.3390/ijerph20021173





