Microbial Profiles of Meat at Different Stages of the Distribution Chain from the Abattoir to Retail Outlets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Procedure
2.3. Microbiological Analyses
2.3.1. Total Bacteria Count
2.3.2. Coliform and E. coli Counts
2.3.3. Staphylococcus aureus
2.4. Statistical Analysis
4. Results and Discussion
4.1. Microbial Counts as Affected by the Abattoir, Meat Type and Distribution Stage
4.2. Microbial Counts as Affected by Shop Class, Storage Period, Distance and Temperature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komba, E.V.G.; Komba, E.V.; Mkupasi, E.M.; Mbyuzi, A.O.; Mshamu, S.; Mzula, A.; Luwumba, D. Sanitary practices and occurrence of zoonotic conditions in cattle at slaughter in Morogoro Municipality, Tanzania: Implications for public health. Tanzan. J. Health Res. 2012, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odetokun, I.A.; Ballhausen, B.; Adetunji, V.O.; Ghali-Mohammed, I.; Adelowo, M.T.; Adetunji, S.A.; Fetsch, A. Staphylococcus aureus in two municipal abattoirs in Nigeria: Risk perception, spread and public health implications. Vet. Microbiol. 2018, 216, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Nel, S.; Lues, J.F.R.; Buys, E.M.; Venter, P. The personal and general hygiene practices in the deboning room of a high throughput red meat abattoir. Food Control 2004, 15, 571–578. [Google Scholar] [CrossRef]
- Mallhi, I.Y.; Sohaib, M.; Khan, A.U.; Nawaz, M. Evaluating food safety knowledge, practices, and microbial profile of meat in abattoirs and butchery shops in Lahore, Pakistan. J. Food Saf. 2019, 39, e12612. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main groups of microorganisms of relevance for food safety and stability: General aspects and overall description. Innov. Technol. food Preserv. 2018, 53–107. [Google Scholar] [CrossRef]
- Zwirzitz, B.; Wetzels, S.U.; Dixon, E.D.; Stessl, B.; Zaiser, A.; Rabanser, I.; Thalguter, S.; Pinior, B.; Roch, F.-F.; Strachan, C. The sources and transmission routes of microbial populations throughout a meat processing facility. Npj Biofilm. Microbiomes 2020, 6, 26. [Google Scholar] [CrossRef]
- Chong, E.S.; Bakar, N.F.A.; Zin, N.M.; Zulfakar, S.S. Presence of Salmonella spp. on Beef Carcasses and Meat Contact Surfaces at Local Abattoirs in Selangor, Malaysia. Malays. J. Health Sci. Sains Kesihat. Malays. 2017, 15, 115–119. [Google Scholar] [CrossRef]
- Smith, A.M.; Gouws, A.-M.; Hoyland, G.; Sooka, A.; Keddy, K.H. Outbreaks of food-borne disease-A common occurrence but rarely reported. S. Afr. Med. J. 2007, 97, 1272. [Google Scholar]
- Bersisa, A.; Tulu, D.; Negera, C. Investigation of bacteriological quality of meat from abattoir and butcher shops in Bishoftu. Cent. Ethiop. 2019, 2019, 6416803. [Google Scholar] [CrossRef] [Green Version]
- Department of Agriculture, Fisheries and Forestry (DAFF). Meat Safety Act No. 40 of 2000. 2000. Available online: https://www.gov.za/documents/meat-safety-act (accessed on 12 January 2023).
- Lambrechts, A.A.; Human, I.S.; Doughari, J.H.; Lues, J.F.R. Bacterial contamination of the hands of food handlers as indicator of hand washing efficacy in some convenient food industries in South Africa. Pak. J. Med. Sci. 2014, 30, 755. [Google Scholar] [CrossRef]
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2008, 78, 3–13. [Google Scholar] [CrossRef]
- Hoffmann, S.; Scallan, E. Epidemiology, cost, and risk analysis of foodborne disease. Foodborne Dis. 2017, 31–63. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015; ISBN 9241565160. [Google Scholar]
- Department of Health (DOH); Department of trade and industry (DTI); Department of Agriculture, Fisheries and Forestry (DAFF). Food Safety and Food Control in South Africa: Specific Reference to Meat Labelling; Government Printers: Pretoria, South Africa, 2013. [Google Scholar]
- Kandasamy, K.; Jannatin, M.; Chen, Y.-C. Rapid Detection of Pathogenic Bacteria by the Naked Eye. Biosensors 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Naidoo, D.; Buys, E.M. Managing meat safety at South African abattoirs. Int. J. Agri. Bios. Sci. Eng. 2013, 7, 3843. [Google Scholar] [CrossRef]
- Bohaychuk, V.M.; Gensler, G.E.; Barrios, P.R. Microbiological baseline study of beef and pork carcasses from provincially inspected abattoirs in Alberta, Canada. Can. Vet. J. 2011, 52, 1095. [Google Scholar] [PubMed]
- Guide, S.A.S.U.; Statistical Analysis System Institute Inc.: Carry, NC, USA, 2010.
- Uzoigwe, N.E.; Nwufo, C.R.; Nwankwo, C.S.; Ibe, S.N.; Amadi, C.O.; Udujih, O.G. Assessment of bacterial contamination of beef in slaughterhouses in Owerri zone, Imo state, Nigeria. Sci. Afr. 2021, 12, e00769. [Google Scholar] [CrossRef]
- Ayçiçek, H.; Aydoğan, H.; Küçükkaraaslan, A.; Baysallar, M.; Başustaoğlu, A.C. Assessment of the bacterial contamination on hands of hospital food handlers. Food Control 2004, 15, 253–259. [Google Scholar] [CrossRef]
- Lues, J.F.R.; Van Tonder, I. The occurrence of indicator bacteria on hands and aprons of food handlers in the delicatessen sections of a retail group. Food Control 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Nyamakwere, F.; Muchenje, V.; Mushonga, B.; Kandiwa, E.; Makepe, M.; Mutero, G. Evaluation of meat safety knowledge, attitudes and practices among slaughter house workers of Amathole District in eastern Cape Province, South Africa. J. Food Saf. Hyg. 2017, 3, 7–15. [Google Scholar]
- Ncoko, P.; Jaja, I.F.; Oguttu, J.W. Microbiological quality of beef, mutton, and water from different abattoirs in the Eastern Cape Province, South Africa. Vet. World 2020, 13, 1363. [Google Scholar] [CrossRef]
- Rani, Z.T. Effect of post-slaughter handling on physico-chemical and microbiological quality of red meat along the distribution chain in the Eastern Cape Province, South Africa. Ph.D. Thesis, University of Fort Hare, Alice, South Africa, 2015. [Google Scholar]
- Fajardo-Guerrero, M.; Rojas-Quintero, C.; Chamorro-Tobar, I.; Zambrano, C.; Sampedro, F.; Carrascal-Camacho, A.K. Exposure assessment of Salmonella spp. in fresh pork meat from two abattoirs in Colombia. Food Sci. Technol. Int. 2020, 26, 21–27. [Google Scholar] [CrossRef]
- Ahmad, M.U.D.; Sarwar, A.; Najeeb, M.I.; Nawaz, M.; Anjum, A.A.; Ali, M.A.; Mansur, N. Assessment of microbial load of raw meat at abattoirs and retail outlets. J. Anim. Plant Sci. 2013, 23, 745–748. [Google Scholar]
- Bradeeba, K.; Sivakumaar, P.K. Assessment of microbiological quality of beef, mutton and pork and its environment in retail shops in Chidambaram, Tamil Nadu. Int. J. Plant Anim. Environ. Sci. 2013, 3, 91–97. [Google Scholar]
- Haileselassie, M.; Taddele, H.; Adhana, K.; Kalayou, S. Food safety knowledge and practices of abattoir and butchery shops and the microbial profile of meat in Mekelle City, Ethiopia. Asian Pac. J. Trop. Biomed. 2013, 3, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Astorga, M.; Capita, R.; Alonso-Calleja, C.; Moreno, B.; García-Fernández, C. Microbiological quality of retail chicken by-products in Spain. Meat Sci. 2002, 62, 45–50. [Google Scholar] [CrossRef]
- Haque, M.A.; Siddique, M.P.; Habib, M.A.; Sarkar, V.; Choudhury, K.A. Evaluation of sanitary quality of goat meat obtained from slaughter yards and meat stalls at late market hours. Bangladesh J. Vet. Med. 2008, 6, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.H.; Farooqui, A.; Khan, A.; Khan, A.Y.; Kazmi, S.U. Microbial contamination of raw meat and its environment in retail shops in Karachi, Pakistan. J. Infect. Dev. Ctries. 2010, 4, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Mouafo, H.T.; Baomog, A.; Adjele, J.J.B.; Sokamte, A.T.; Mbawala, A.; Ndjouenkeu, R. Microbial profile of fresh beef sold in the markets of ngaoundéré, cameroon, and antiadhesive activity of a biosurfactant against selected bacterial pathogens. J. Food Qual. 2020, 2020, 5989428. [Google Scholar] [CrossRef]
- Agbodaze, D.; Nmai, P.N.A.; Robertson, F.C.; Yeboah-Manu, D.; Owusu-Darko, K.; Addo, K.K. Microbiological quality of “khebab” consumed in the Accra metropolis. Ghana Med. J. 2005, 39, 46–49. [Google Scholar] [CrossRef]
- Hill, A.A.; Simons, R.L.; Swart, A.N.; Kelly, L.; Hald, T.; Snary, E.L. Assessing the Effectiveness of On-Farm and Abattoir Interventions in Reducing Pig Meat–Borne Salmonellosis within EU Member States. Risk Anal. 2016, 36, 546–560. [Google Scholar] [CrossRef]
- Bhandare, S.G.; Sherikar, A.T.; Paturkar, A.M.; Waskar, V.S.; Zende, R.J. A comparison of microbial contamination on sheep/goat carcasses in a modern Indian abattoir and traditional meat shops. Food Control 2007, 18, 854–858. [Google Scholar] [CrossRef]
- Jaja, I.F.; Jaja, C.-J.I.; Chigor, N.V.; Anyanwu, M.U.; Maduabuchi, E.K.; Oguttu, J.W.; Green, E. Antimicrobial resistance phenotype of Staphylococcus aureus and Escherichia coli isolates obtained from meat in the formal and informal sectors in South Africa. BioMed Res. Int. 2020, 2020, 3979482. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.O.; Keen, J.E.; Siragusa, G.R.; Barkocy-Gallagher, G.A.; Koohmaraie, M.; Laegreid, W.W. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 2000, 97, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.; Cummins, E.; Nally, P.; O’Brien, S.; Butler, F. A review of quantitative microbial risk assessment in the management of Escherichia coli O157: H7 on beef. Meat Sci. 2006, 74, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Kilgannon, A.K.; Holman, B.W.B.; Mawson, A.J.; Campbell, M.; Collins, D.; Hopkins, D.L. The effect of different temperature-time combinations when ageing beef: Sensory quality traits and microbial loads. Meat Sci. 2019, 150, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.; Castro, R.; Delgadillo, I.; Saraiva, J.A. Improvement of the refrigerated preservation technology by hyperbaric storage for raw fresh meat. J. Sci. Food Agric. 2020, 100, 969–977. [Google Scholar] [CrossRef]
- Mashilo, E.N.; Agigi, A.F.A.; Mocke, K.; Jaqueta, S.D.J. Physical distribution challenges and adaptations: A qualitative study of South Africa-based organisations operating in emerging African markets. J. Transp. Supply Chain Manag. 2020, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mercier, S.; Villeneuve, S.; Mondor, M.; Uysal, I. Time–temperature management along the food cold chain: A review of recent developments. Compr. Rev. Food Sci. Food Saf. 2017, 16, 647–667. [Google Scholar] [CrossRef] [Green Version]
- Nørrung, B.; Andersen, J.K.; Buncic, S. Main concerns of pathogenic microorganisms in meat. Saf. Meat Process. Meat 2009, 3–29. [Google Scholar] [CrossRef]
- Naas, H.T.; Edarhoby, R.A.; Garbaj, A.M.; Azwai, S.M.; Abolghait, S.K.; Gammoudi, F.T.; Moawad, A.A.; Barbieri, I.; Eldaghayes, I.M. Occurrence, characterization, and antibiogram of Staphylococcus aureus in meat, meat products, and some seafood from Libyan retail markets. Vet. World 2019, 12, 925. [Google Scholar] [CrossRef]
- da Silva, A.C.; Rodrigues, M.X.; Silva, N.C.C. Methicillin-resistant Staphylococcus aureus in food and the prevalence in Brazil: A review. Braz. J. Microbiol. 2020, 51, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, M. Microbial flora and incidence of some food-borne pathogens on fresh raw beef from butcher’s shops in Awassa, Ethiopia. Bull. Anim. Health Prod. Afr. 1994, 42, 273–277. [Google Scholar]
- Adetunde, L.A.; Glover, R.L.K.; Oliver, A.W.O.; Samuel, T. Source and distribution of microbial contamination on beef and Chevron in Navrongo, Kassena Nankana district of Upper East region in Ghana. J. Anim. Prod. Adv. 2011, 1, 21–28. [Google Scholar]
- Rortana, C.; Nguyen-Viet, H.; Tum, S.; Unger, F.; Boqvist, S.; Dang-Xuan, S.; Koam, S.; Grace, D.; Osbjer, K.; Heng, T. Prevalence of Salmonella spp. and Staphylococcus aureus in chicken meat and pork from Cambodian Markets. Pathogens 2021, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Buzby, J.; Lichtenberg, E. Economic consequences of Foodborne hazards. In Food Safety Handbook. RH Schmidt and GE Rodrick; John and Wiley and Sons: Hoboken, NJ, USA, 2003; pp. 89–124. [Google Scholar] [CrossRef]
- Roose, G.; Mulier, L. Healthy advertising coming to its senses: The effectiveness of sensory appeals in healthy food advertising. Foods 2020, 9, 51. [Google Scholar] [CrossRef]
- Becker, T.; Benner, E.; Glitsch, K. Consumer perception of fresh meat quality in Germany. Br. Food J. 2000, 103, 246–266. [Google Scholar] [CrossRef]
- Rani, Z.T.; Hugo, A.; Hugo, C.J.; Vimiso, P.; Muchenje, V. Effect of post-slaughter handling during distribution on microbiological quality and safety of meat in the formal and informal sectors of South Africa: A review. S. Afr. J. Anim. Sci. 2017, 47, 255–267. [Google Scholar] [CrossRef] [Green Version]
- McWhorter, A.R.; Weerasooriya, G.; Willson, N.-L.; Chousalkar, K.K. Peroxyacetic acid and acidified sodium chlorite reduce microbial contamination on whole chicken carcasses obtained from two processing points. Food Microbiol. 2022, 106, 104035. [Google Scholar] [CrossRef]
- Chen, X.; Dong, P.; Li, K.; Zhu, L.; Yang, X.; Mao, Y.; Niu, L.; Hopkins, D.L.; Luo, X.; Liang, R. Effect of the combination of superchilling and super-chilled storage on shelf-life and bacterial community dynamics of beef during long-term storage. Meat Sci. 2022, 192, 108910. [Google Scholar] [CrossRef]
- Gill, C.O. Extending the storage life of raw chilled meats. Meat Sci. 1996, 43, 99–109. [Google Scholar] [CrossRef]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, L.; Purnell, G.; James, S.J.; James, C. Operating temperatures of supermarket frozen retail display cabinets. Int. J. Refrig. 2020, 117, 81–93. [Google Scholar] [CrossRef]
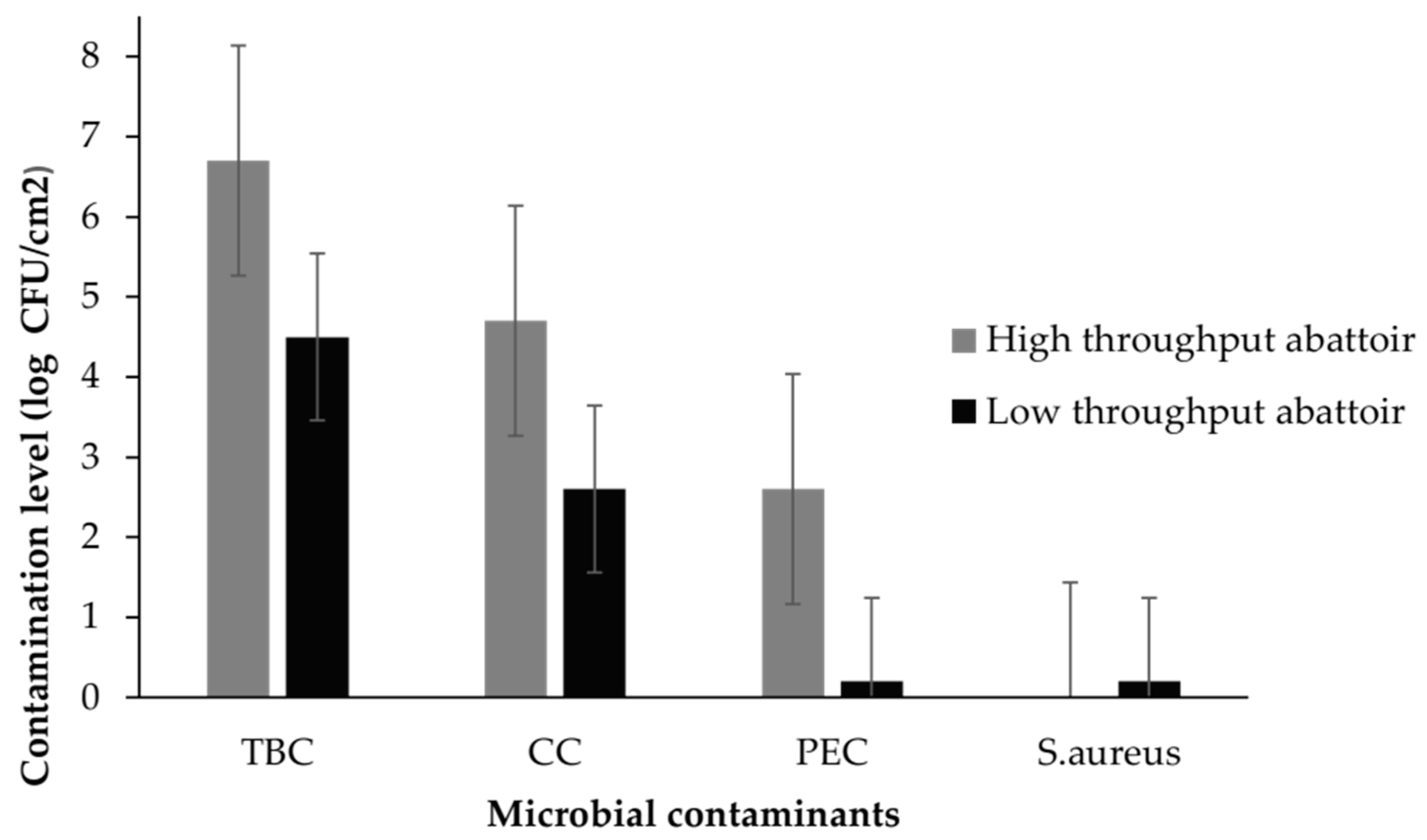
| HTA | LTA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microbial Contaminants | Microbial Contaminants | ||||||||
| Stage | Meat Type | Total Bacteria Count (log CFU/cm2) | Coliform Count (log CFU/cm2) | Presumptive E. coli (log CFU/cm2) | S. aureus (log CFU/cm2) | Total Bacteria Count (log CFU/cm2) | Coliform Count (log CFU/cm2) | Presumptive E. coli (log CFU/cm2) | S. aureus (log CFU/cm2) |
| Loading | Mutton | 7.2 ± 0.16 b | 4.2 ± 0.39 a | 1.2 ± 0.34 a | 0.0 ± 0.00 a | 4.9 ± 0.42 c | 2.6 ± 0.34 b | 0.2 ± 0.15 a | 0.0 ± 0.00 a |
| Pork | 5.9 ± 0.35 a | 3.9 ± 0.29 a | 1.8 ± 0.44 a | 0.0 ± 0.00 a | 2.9 ± 0.20 a | 0.7 ± 0.29 a | 0.2 ± 0.14 a | 0.3 ± 0.24 b | |
| Beef | 7.7 ± 0.17 b | 5.8 ± 0.30 b | 4.7 ± 0.26 c | 0.0 ± 0.00 a | 4.0 ± 0.39 b | 2.3 ± 0.28 b | 0.4 ± 0.23 b | 0.0 ± 0.00 a | |
| Offloading | Mutton | 6.1 ± 0.36 a | 3.6 ± 0.30 a | 3.2 ± 0.92 b | 0.0 ± 0.00 a | 4.80 ± 0.45 b | 2.6 ± 0.30 b | 0.1 ± 0.14 b | 0.0 ± 0.00 a |
| Pork | 6.5 ± 0.40 a | 4.8 ± 0.41 b | 2.7 ± 0.43 b | 0.0 ± 0.00 a | 3.2 ± 0.23 a | 0.9 ± 0.27 a | 0.0 ± 0.00 a | 0.4 ± 0.23 b | |
| Beef | 7.7 ± 0.20 b | 5.8 ± 0.22 b | 4.9 ± 0.16 c | 0.0 ± 0.00 a | 5.0 ± 0.27 b | 2.7 ± 0.48 b | 0.7 ± 0.27 c | 0.0 ± 0.00 a | |
| Display | Mutton | 5.4 ± 0.35 a | 4.1 ± 0.35 a | 1.2 ± 0.49 a | 0.0 ± 0.00 a | 6.1 ± 0.40 b | 5.2 ± 0.36 b | 0.1 ± 0.15 b | 0.5 ± 0.32 b |
| Pork | 7.0 ± 0.37 b | 4.5 ± 0.25 b | 2.5 ± 0.56 b | 0.0 ± 0.00 a | 4.7 ± 0.35 a | 2.3 ± 0.31 a | 0.0 ± 0.00 a | 0.4 ± 0.35 b | |
| Beef | 6.7 ± 0.40 ab | 6.1 ± 0.36 c | 1.8 ± 0.68 a | 0.0 ± 0.00 a | 4.8 ± 0.47 a | 4.8 ± 0.66 b | 0.1 ± 0.17 b | 0.0 ± 0.00 a | |
| Description | Total Bacteria Count (log CFU/cm2) | Coliform Count (log CFU/cm2) | Presumptive E. coli (log CFU/cm2) | S. aureus (log CFU/cm2) |
|---|---|---|---|---|
| Class | ||||
| Top-class shop | 4.8 ± 0.61 a | 2.9 ± 0.61 a | 1.9 ± 0.67 a | 0.1 ± 0.23 a |
| Middle-class shop | 5.7 ± 0.58 ab | 4.2 ± 0.57 b | 2.3 ± 0.63 b | 0.0 ± 0.21 a |
| Butchery | 6.1 ± 0.61 b | 4.1 ± 0.60 b | 1.6 ± 0.66 a | 0.1 ± 0.22 a |
| Storage period | ||||
| Commercial | ||||
| >1 h | 6.8 ± 0.22 b | 4.7 ± 1.44 a | 3.5 ± 0.36 c | 0.0 ± 0.000 a |
| 24 h | 6.1 ± 0.27 a | 4.6 ± 0.24 a | 1.5 ± 0.35 a | 0.0 ± 0.000 a |
| 48 h | 6.9 ± 0.27 c | 5.1 ± 0.38 b | 2.3 ± 0.53 ab | 0.0 ± 0.000 a |
| 72 h | 7.2 ± 0.23 b | 4.7 ± 0.28 a | 2.9 ± 0.39 b | 0.0 ± 0.000 a |
| Communal | ||||
| >1 h | 4.3 ± 0.23 b | 2.1 ± 0.23 a | 0.3 ± 0.11 b | 0.1 ± 0.08 a |
| 2 h | 3.9 ± 0.25 a | 1.9 ± 0.22 a | 0.3 ± 0.10 b | 0.1 ± 0.07 a |
| 48 h | 5.3 ± 0.26 c | 3.9 ± 0.33 b | 0.1 ± 0.07 a | 0.3 ± 0.16 b |
| Distance | ||||
| Commercial | ||||
| 0 km | 6.9 ± 0.17 b | 5.0 ± 0.21 b | 2.4 ± 0.29 a | 0.0 ± 0.000 a |
| 1.8 km | 5.9 ± 0.23 a | 3.9 ± 1.14 a | 2.2 ± 0.46 a | 0.0 ± 0.000 a |
| 2 km | 7.8 ± 0.23 c | 5.6 ± 0.22 c | 4.7 ± 0.19 c | 0.0 ± 0.000 a |
| 25 km | 7.0 ± 0.31 b | 4.9 ± 0.29 b | 3.0 ± 0.45 b | 0.0 ± 0.000 a |
| Communal | ||||
| 0 km | 4.6 ± 0.19 b | 2.9 ± 0.23 b | 0.2 ± 0.06 a | 0.2 ± 0.09 a |
| 1.5 km | 4.3 ± 0.23 a | 2.1 ± 0.24 a | 0.3 ± 0.11 a | 0.1 ± 0.08 a |
| Temperature | Meat Type | Stage | Shop Class | Storage Period | TBC | CC | PEC | S. aureus | |
|---|---|---|---|---|---|---|---|---|---|
| Distance | −0.19 ** | 0.01 | −0.19 ** | −0.09 | −0.01 | 0.24 ** | 0.18 ** | 0.25 *** | −0.06 |
| Temperature | −0.01 | 0.34 *** | 0.55 *** | −0.43 *** | −0.46 *** | −0.53 *** | −0.31 *** | 0.01 | |
| Meat Type | −0.01 | 0.01 | 0.12 | 0.05 | 0.18 ** | 0.22 *** | −0.06 | ||
| Stage | 0.80 *** | −0.34 *** | −0.49 *** | −0.36 *** | −0.57 *** | 0.21 * | |||
| Shop Class | −0.31 *** | −0.52 *** | −0.45 *** | −0.53 *** | 0.17 * | ||||
| Storage period | 0.37 *** | 0.43 *** | 0.22 ** | −0.05 | |||||
| TBC | 0.75 *** | 0.57 *** | −0.07 | ||||||
| CC | 0.54 *** | −0.13 * | |||||||
| PEC | −0.13 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, Z.T.; Mhlongo, L.C.; Hugo, A. Microbial Profiles of Meat at Different Stages of the Distribution Chain from the Abattoir to Retail Outlets. Int. J. Environ. Res. Public Health 2023, 20, 1986. https://doi.org/10.3390/ijerph20031986
Rani ZT, Mhlongo LC, Hugo A. Microbial Profiles of Meat at Different Stages of the Distribution Chain from the Abattoir to Retail Outlets. International Journal of Environmental Research and Public Health. 2023; 20(3):1986. https://doi.org/10.3390/ijerph20031986
Chicago/Turabian StyleRani, Zikhona Theodora, Lindokuhle Christopher Mhlongo, and Arno Hugo. 2023. "Microbial Profiles of Meat at Different Stages of the Distribution Chain from the Abattoir to Retail Outlets" International Journal of Environmental Research and Public Health 20, no. 3: 1986. https://doi.org/10.3390/ijerph20031986
APA StyleRani, Z. T., Mhlongo, L. C., & Hugo, A. (2023). Microbial Profiles of Meat at Different Stages of the Distribution Chain from the Abattoir to Retail Outlets. International Journal of Environmental Research and Public Health, 20(3), 1986. https://doi.org/10.3390/ijerph20031986






