3.1. Element Concentrations in the Amara Lake Sediment Core
The results of the analysis of 34 elements show for most of them a distinct pattern, in which some of them occur in the largest amount in the upper part of the sediment core, down to 20 cm, and occur in much lower concentrations below this depth. Other elements show an opposite trend, occurring in much smaller amounts in the upper 20 cm, and have their highest concentration under this level. In addition, some elements do not fit any of these two main patterns mentioned above; other trends can also be distinguished within these two main patterns. The section/border between 17 and 21 cm seems to be significant for understanding the chemical stratigraphy of the investigated sediment core.
The concentration profiles are presented in
Figure 2,
Figure 3,
Figure 4 and
Figure 5, with the analysed elements divided into main elements, heavy metals (HM), light rare earth elements (LREE), heavy rare earth elements (HREE), and trace elements (TE).
The concentrations of main elements measured in the sediment core are shown in
Figure 2. The Na concentration ranged from 7.17 to 12.00 g·kg
−1, with an average value of 9.60 g·kg
−1; a gradual increase in concentration with three levels of variation structured on the depth, from the bottom to the surface, of the sediment core can be noticed: 94–65 cm (average value of 8.25 g·kg
−1), 63–23 cm (average value of 9.77 g·kg
−1), and 21–1.0 cm (average value of 11.14 g·kg
−1). The concentration of Mg had a range from 16.20 to 36.30 g·kg
−1, with an average value of 29.40 g·kg
−1; a variation of the concentration can be observed on two depth levels, from the bottom to the surface, of the sediment core, with a gradual decrease in the second (surface) level: 94–19 cm (average value of 31.36 g·kg
−1) and 17–1.0 cm (average value of 21.12 g·kg
−1).
The Al concentration profile (39.50 to 68.10 g·kg−1 range, with an average value of 59.04 g·kg−1) has a similar trend with that of Mg observed on two depth levels, from the bottom to the surface, of the sediment core: 94–19 cm (average value of 62.02 g·kg−1) and 17–1.0 cm (average value of 46.44 g·kg−1).
The concentration of K had a range from 11.90 to 18.40 g·kg−1, with an average value of 15.60 g·kg−1, and a relatively similar trend with that of Na (three levels of variation structured on depth, from the bottom to the surface) in the sediment core: 94–67 cm (average value of 16.59 g·kg−1), 65–17 cm (average value of 15.44 g·kg−1), and 15–1.0 cm (average value of 14.38 g·kg−1).
The Ca concentration profile ranged from 27.20 to 60.20 g·kg−1, with an average value of 47.97 g·kg−1; four levels of variation (from the bottom to the surface of the sediment core) can be noticed: 94–77 cm (average value of 44.24 g·kg−1), 75–25 cm (average value of 53.30 g·kg−1), 23–11 cm (average value of 31.77 g·kg−1), and 9–1.0 cm (average value of 49.68 g·kg−1).
A similar variation (a notable decrease in the sediment core) with Ca concentration in the 23–11 cm layer is also observed for Al, Ni, Co, Zn, and Fe; this pattern suggests a significant reduction in pedological input. The decrease in the content of Fe correlated with that of Ca may be due to the intensification of the reducing conditions in the hypolimnion (which increases the solubility of Fe), due either to the accumulation of humus in a large amount or to the existence of a stratification of water. The content of Sr does not register, in the same sediment layer, a significant variation compared to Ca; as a result, the increase in the Sr:Ca ratio indicates a progressive increase in salinity, also observed by the variation in the content of Cl and Br (it must be taken into account that part of the Sr can come from the aeolian input of clastic material).
The concentration of Ti ranged from 3.28 to 6.29 g·kg−1, with an average value of 4.81 g·kg−1; a variation of the concentration can be observed on two depth levels, from the bottom to the surface, of the sediment core, with a gradual decrease in the second (surface) level: 94–11 cm (average value of 4.94 g·kg−1) and 9.0–1.0 cm (average value of 3.78 g·kg−1).
Both the Mn and Fe profile concentrations share almost a similar trend, except younger sediment layers (17–1.0 cm depth), where Fe (average value 23.62 g·kg
−1) and Mn (average value 0.84 g·kg
−1) concentrations increase and decrease (from the bottom to the surface), respectively; a relatively small variation of the concentration can be observed also for both elements on three depth levels, from the bottom to the surface, of the sediment core: 94–85 cm (average values of 1.04 g·kg
−1 for Mn and 25.22 g·kg
−1 for Fe), 83–67 cm (average values of 1.06 g·kg
−1 for Mn and 27.67 g·kg
−1 for Fe), and 65–19 cm (average values of 0.87 g·kg
−1 for Mn and 24.36 g·kg
−1 for Fe). Surface processes, diagenetic transformation, and humic matter interactions influence the net burial rates of Fe and Mn and can obscure the interpretation of Mn/Fe ratio as a redox proxy [
72]. Residual Fe and Mn, and crystalline Fe oxy(hydr)oxides and Mn oxides, are primarily sourced from the catchment soils, whereas humic Fe and Mn is related to dissolved organic carbon in the lake and leaching from soils and wetlands. Fe and Mn seem to behave similarly to catchment processes, though Fe accumulation is considerably higher; crystalline Fe oxy(hydr)oxides and Mn oxides are generally stable under all prevailing redox conditions [
72,
73]. The Mn/Fe ratio is lower, mainly due to the higher allogenic input of the crystalline Fe oxy(hydr)oxides in the sediments. Periods of higher detrital input effects can be distinguished and corrected by normalization with Ti [
74,
75], as can be seen in the Fe/Ti and Mn/Ti ratios [
72].
The concentration profile of Cl (0.17 to 2.63 g·kg−1 range, with an average value of 0.68 g·kg−1) and Br (0.17 to 2.63 mg·kg−1 range, with an average value of 0.68 mg·kg−1) have almost a similar pattern, with three depth levels, from the bottom to the surface, of the sediment core: 94–55 cm (average values of 0.30 g·kg−1 for Cl and 6.45 mg·kg−1 for Br), 53–21 cm (average values of 0.48 g·kg−1 for Cl and 10.92 mg·kg−1 for Br), and 19–1.0 cm (average values of 1.78 g·kg−1 for Cl and 20.06 mg·kg−1 for Br). For both Cl and Br, a significant increase of concentration can be observed in the young sediment layers (the last level from the surface previously mentioned).
The concentrations of heavy metals/potentially toxic elements measured in the sediment core are shown in
Figure 3. The V concentration ranged from 51.20 to 107.00 mg·kg
−1, with an average value of 87.08 mg·kg
−1; a decrease in concentration in the upper layers of the sediment core can be noticed: 94–19 cm (average value of 93.91 mg·kg
−1) and 17–1.0 cm (average value of 60.79 mg·kg
−1).
The concentration of Cr had a range from 86.10 to 131.00 mg·kg−1, with an average value of 104.58 mg·kg−1; an increase in concentration can be seen in the upper layers of the sediment core: 94–17 cm (average value of 102.91 mg·kg−1) and 15–1.0 cm (average value of 114.11 mg·kg−1).
The concentration profiles of Ni (22.60 to 48.30 mg·kg−1 concentration range, with an average value of 40.45 mg·kg−1) and Co (7.58 to 14.30 mg·kg−1 concentration range, with an average value of 11.53 mg·kg−1) share a similar pattern from the bottom to the surface of the sediment core: 94–21 cm (Ni—average value of 42.63 mg·kg−1 and Co—average value of 11.98 mg·kg−1), 17–11 cm (Ni—average value of 23.68 mg·kg−1 and Co—average value of 8.09 mg·kg−1), and 9–1.0 cm (Ni—average value of 39.14 mg·kg−1 and Co—average value of 10.88 mg·kg−1). Similarities in the sediment core variability of iron and manganese (especially iron) and heavy metals like Ni, Co, and Zn indicate sorption or co-precipitation of these elements with Fe and Mn hydroxides and oxides, as well as sorption by clay minerals, chemical processes involving hydrolytic reactions and both complexed and dissolved ions. Zn, V, Ni, and Co can be linked mostly to the lake catchment rocks, implying a source-rock effect controlling the vertical distribution of these elements, especially in the lower bottom layers.
The Zn concentration ranged from 38.00 to 95.40 mg·kg
−1, with an average value of 62.42 mg·kg
−1; the concentration profile looks almost similar to those of Ni and Co. The concentration of As had a range from 5.53 to 13.20 mg·kg
−1, with an average value of 8.62 mg·kg
−1. Most of the potentially toxic elements, including V, Ni, Co, and Zn, exhibited similar distribution patterns to Al in the bottom sediment layers (94–19 cm). The concentration profiles of LREEs and HREEs in the sediment core are shown in
Figure 4. Most of the LREEs, including La, Ce, and Sm, exhibited similar distribution patterns. The La concentration ranged from 29.60 to 41.20 mg·kg
−1, with an average value of 34.33 mg·kg
−1. The concentration of Ce had a range from 52.00 to 81.40 mg·kg
−1, with an average value of 63.44 mg·kg
−1. Nd and Sm concentrations ranged from 20.50 to 37.40 mg·kg
−1, with an average value of 28.17 mg·kg
−1 and from 3.64 to 6.89 mg·kg
−1, with an average value of 5.23 mg·kg
−1, respectively. HREEs show a similar pattern of concentration profiles in the bottom section (94–23 cm) of the sediment core, and visible differences can be observed in the younger layers of the sediment core.
The Tb concentration ranged from 0.75 to 0.98 mg·kg−1, with an average value of 0.85 mg·kg−1. The concentration profiles of Tm (0.28 to 0.50 mg·kg−1 range, with an average value of 0.39 mg·kg−1) and Yb (2.05 to 4.39 mg·kg−1 range, with an average value of 3.01 mg·kg−1) looks almost similar to that of Tb, even in upper layers of the sediment core. The concentration of Sc had a range from 8.81 to 12.10 mg·kg−1, with an average value of 11.10 mg·kg−1.
The concentrations of TEs measured in the sediment core are shown in
Figure 5. Rb (64.30 to 93.80 mg·kg
−1 range, with an average value of 84.13 mg·kg
−1) and Cs (2.69 to 4.70 mg·kg
−1 range, with an average value of 4.17 mg·kg
−1) concentrations share a similar pattern, with almost stable values in the bottom sediment core (94–19 cm) and variations in the upper layers (17–1.0 cm). From trace elements, Sr (174.00 to 638.00 mg·kg
−1 range, with an average value of 245.38 mg·kg
−1) and Hf (7.38 to 15.10 mg·kg
−1 range, with an average value of 9.11 mg·kg
−1) show also a similar concentration profile in the bottom sediment cores (Sr—average value of 201.08 mg·kg
−1 and Hf—average value of 8.76 mg·kg
−1). Significant variations can be seen in the upper layers: 17–11 cm (Sr—average value of 253.00 mg·kg
−1 and Hf—average value of 13.55 mg·kg
−1, with a maximum value at about 16 cm) and 9–1.0 cm (Sr—average value of 574.00 mg·kg
−1, reaching a maximum at about 6 cm and Hf—average value of 8.22 mg·kg
−1).
The concentration profiles of Sb, Ba, Ta, and W do not present distinct features over the sediment core, except for the increase of W concentration in the upper layer: Sb—0.70 to 1.18 mg·kg−1 range, with an average value of 0.89 mg·kg−1; Ba—201.00 to 528.00 mg·kg−1 range, with an average value of 412.79 mg·kg−1; Ta—0.89 to 1.19 mg·kg−1 range, with an average value of 1.02 mg·kg−1, and W—1.32 to 2.46 mg·kg−1 range, with an average value of 1.63 mg·kg−1. Two elements—Th (8.42 to 12.30 mg·kg−1 range, with an average value of 10.00 mg·kg−1) and U (2.80 to 4.47 mg·kg−1 range, with an average value of 3.46 mg·kg−1)—exhibit almost similar trends over the entire core sediment.
3.2. Element Concentrations in the Caineni Lake Sediment Core
The results of the analysis of the main elements HM, LREE, and HREE and the trace elements show for most of them a distinct pattern, in which some of them occur in the smallest amount in the upper part of the sediment core, down to 10–12 cm, and occur in much higher concentrations below this depth. Other elements show an opposite trend, occurring in much higher amounts in the upper layer, and they have their highest concentration under this level. Additionally, some elements do not fit any of these two main patterns mentioned above; other trends can also be distinguished within these two main patterns. The borders between 28 and 32 cm and 58 and 54 cm seem to be significant to understanding the chemical stratigraphy of the investigated sediment core. The concentration profiles for the main elements, heavy metals (HM), light rare earth elements (LREE), heavy rare earth elements (HREE), and trace elements (TE) are presented in
Figure 6,
Figure 7,
Figure 8 and
Figure 9.
The concentrations of the main elements measured in the sediment core are shown in
Figure 6. The Na concentration ranged from 4.97 to 16.40 g·kg
−1, with an average value of 11.45 g·kg
−1; a gradual increase in concentration structured on the depth, from the bottom to the surface, of the sediment core can be noticed: 82–58 cm (average value of 11.16 g·kg
−1; notable variations between analysed sections), 56–30 cm (average value of 9.77 g·kg
−1; uniform variation with gradual increase), 28–20 cm (average value of 13.74 g·kg
−1; uniform variation with gradual decrease), 18–10 cm (average value of 11.20 g·kg
−1; constant variation trend), and 8–0 cm (average value of 13.80 g·kg
−1; gradual increase in uppermost sediment layer).
The Mg (ranged from 11.20 to 55.20 g·kg−1, with an average value of 42.03 g·kg−1) and Al (ranged from 15.20 to 105.00 g·kg−1, with an average value of 73.21 g·kg−1) concentration profiles display a similar trend structured on three levels: 82–38 cm (Mg—average value of 44.19 g·kg−1 and Al—average value of 77.33 g·kg−1, with almost constant variation between sections), 36–28 cm (Mg—average value of 26.06 g·kg−1 and Al—average value of 41.82 g·kg−1, with gradual decrease and a minimum value at 28 cm for both elements), and 26–0.0 cm (gradual decrease of concentration; Mg—average value of 44.37 g·kg−1 and Al—average value of 78.01 g·kg−1).
The notable Mg and Al variation at 28 cm depth in the sediment core suggests a possible pH-dependent dissolution of aluminium hydroxides to form aluminate (process present in shallow lakes, such as the present one) where resuspension at pH increase in the water occurs frequently. The decrease in Fe and especially Mn content, at the same time, may be due to the presence of reducing conditions (which increase the solubility of Fe and Mn; manganese is more soluble than iron). Furthermore, the same sudden variation observed for Ca, Ti, Cl, and V suggests a significant supply of fresh water.
In addition, the K geochemical profile (ranged from 10.90 to 29.60 g·kg−1, with an average value of 20.21 g·kg−1) shows a similar variation to that of Na in the bottom sediment layers (82–58 cm) and an opposite trend in the upper layers (56–0 cm): 82–58 cm (average value of 23.02 g·kg−1; notable variations between analysed sections), 56–30 cm (average value of 15.19 g·kg−1; uniform variation with gradual decrease), 28–12 cm (average value of 15.19 g·kg−1; constant variation), and 10–0 cm (average value of 17.68 g·kg−1; significant decrease in the youngest sediment sections).
The concentration profile of Ca (ranged from 15.50 to 130.00 g·kg−1, with an average value of 55.38 g·kg−1) displays almost a constant variation in the bottom sediment layers, with notable changes in the upper layers (38–0 cm): 82–38 cm (average value of 47.43 g·kg−1; gradual slow increase), 36–30 cm (average value of 84.58 g·kg−1; constant variation), 28–8 cm (average value of 42.40 g·kg−1; gradual increase in variation), and 6.0–0.0 cm (average value of 125.00 g·kg−1).
The Ti concentration (ranged from 1.15 to 6.44 g·kg−1, with an average value of 4.21 g·kg−1) presents almost a similar pattern as Mg and Al: 82–30 cm (average value of 4.31 g·kg−1, with maximum and minimum values at 66 cm and 28 cm, respectively), 26–0.0 cm (average value of 4.24 g·kg−1; gradual decrease).
The concentration profile of Mn (ranged from 0.30 to 1.31 g·kg−1, with an average value of 0.87 g·kg−1) follows almost the same pattern (except for the 44–36 cm section core) in the bottom sediment layers with Mg, Al, and Ti: 82–48 cm (average value of 0.75 g·kg−1; gradual decrease), 46–22 cm (average value of 0.87 g·kg−1; gradual increase with a minimum at 28 cm/0.30 g·kg−1), 20–14 cm (average value of 0.97 g·kg−1), and 12–0 cm (average value of 1.20 g·kg−1).
The Fe concentration (ranged from 19.3 to 52.9 g·kg−1, with an average value of 40.76 g·kg−1) displays a similar trend as K on the depth of the sediment core: 82–54 cm (average value of 41.15 g·kg−1), 52–32 cm (average value of 36.29 g·kg−1; uniform variation with gradual decrease), 26–10 cm (average value of 49.41 g·kg−1; almost constant variation), and 8–0 cm (average value of 30.17 g·kg−1; gradual decrease).
Both concentration profiles of Cl (ranged from 4.16 to 23.00 g·kg−1, with an average value of 11.43 g·kg−1) and Br (ranged from 9.27 to 75.70 mg·kg−1, with an average value of 26.43 mg·kg−1) show a similar trend of variation. On the contrary, the Cl concentration displays notable variations, especially in the upper layers of the sediment core: 82–30 cm (average value of 4.31 g·kg−1; almost constant between analysed sections), 24–18 cm (average value of 13.91 g·kg−1; gradual decrease), and 16–0 cm (average value of 13.91 g·kg−1; gradual increase). Moreover, for the Br concentration profile, notable variations can be observed in the upper layers of the sediment core: 82–14 cm (average value of 22.77 mg·kg−1; almost constant between analysed sections) and 12–0.0 cm (average value of 47.83 mg·kg−1; gradual increase).
The concentration profiles of the heavy metals (V, Cr, Ni, Co, Zn, and As) measured in the sediment core are shown in
Figure 7. In this respect, it can be seen from
Figure 7 that V and particularly As display a different pattern relative to the rest of the analysed HM (Cr, Ni, Co, and Zn show almost a similar trend on the depth of the sediment core). In addition, the V concentration profile (ranged from 31.30 to 193.00 mg·kg
−1, with an average value of 138.35 mg·kg
−1), from the bottom to the surface of the sediment core, shows a relative slow variation (decrease), followed by a notable increase in the upper layers: 82–30 cm (average value of 135.09 mg·kg
−1; slow gradual decrease), 26–12 cm (average value of 177.50 mg·kg
−1; constant variation), and 10–0.0 cm (average value of 114.74 mg·kg
−1; uniform variation with gradual decrease).
The concentration profile of As (ranged from 3.72 to 29.60 mg·kg−1, with an average value of 12.56 mg·kg−1) shows a similar variation as the rest of the HM, mostly in the 46–14 cm sediment core, with significant variations noticed almost all along the analysed sections: 82–14 cm (average value of 12.18 mg·kg−1) and 10–0.0 cm (average value of 13.37 mg·kg−1). Cr (ranged from 62.30 to 170.00 mg·kg−1, with an average value of 117.39 mg·kg−1), Ni (ranged from 3.05 to 90.08 mg·kg−1, with an average value of 66.74 mg·kg−1), Co (ranged from 7.39 to 20.04 mg·kg−1, with an average value of 16.91 mg·kg−1), and Zn (ranged from 44.1 to 115.00 mg·kg−1, with an average value of 95.59 mg·kg−1) concentrations present a similar pattern in the following core sections: 82–56 cm (Cr—average value of 135.16 mg·kg−1; Ni—average value of 67.90 mg·kg−1; Co—average value of 16.14 mg·kg−1; Zn—average value of 92.44 mg·kg−1), 54–32 cm (Cr—average value of 117.28 mg·kg−1; Ni—average value of 66.11 mg·kg−1; Co—average value of 17.65 mg·kg−1; Zn—average value of 98.06 mg·kg−1), 26–12 cm (Cr—average value of 123.13 mg·kg−1; Ni—average value of 72.65 mg·kg−1; Co—average value of 18.25 mg·kg−1; Zn—average value of 106.38 mg·kg−1), and 10–0.0 cm (Cr—average value of 91.60 mg·kg−1; Ni—average value of 55.16 mg·kg−1; Co—average value of 14.78 mg·kg−1; Zn—average value of 88.28 mg·kg−1).
The vertical distribution of LREEs and HREEs concentrations in the sediment core is illustrated in
Figure 8. All four LREEs present almost a similar concentration profile, with the main differences noticed for Nd and Sm in the upper sediment layers. La (ranged from 15.90 to 43.70 mg·kg
−1, with an average value of 30.53 mg·kg
−1) and Ce (ranged from 27.10 to 80.90 mg·kg
−1, with an average value of 64.95 mg·kg
−1) concentration profiles show four notable sections, starting from the bottom sediment core: 82–58 cm (La—average value of 35.78 mg·kg
−1 and Ce—average value of 64.32 mg·kg
−1; significant variation between sections), 56–30 cm (La—average value of 24.74 mg·kg
−1 and Ce—average value of 61.91 mg·kg
−1), 28–8 cm (La—average value of 34.04 mg·kg
−1 and Ce—average value of 74.35 mg·kg
−1), and 6–0.0 cm (La—average value of 21.93 mg·kg
−1 and Ce—average value of 47.33 mg·kg
−1).
The concentration of Nd (ranged from 8.22 to 35.60 mg·kg−1, with an average value of 25.34 mg·kg−1) displays a notable variation, especially in the upper and bottom sediment core layers: 82–58 cm (average value of 29.26 mg·kg−1) and 56–0 cm (average value of 24.59 mg·kg−1). The Sm profile (ranged from 2.21 to 6.47 mg·kg−1, with an average value of 4.69 mg·kg−1) shows small differences relative to La and Ce, mostly in the upper layers of the sediment core: 28–8 cm (average value of 5.62 mg·kg−1) and 6–0 cm (average value of 3.44 mg·kg−1).
The concentration profile of HREEs follows generally the same pattern (except for Tm and Yb) as LREEs. Tb (ranged from 0.36 to 0.94 mg·kg−1, with an average value of 0.72 mg·kg−1) and Sc (ranged from 8.06 to 22.20 mg·kg−1, with an average value of 15.34 mg·kg−1) concentrations display almost the same profile along the sediment core: 82–58 cm (Tb—average value of 0.79 mg·kg−1 and Sc—average value of 17.87 mg·kg−1; significant variation between sections), 56–10 cm (Tb—average value of 0.71 mg·kg−1 and Sc—average value of 14.66 mg·kg−1; small variations between sections), and 8–0 cm (Tb—average value of 0.54 mg·kg−1 and Sc—average value of 11.16 mg·kg−1).
The concentration profile of Tm (ranged from 0.19 to 0.43 mg·kg−1, with an average value of 0.32 mg·kg−1) shows a nonspecific variation, with a constant trend noticed only in the 36–30 cm range (average value of 0.23 mg·kg−1). The concentration of Yb (ranged from 1.41 to 3.67 mg·kg−1, with an average value of 2.71 mg·kg−1) also displays nonspecific variation, with a general profile close to that of Tm.
The concentration profiles of the trace elements measured in the sediment core are shown in
Figure 9. Sr and U display a different pattern relative to the rest of analysed elements. On the contrary, the other elements display generally the same trend, with most similarities noticed between Rb (ranged from 70.50 to 187.00 mg·kg
−1, with an average value of 129.97 mg·kg
−1), Cs (ranged from 4.38 to 11.90 mg·kg
−1, with an average value of 8.15 mg·kg
−1), Ba (ranged from 123.00 to 464.00 mg·kg
−1, with an average value of 345.61 mg·kg
−1), and Sb (ranged from 0.47 to 1.70 mg·kg
−1, with an average value of 1.13 mg·kg
−1).
However, the concentration profiles of Hf (ranged from 1.17 to 4.93 mg·kg−1, with an average value of 3.42 mg·kg−1), Ta (ranged from 0.42 to 1.19 mg·kg−1, with an average value of 0.89 mg·kg−1), Th (ranged from 4.65 to 13.30 mg·kg−1, with an average value of 10.62 mg·kg−1), and W (ranged from 0.68 to 2.21 mg·kg−1, with an average value of 1.50 mg·kg−1) allow them to be relatively differentiated from the previous group.
In addition, the Sr concentration (ranged from 74.30 to 2870.00 mg·kg−1, with an average value of 534.20 mg·kg−1) profile, from the bottom to the surface of the sediment core, shows a relatively slow variation, followed by a notable increase in the upper layers: 82–38 cm (average value of 235.71 mg·kg−1), 36–28 cm (maximum at 32 cm/1360.00 mg·kg−1), 28–10 cm (average value of 306.00 mg·kg−1), and 8–0 cm (average value of 2201.75 mg·kg−1; significant increase in most upper layer).
The concentration profile of U (ranged from 1.19 to 8.77 mg·kg−1, with an average value of 3.21 mg·kg−1) shows gradual increase from the bottom to the upper layers of the sediment core: 82–12 cm (average value of 2.76 mg·kg−1) and 10–0.0 cm (average value of 6.45 mg·kg−1).
3.3. Element Concentrations in the Movila Miresii Lake Sediment Core
The concentration profiles for main elements, heavy metals (HM), light rare earth elements (LREE), heavy rare earth elements (HREE), and trace elements (TE) are presented in
Figure 10,
Figure 11,
Figure 12 and
Figure 13. The results of the analysis did not show a distinct pattern for most of them; some of them occur in the smallest amount in the upper part of the sediment core, down to around 10 cm, and then occur in much higher concentrations below this depth. Other elements show an opposite trend, occurring in much higher amounts in the upper layer, with their highest concentration under this level.
The concentrations of the main elements measured in the sediment core are shown in
Figure 10. It can be noticed that most of the analysed elements do not follow a distinct pattern, except for Na, Cl, and Br.
The concentration profiles of Na (ranged from 11.20 to 42.40 g·kg−1, with an average value of 18.45 g·kg−1), Cl (ranged from 1.75 to 38.40 g·kg−1, with an average value of 10.34 g·kg−1), and Br (ranged from 8.57 to 134.00 mg·kg−1, with an average value of 40.78 mg·kg−1) show a gradual increase in concentration structured on the depth, from the bottom to the surface, of the sediment core: 90–17 cm (Na—average value of 16.21 g·kg−1; Cl—average value of 7.45 g·kg−1; Br—average value of 31.77 mg·kg−1), 15–7 cm (Na—average value of 24.38 g·kg−1; Cl—average value of 19.50 g·kg−1; Br—average value of 69.36 mg·kg−1), and 7–0 cm (Na—average value of 34.90 g·kg−1; Cl—average value of 29.45 g·kg−1; Br—average value of 101.20 mg·kg−1).
On the contrary, Mg (ranged from 24.40 to 40.80 g·kg−1, with an average value of 32.04 g·kg−1), Ca (ranged from 36.80 to 86.20 g·kg−1, with an average value of 63.56 g·kg−1), and Ti (ranged from 2.48 to 4.82 g·kg−1, with an average value of 3.68 g·kg−1) concentrations do not show a specific pattern.
Concentration of Al (ranged from 39.00 to 70.50 g·kg−1, with an average value of 57.69 g·kg−1) and K (ranged from 13.40 to 19.80 g·kg−1, with an average value of 16.82 g·kg−1) share a similar pattern, except for the uppermost layer of sediment core. Mn (ranged from 0.66 to 1.36 g·kg−1, with an average value of 1.01 g·kg−1) and Fe (ranged from 19.50 to 40.20 g·kg−1, with an average value of 29.41 g·kg−1) also present a similar trend of concentration profile.
The concentration profiles of heavy metals (V, Cr, Ni, Co, Zn, and As) measured in the sediment core are shown in
Figure 11. Zn and As display a different pattern relative to the rest of the analysed HM (V, Cr, Ni, and Co show almost a similar trend in the depth of the sediment core, especially in the upper sediment layers above 35 cm). V (ranged from 55.50 to 115.00 mg·kg
−1, with an average value of 90.23 mg·kg
−1), Cr (ranged from 62.30 to 97.20 mg·kg
−1, with an average value of 81.33 mg·kg
−1), Ni (ranged from 27.20 to 53.30 mg·kg
−1, with an average value of 40.23 mg·kg
−1), and Co (ranged from 8.75 to 17.80 mg·kg
−1, with an average value of 13.48 mg·kg
−1) concentrations show the highest variations in the half bottom of the sediment core.
The concentration profile of Zn (ranged from 3.46 to 82.80 mg·kg−1, with an average value of 54.34 mg·kg−1) displays significant variations in the upper (13–0.0 cm) and bottom (90–55 cm) sediment layers. The As concentration (ranged from 2.88 to 22.50 mg·kg−1, with an average value of 13.68 mg·kg−1) also does not present a specific pattern.
The vertical distribution of the LREEs and HREEs concentrations in the sediment core is illustrated in
Figure 12. All four LREEs (La—ranged from 21.10 to 29.10 mg·kg
−1, with an average value of 24.73 mg·kg
−1; Ce—ranged from 48.10 to 65.80 mg·kg
−1, with an average value of 56.52 mg·kg
−1; Nd—ranged from 13.40 to 29.30 mg·kg
−1, with an average value of 21.41 mg·kg
−1; Sm—ranged from 3.26 to 8.34 mg·kg
−1, with an average value of 4.66 mg·kg
−1) do not show a specific pattern or a similar concentration profile, with the main differences noticed for La, Ce, and Nd in the upper 20 cm sediment layer.
The concentration profiles of HREEs (Tb—ranged from 0.53 to 0.80 mg·kg−1, with an average value of 0.68 mg·kg−1; Tm—ranged from 0.27 to 0.42 mg·kg−1, with an average value of 0.32 mg·kg−1; Yb—ranged from 1.95 to 2.74 mg·kg−1, with an average value of 2.35 mg·kg−1; Sc—ranged from 7.13 to 12.6 mg·kg−1, with an average value of 10.38 mg·kg−1) suggest the same general pattern, with a decrease in the upper 20 cm sediment layer.
The concentration profiles of the trace elements measured in the sediment core are shown in
Figure 13.
Sr (ranged from 271.00 to 686.00 mg·kg−1, with an average value of 499.10 mg·kg−1), Th (ranged from 7.89 to 11.1 mg·kg−1, with an average value of 9.25 mg·kg−1), and U (ranged from 1.80 to 3.67 mg·kg−1, with an average value of 2.47 mg·kg−1) display a different trend relative to the rest of analysed elements (gradually increase from the bottom to the surface of the sediment core).
In addition, the other elements display the same trend. The most similarities are noticed between Rb (ranged from 56.90 to 90.60 mg·kg−1, with an average value of 79.53 mg·kg−1) and Cs (ranged from 2.87 to 5.73 mg·kg−1, with an average value of 4.46 mg·kg−1). On the contrary, Ba (ranged from 312.00 to 689.00 mg·kg−1, with an average value of 440.35 mg·kg−1), W (ranged from 1.02 to 1.79 mg·kg−1, with an average value of 1.35 mg·kg−1), and Sb (ranged from 0.04 to 1.75 mg·kg−1, with an average value of 0.89 mg·kg−1) also follow a relatively similar pattern, except for the bottom half of the sediment core.
The concentration profiles of Hf (ranged from 4.11 to 13.10 mg·kg−1, with an average value of 6.19 mg·kg−1) and Ta (ranged from 0.64 to 0.96 mg·kg−1, with an average value of 0.80 mg·kg−1) have an almost similar pattern, except for the uppermost layer of the sediment core (above 20 cm depth).
3.6. Correlation Analysis and Principal Component Analysis
The correlations between the 34 investigated elements (Person correlation coefficients) in the Amara Lake sediment core are presented in
Figure 20. Significant positive correlations (significance level alpha = 0.05) for V with Ni (0.68), Co (0.65), Mn (0.39), Rb (0.75), and Cs (0.78) might indicate that they originate from the same sources and have similar transformation and migration processes.
Ni was significantly correlated with Co (0.89), Rb (0.85), and Cs (0.90). Zn was significantly negatively correlated with all REEs, except for Tm (−0.03). As was positively correlated with all LREEs (La: 0.56; Ce: 0.49; Nd: 0.30, and Sm: 0.69) and some of TEs (Hf: 0.44; Ta: 0.32, and Th: 0.60). As a major component of clay minerals, Al showed significantly positive correlations with V (0.95), Ni (0.55), and Co (0.57) as well, thus suggesting a natural origin of these HMs. High values of the Pearson coefficients (positive correlation) were obtained between LREEs (0.56÷0.91) and HREEs (0.50÷0.71), except for Sc, and significantly positive correlations were obtained for Sc with HMs (V: 0.77; Ni: 0.87, and Co: 0.87), Rb (0.95), and Cs (0.97).
The PCA performed on the database aimed to identify the most important sources for HMs, REEs, and TEs in the sediment core of Amara Lake. The results from the PCA applied to the database are presented in
Figure 21 and
Table 6. The first three principal components with eigenvalues greater than 1.00 were selected, with 70.33% of the variance explained. The measure of sampling adequacy by KMO statistics provided the value of 0.71, while the significance of Bartlett’s test of sphericity was less than 0.001, which led to the conclusion that the database size was suitable for evaluation by the PCA. As presented in
Table 6, the first three principal components loadings were classified as strong (loadings values > 0.60) and moderate (loading values 0.60 ÷ 0.30).
Figure 21 presents the PCA box plots (correlations between variables and the principal components after Varimax rotation), with the loading of PC1 versus PC2, PC1 versus PC3, and PC2 versus PC3 from the PCA applied to the investigated dataset.
PC1 had strong loadings of most LREEs (except for Tm, with moderate loading), HREEs (except for Sr), Ta, Hf, Th, and As and moderate loadings of U, corresponding to the pedological characteristics of the area. PC2 highlights strong loadings for Na, Cl, Br, Sr, Zn, Cr, and W and moderate loadings for U and Yb; this principal component can be attributed to a mixed source of salts (due to the lack of a constant supply of freshwater and through evaporation during dry periods) and dry or wet atmospheric deposition. The moderate/strong loadings for Zn (0.58), Cr (0.72), and W (0.87) indicate an anthropogenic input; other studies [
34,
104,
105,
106] indicated Zn, W, and Cr as tracers of anthropogenic factor (pollution from the urban roads dust). PC3 with strong loadings of K, Sc, Cs, Co, Fe, Ni, Rb, and Sb and moderate loadings of Al, Ca, Mg, Mn, Zn, V, and Cr can be attributed to leaching from soil surface/denudation, rock weathering and mixed anthropogenic input (e.g., agricultural runoff, wastewater discharges).
PCA analysis was followed by AHC applied to the factors/principal components score of the sediment core sections, i.e., the 47 sections (from A 1–3 cm to A 93–94 cm) in which investigated elements were analysed in order to group similar sections (vertical variability). The AHC results were rendered as a dendrogram (
Figure 22), where all the sediment sections were clustered in four statistically significant groups/clusters—inertia decomposition for the optimal classification: within-cluster 18.03% and between-clusters 81.97%, H(k − 1)–H(k) at 12.31, the Hartigan index (H) of a clustering with k clusters and a clustering with (k − 1) clusters.
The four clusters obtained, C1, C2, C3, and C4 (
Figure 22), can offer a better view of sediment core stratification based on the statistical approach to the dataset. A clear differentiation of C1 (sections from A 1–3 cm to A 9–11 cm) and C2 (sections from A 11–13 cm to A 17–19 cm) clusters, which includes sections from the upper layers of the sediment core, can be observed. C3 and C4 clusters’ relatively lower homogeneity (within-cluster variance 5.90 for C3 and 4.14 for C4) suggests certain elements’ migration or geochemical and weathering events that require further investigation.
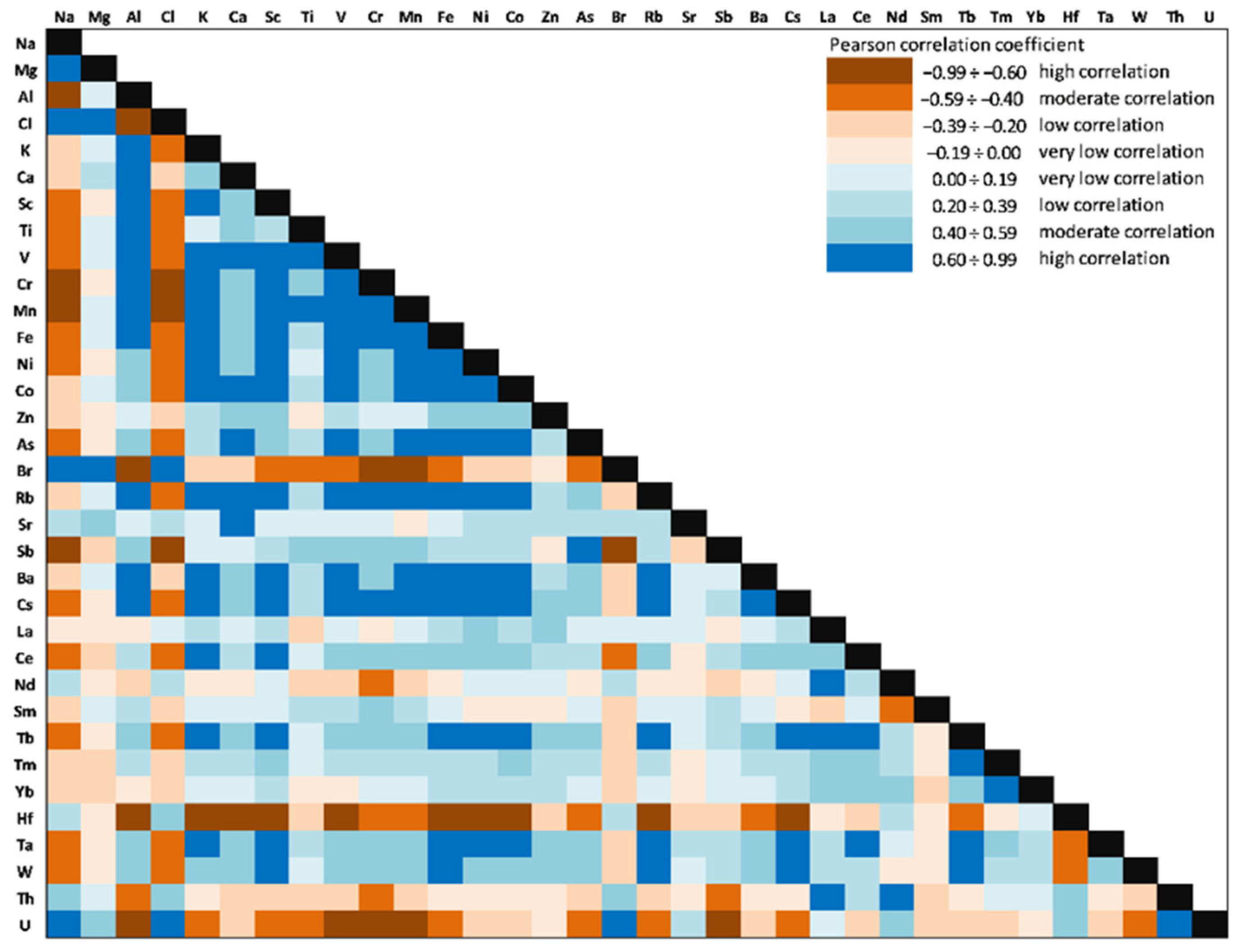
The correlations between investigated elements (Person correlation coefficients) in the Caineni Lake sediment core are presented in
Figure 23.
Significantly positive correlations (significance level alpha = 0.05) were obtained for V with Al (0.99), Mg (0.94), Ti (0.88), Fe (0.65), Rb(0.64), and Cs(0.69), which might indicate that they originate from the same sources and have similar migration processes. Cr was significantly correlated with K (0.81, Sc (0.97), Ni (0.90), Co (0.71), Zn (0.71), Rb (0.97), Sb (0.75), and REEs (0.61÷0.90). For Fe, Ni, Co, and Zn, an almost similar pattern was obtained, with positive high correlation for Rb (0.70÷0.89), Sb (0.76÷0.81), Ba (0.58÷0.75), Cs (0.67÷0.83), REEs (0.42÷0.92), Ta (0.72÷0.88), W (0.63÷0.80), and Th (0.90÷0.94). Aluminium showed significantly positive correlations with Ti (0.91), V (0.99), Cr (0.60), Fe (0.62), Rb (0.63), Cs (0.67), and REEs (0.40÷0.56). High values of Pearson coefficients (positive correlation) were obtained between LREEs (0.65÷0.96) and HREEs (0.45÷0.81). Significantly positive correlations were obtained for Sc with HMs (0.62÷0.97), Sb (0.78), Cs (0.99), and LREEs (0.68÷0.96).
The results from the PCA applied to the Caineni Lake database are presented in
Figure 24 and
Table 7. The first three principal components with eigenvalues greater than 1.00 were selected, with 79.92% of the variance explained. The measure of sampling adequacy by KMO statistics provided the value of 0.78, while the significance of Bartlett’s test of sphericity was less than 0.001; the database size was suitable for evaluation by the PCA. As presented in
Table 7, the first three principal components loadings were classified as strong (loadings values > 0.60) and moderate (loading values 0.60 ÷ 0.30).
Figure 24 presents the PCA box plots for the Caineni Lake dataset (correlations between variables and the principal components after Varimax rotation), with the loading of PC1 versus PC2, PC1 versus PC3, and PC2 versus PC3 from the PCA applied to the dataset.
PC1 reveals strong loadings for LREEs, HREEs, TEs (except for U and Hf), HMs (except for V), K, Sc, Fe, and Rb, corresponding to pedological characteristics of the area. PC2 highlights strong loadings for Na, Cl, Br, Sr, Ca, Mn, and U; this principal component can be attributed to a mixed source of salts, loess fingerprint, and dry or wet atmospheric deposition. PC3, with strong loadings of Al, Mg, Ti, and V, can be attributed to leaching from soil surface/denudation and rock weathering. PCs plots and the Pearson correlation map (
Figure 23) also reveal strong negative loading and low correlations of Hf; this may suggest element sensitivity to the redox status of the sediment [
107,
108,
109].
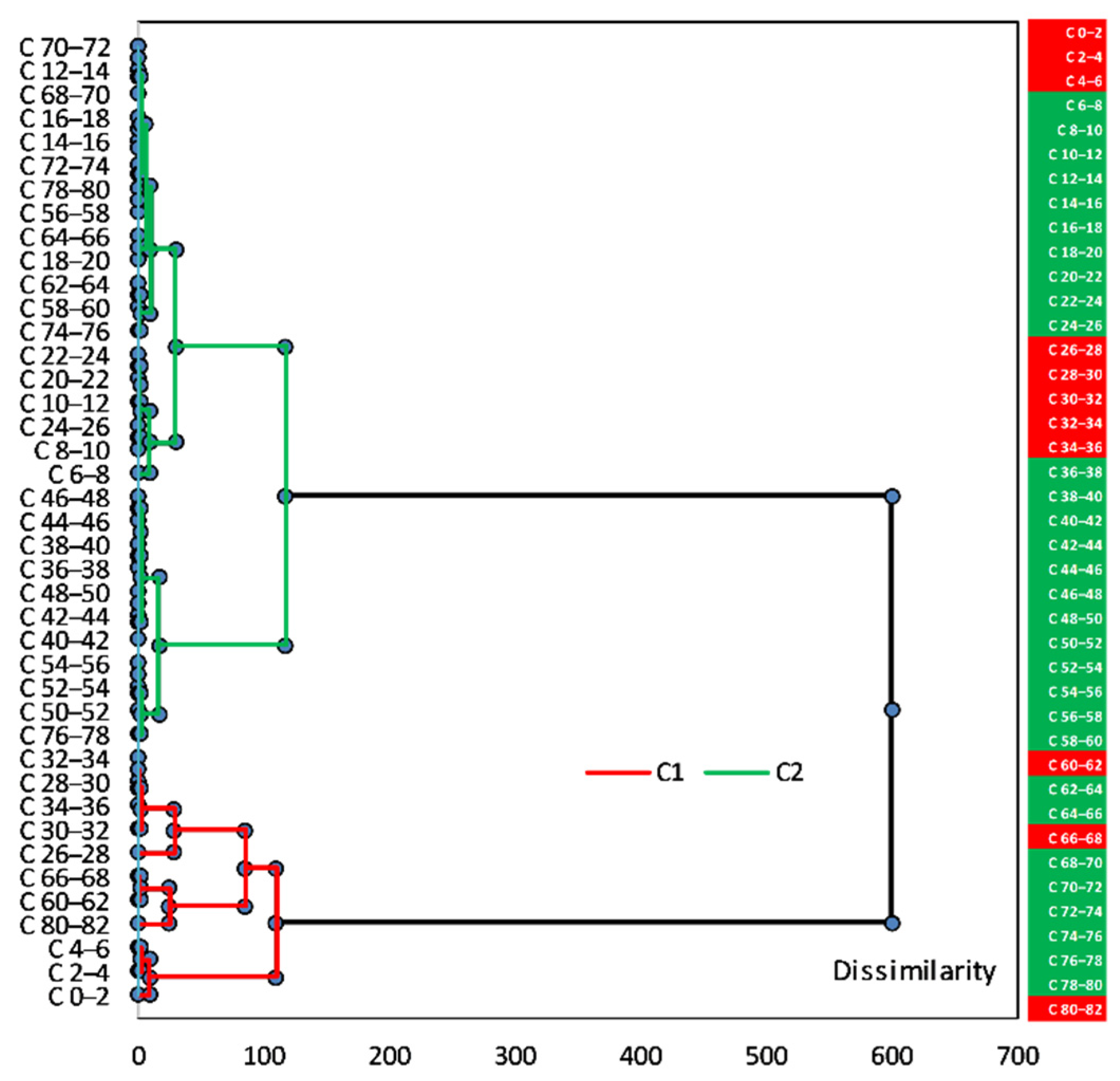
Agglomerative hierarchical clustering (AHC) analysis was applied to the principal components score of sediment core sections, i.e., the 41 sections (from C 0–2 cm to C 80–82 cm) in which investigated elements were analysed, to group similar sections (vertical variability). The AHC results were rendered as a dendrogram (
Figure 25), where all the sediment sections were clustered in two statistically significant groups/clusters—inertia decomposition for the optimal classification: within-cluster 44.80% and between-clusters 55.20%, H(k − 1)–H(k) at 35.91, the Hartigan index (H) of a clustering with k clusters and a clustering with (k − 1) clusters.
Based on the statistical approach to dataset, the two clusters generated, C1 (within-cluster variance 27.02) and C2 (within-cluster variance 7.47), can provide a representation of the sediment core stratification. An alternation between the layers that group sections belonging to the same cluster, suggesting weathering events that can lead to alternating depositional sequences, can be observed.
Figure 26 highlights the correlations between the 34 investigated elements (Person correlation coefficients) in the Movila Miresii Lake sediment core (90 cm depth).
Significantly positive correlations (significance level alpha = 0.05) for HMs (V: 0.94, 0.70, 0.71, 0.67; Cr: 0.79, 0.66, 0.43, 0.57; Ni: 0.58, 0.74, 0.55; Co: 0.59, 0.69, 0.64) with Al, K, Ca, and Ti, respectively, suggest their primary source is the lithological one. Ba and Cs also show significantly positive correlations (ranged from 0.57÷0.98 and 0.57÷0.86) with Al, K, Ca, Mn, Fe, and HMs.
High values of Pearson coefficients (positive correlation) for LREEs and HREEs were obtained, especially for Ce (0.60, 0.61, and 0.59) and Tb (0.62, 0.80, and 0.65) with K, Sc, and La. As was significantly positively correlated with Ca (0.60), Sc (0.58), V (0.61), Mn (0.63), Fe (0.67), Ni (0.67), and Co (0.76).
The PCA performed on the database aimed to identify the most important sources for HMs, REEs, and TEs in the sediment core of Movila Miresii Lake. The results from the PCA applied to the database are presented in
Figure 27 and
Table 8. The first three principal components with eigenvalues greater than 1.00 were selected, with 71.42% of the variance explained. The measure of sampling adequacy by KMO statistics provided the value of 0.69, while the significance of Bartlett’s test of sphericity was less than 0.001, which led to the conclusion that the database size was suitable for evaluation by the PCA.
Table 8 summarizes the loading values (classified as strong: loadings values > 0.60, and moderate with loading values 0.60 ÷ 0.30) for the first three principal components.
Figure 27 presents the PCA box plots (correlations between variables and the principal components after Varimax rotation), with the loading of PC1 versus PC2, PC1 versus PC3, and PC2 versus PC3 from the PCA applied to the investigated dataset.
The PC1 versus PC2 box plot (
Figure 27a) allows correlating, due to the PC1 strong positive loadings, Al, K, Ca, Mn, Fe, HMs and Sc, Tb, and Ce from REEs. PC1 can be attributed to leaching from the soil surface/denudation, rock weathering, dry or wet atmospheric deposition, and mixed anthropogenic input. PC2 has significant negative loadings for Ti, Cr, Sb, and Sm and high positive loadings for La, Nd, Th, and U. This pattern, together with PC3 moderate/high loading of Tm and Yb, respectively, suggests a mixed contribution from both pedological and anthropogenic inputs. PC3 highlights strong negative loadings for Na, Cl, Br, Sr, and Mg; this principal component can be attributed mainly to salts deposition and denudation.
PCA analysis was followed by AHC, which was applied to the factors/principal components score of the sediment core sections, i.e., the 41 sections (from MM 0–2 cm to MM 89–90 cm) in which investigated elements were analysed, in order to group similar sections (vertical variability). The AHC results were rendered as a dendrogram (
Figure 28), where all the sediment sections were clustered in two statistically significant groups/clusters—inertia decomposition for the optimal classification: within-cluster 52.07% and between-clusters 47.93%, H(k − 1)–H(k) at 24.95, the Hartigan index (H) of a clustering with k clusters and a clustering with (k − 1) clusters.
Based on the statistical approach to the dataset, the two clusters generated, C1 (within-cluster variance 10.90) and C2 (within-cluster variance 13.22), can provide a representation of the sediment core stratification. A clear differentiation between two regions of the sediment core can be observed: upper area included in C1 (from sections MM 0–2 cm to MM 12–14 cm) and bottom area (from sections MM 14–16 cm to MM 89–90 cm). The vertical distribution based on AHC analysis suggests a higher stability in terms of elemental composition and elements migration without significant geochemical or weathering events.