Identification and Risk Assessment of Priority Control Organic Pollutants in Groundwater in the Junggar Basin in Xinjiang, P.R. China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological and Hydrogeological Conditions
2.1.1. Geological Conditions
2.1.2. Hydrogeological Conditions
2.2. Sample Collection and Measurement
2.3. Screening Methods for PCOPs
2.3.1. Prevalence
2.3.2. Occurrence
2.3.3. PBT
2.3.4. PvOPBT Scores
2.4. Risk Assessment Methods
2.4.1. Ecological Risk Assessment
2.4.2. Health Risk Assessment
3. Results
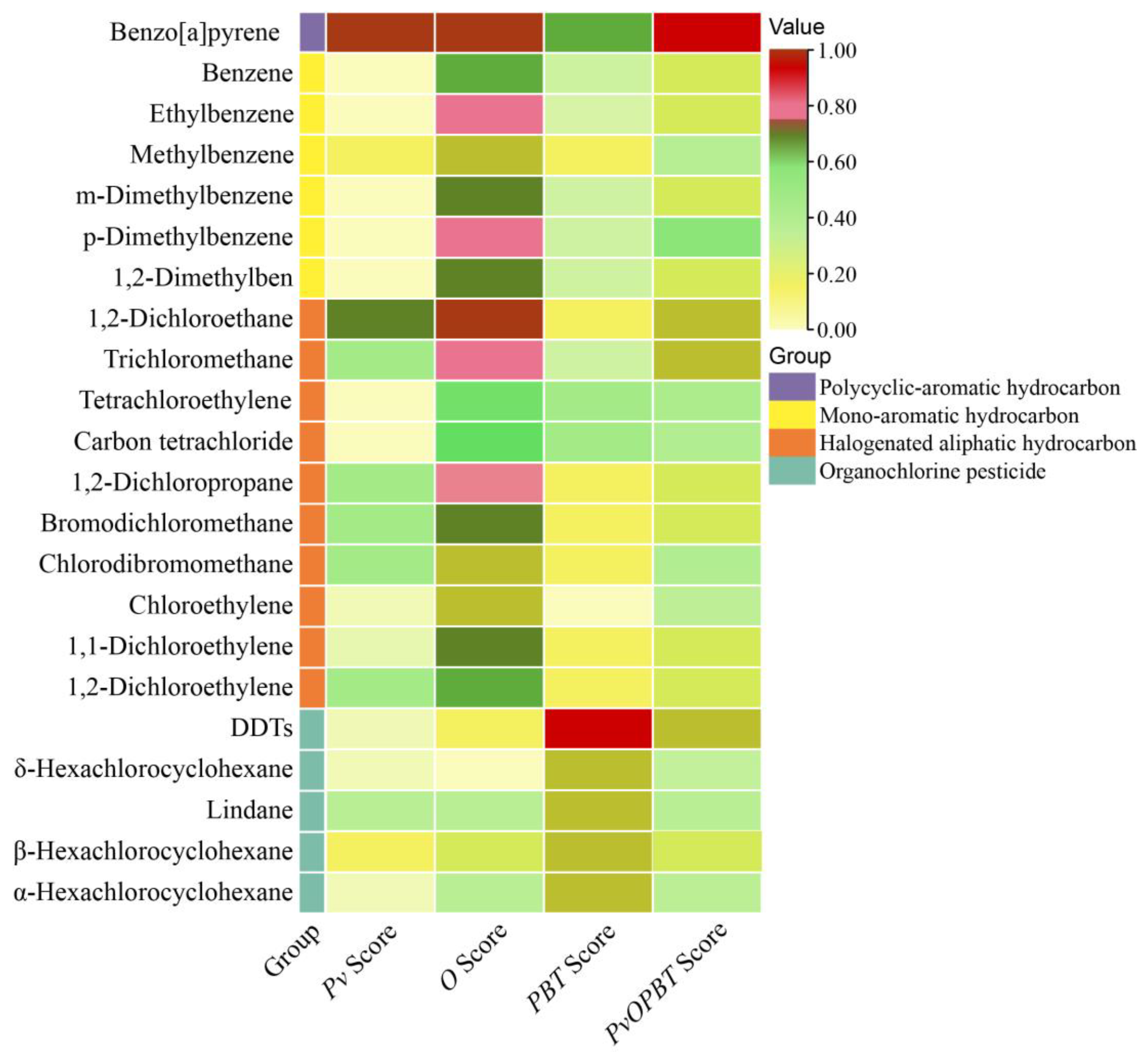
3.1. Evaluation Scores of Organic Pollutants
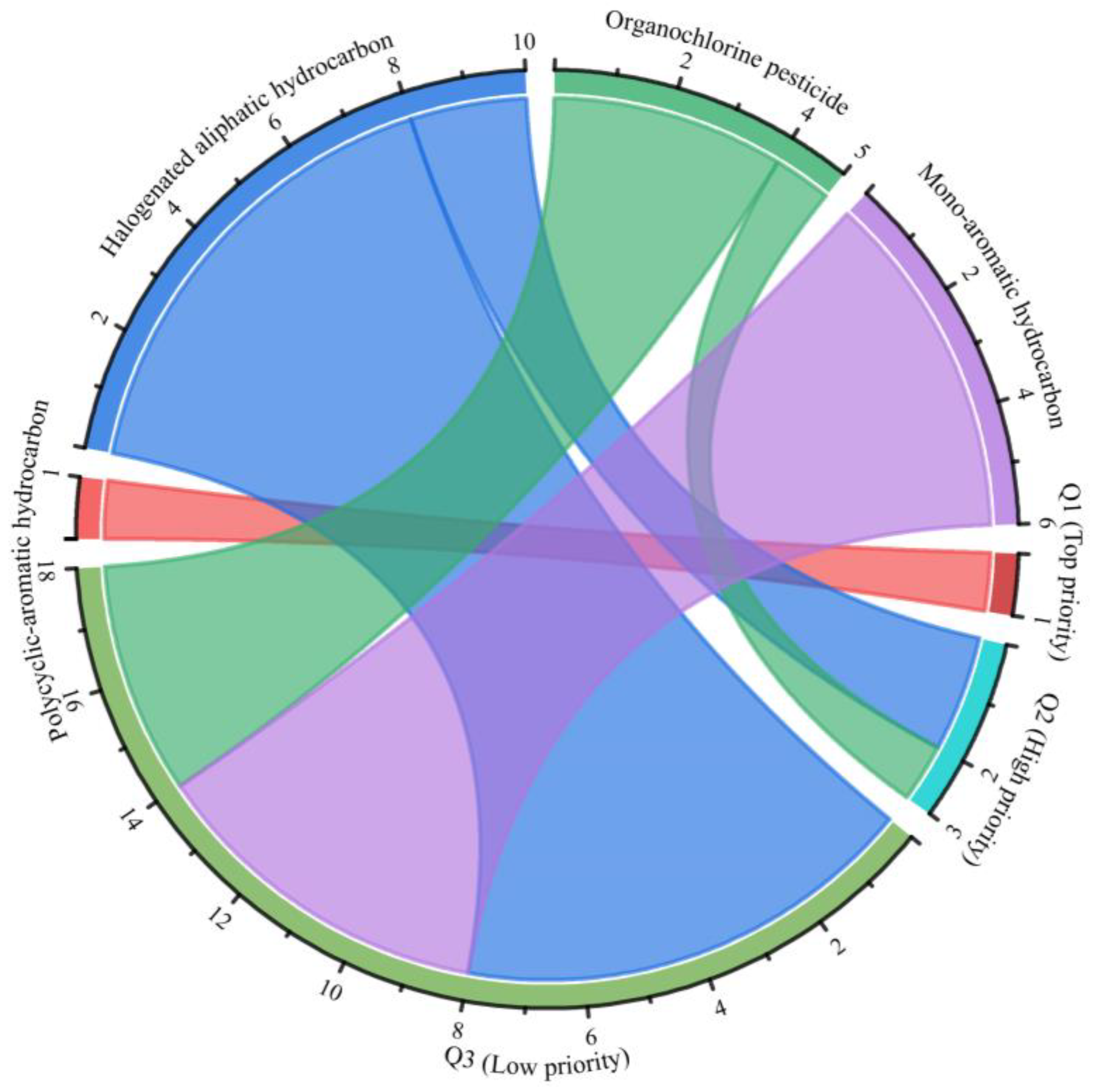
3.2. Priority Ranking of Organic Pollutant Categories and Scores of Priority Organic Pollutants
3.3. Spatial Distribution of PCOPs in Groundwater
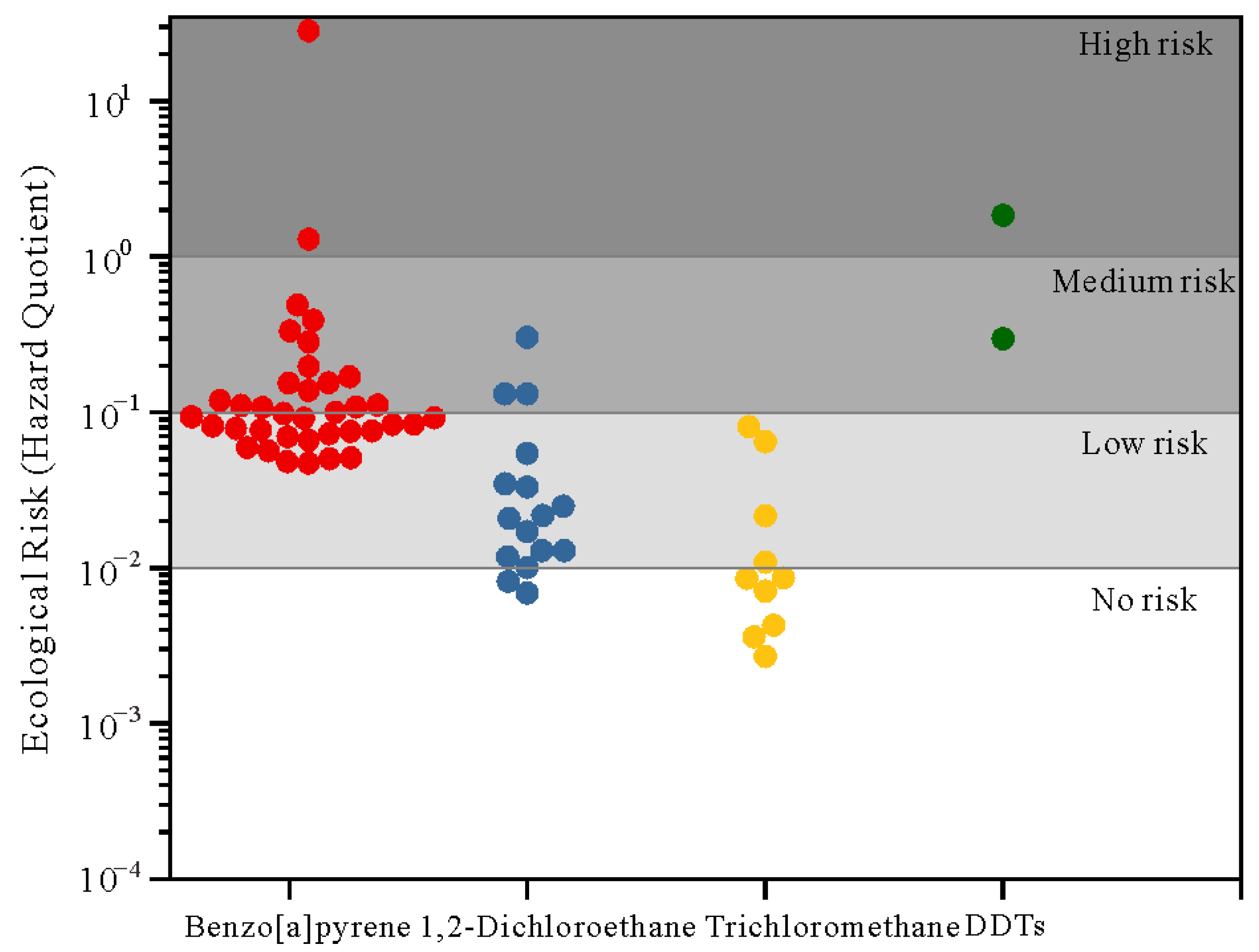
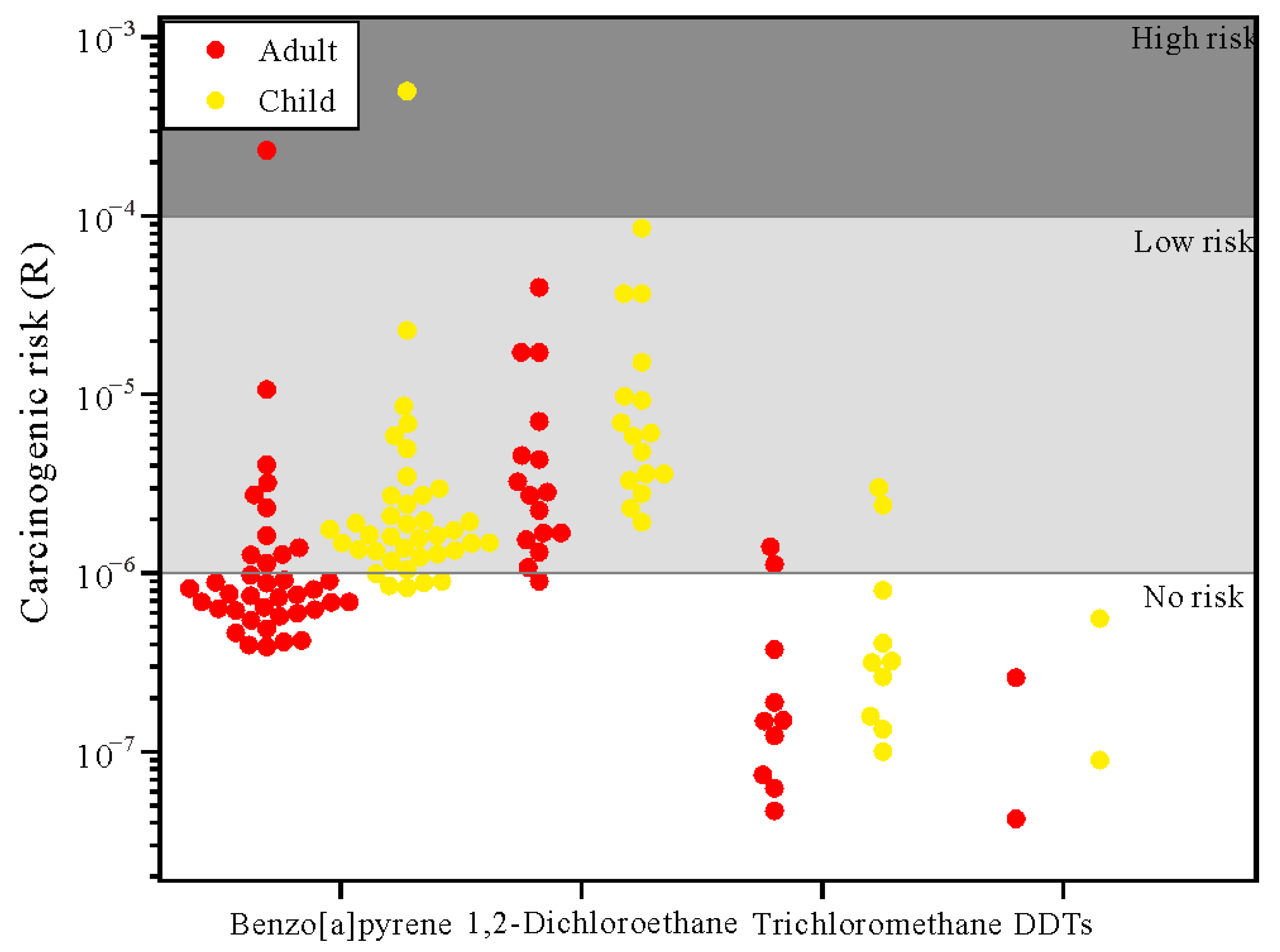
3.4. Results of Risk Assessment
3.4.1. Ecological Risk Assessment
3.4.2. Health Risk Assessment
4. Discussion
4.1. Benzo[a]pyrene
4.2. 1,2-Dichloroethane
4.3. Trichloromethane
4.4. DDTs
4.5. Risk Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talib, M.A.; Tang, Z.; Shahab, A.; Siddique, J.; Faheem, M.; Fatima, M. Hydrogeochemical Characterization and Suitability Assessment of Groundwater: A Case Study in Central Sindh, Pakistan. Int. J. Environ. Res. Public Health 2019, 16, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.W.; Zhang, Q.Q. Assessment of the Evolution of Groundwater Chemistry and Its Controlling Factors in the Huangshui River Basin of Northwestern China, Using Hydrochemistry and Multivariate Statistical Techniques. Int. J. Environ. Res. Public Health 2021, 18, 7551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, R.; Wang, W.; Su, X. Preface: Groundwater sustainability in fast-developing China. Hydrogeol. J. 2018, 26, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Feng, F.; Jia, Y.F.; Yang, Y.; Huan, H.; Lian, X.Y.; Xu, X.J.; Xia, F.; Han, X.; Jiang, Y.H. Hydrogeochemical and statistical analysis of high fluoride groundwater in northern China. Environ. Sci. Pollut. Res. 2020, 27, 34840–34861. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.D.; Zhao, Z.Q.; Liu, C.Q. Using dual isotopic data to track the sources and behaviors of dissolved sulfate in the western North China Plain. Appl. Geochem. 2015, 52, 43–56. [Google Scholar] [CrossRef]
- Kath, J.; Dyer, F.J. Why groundwater matters: An introduction for policy-makers and managers. Policy Stud. 2017, 38, 447–461. [Google Scholar] [CrossRef]
- Su, H.; Kang, W.D.; Xu, Y.J.; Wang, J.D. Evaluation of groundwater quality and health risks from contamination in the north edge of the Loess Plateau, Yulin City, Northwest China. Environ. Earth Sci. 2017, 76, 467. [Google Scholar] [CrossRef]
- Li, P. Groundwater Quality in Western China: Challenges and Paths Forward for Groundwater Quality Research in Western China. Expo. Health 2016, 8, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Lapworth, D.J.; Das, P.; Shaw, A.; Mukherjee, A.; Civil, W.; Petersen, J.O.; Gooddy, D.C.; Wakefield, O.; Finlayson, A.; Krishan, G.; et al. Deep urban groundwater vulnerability in India revealed through the use of emerging organic contaminants and residence time tracers. Environ. Pollut. 2018, 240, 938–949. [Google Scholar] [CrossRef]
- Jurado, A.; Vázquez-Suñé, E.; Pujades, E. Urban Groundwater Contamination by Non-Steroidal Anti-Inflammatory Drugs. Water 2021, 13, 720. [Google Scholar] [CrossRef]
- Machiwal, D.; Jha, M.K.; Singh, V.P.; Mohan, C. Assessment and mapping of groundwater vulnerability to pollution: Current status and challenges. Earth Sci. Rev. 2018, 185, 901–927. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Ren, W.; Wang, H.; Liu, F.; Yang, F. Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 2020, 260, 121006. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Liang, D.; Zhu, H.M. Optimal control of groundwater pollution combined with source abatement costs and taxes. J. Comput. Sci. 2017, 20, 17–29. [Google Scholar] [CrossRef]
- Qiao, X.Y.; Wang, W.K.; Duan, L.; Wang, Y.L.; Xiao, P. Regional groundwater cycle patterns and renewal capacity assessment at the south edge of the Junggar Basin, China. Environ. Earth Sci. 2020, 79, 334. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Xu, H.; Ding, Z.; Shi, Y.; Lu, Z.; Cheng, Z. Groundwater pollution risk assessment based on groundwater vulnerability and pollution load on an isolated island. Chemosphere 2022, 289, 133134. [Google Scholar] [CrossRef]
- Zait, R.; Sluser, B.; Fighir, D.; Plavan, O.; Teodosiu, C. Priority Pollutants Monitoring and Water Quality Assessment in the Siret River Basin, Romania. Water 2022, 14, 129. [Google Scholar] [CrossRef]
- EPA (United States Environmental Protection Agency). Toxic and Priority Pollutants under the Clean Water Act. Available online: https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 20 May 1977).
- Zhou, W.M.; Fu, D.Q.; Sun, Z.G. Determination of Black List of China’s Priority Pollutants in Water. Res. Environ. Sci. 1991, 4, 9–12. [Google Scholar] [CrossRef]
- Babut, M.; Corinne, B.; Marc, B.; Patrick, F.; Jeanne, G.; Geneviève, G. Developing environmental quality standards for various pesticides and priority pollutants for French freshwaters. J. Environ. Manag. 2003, 69, 139–147. [Google Scholar] [CrossRef]
- Coquery, M.; Morin, A.; Bécue, A.; Lepot, B. Priority substances of the European Water Framework Directive: Analytical challenges in monitoring water quality. TrAC Trends Anal. Chem. 2005, 24, 117–127. [Google Scholar] [CrossRef]
- Amirabdollahian, M.; Datta, B.; Beck, P.H. Application of a link simulation optimization model utilizing quantification of hydrogeologic uncertainty to characterize unknown groundwater contaminant sources. Model. Earth Syst. Environ. 2019, 5, 119–131. [Google Scholar] [CrossRef]
- Shofstahl, J.H.; Hardy, J.K. Method for the Determination of the Priority Pollutant Metals by HPLC. J. Chromatogr. Sci. 1990, 28, 225–229. [Google Scholar] [CrossRef]
- Khan, F.I.; Sadiq, R. Risk-Based Prioritization of Air Pollution Monitoring Using Fuzzy Synthetic Evaluation Technique. Environ. Monit. Assess. 2005, 105, 261–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Xu, P.; Qian, H. Application of fuzzy comprehensive evaluation method using entropy weight in groundwater quality evaluation: A case study on Xianyang, China. IOP Conf. Ser. Earth Environ. Sci 2020, 467, 012146. [Google Scholar] [CrossRef]
- Gramatica, P.; Cassani, S.; Sangion, A. PBT assessment and prioritization by PBT Index and consensus modeling: Comparison of screening results from structural models. Environ. Int. 2015, 77, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sangion, A.; Gramatica, P. PBT assessment and prioritization of contaminants of emerging concern: Pharmaceuticals. Environ. Res. 2016, 147, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Wang, T.; Zhao, W.; Huang, J.; Wang, B.; Blaney, L.; Bu, Q.; Yu, G. Emerging Organic Contaminants in Chinese Surface Water: Identification of Priority Pollutants. Engineering 2022, 11, 111–125. [Google Scholar] [CrossRef]
- Gonçalves, S.F.; Silva, A.R.R.; Pavlaki, M.D.; Morgado, R.G.; Loureiro, S. Site-specific hazard evaluation for improved groundwater risk assessment. Chemosphere 2021, 274, 129742. [Google Scholar] [CrossRef]
- Fan, W.; Zhou, J.L.; Zeng, Y.Y.; Jia, R.L. Determination of Priority Control Pollutants of Groundwater in Shihezi Area. Yellow River 2018, 40, 69–71. (In Chinese) [Google Scholar] [CrossRef]
- Tu, Z.; Zhou, J.L.; Sun, Y.; Zeng, Y.Y.; Lei, M. Determination of Priority Control Pollutants in Groundwater of Aksu Prefecture, Xinjiang. Saf. Environ. Eng. 2022, 29, 151–159. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, H.Q.; Miao, S.; Liu, C.; Miao, Q. Screening methodology of groundwater priority-control pollutants: A case study. IOP Conf. Ser. Earth Environ. Sci 2020, 612, 12014. [Google Scholar] [CrossRef]
- Huang, F.Y.; Chen, L.P.; Zhang, C.; Liu, F.; Li, H. Prioritization of antibiotic contaminants in China based on decennial national screening data and their persistence, bioaccumulation and toxicity. Sci. Total Environ. 2022, 806, 150636. [Google Scholar] [CrossRef]
- Li, S.Y.; Tang, Q.L.; Lei, J.Q.; Xu, X.W.; Jiang, J.; Wang, Y.D. An overview of non-conventional water resource utilization technologies for biological sand control in Xinjiang, northwest China. Environ. Earth Sci. 2015, 73, 873–885. [Google Scholar] [CrossRef]
- Wang, W.K.; Wang, Z.; Hou, R.Z.; Guan, L.Y.; Dang, Y.; Zhang, Z.Y.; Wang, H.; Duan, L.; Wang, Z.F. Modes, hydrodynamic processes and ecological impacts exerted by river–groundwater transformation in Junggar Basin, China. Hydrogeol. J. 2018, 26, 1547–1557. [Google Scholar] [CrossRef]
- Zhou, Y.; Tu, Z.; Zhou, J.; Han, S.; Sun, Y.; Liu, X.; Liu, J.; Liu, J. Distribution, dynamic and influence factors of groundwater arsenic in the Manas River Basin in Xinjiang, P.R.China. Appl. Geochem. 2022, 146, 105441. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, P.Y.; Wang, D.; Ren, X.F.; Wei, M.J. Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. Int. J. 2019, 26, 1603–1621. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Yang, Y.; Ma, H.; Wang, H.; Luo, J.; Bian, J.; Martin, J.D. Isotopic and geochemical interpretation of groundwater under the influences of anthropogenic activities. J. Hydrol. 2019, 576, 685–697. [Google Scholar] [CrossRef]
- Azzellino, A.; Colombo, L.; Lombi, S.; Marchesi, V.; Piana, A.; Andrea, M.; Alberti, L. Groundwater diffuse pollution in functional urban areas: The need to define anthropogenic diffuse pollution background levels. Sci. Total Environ. 2019, 656, 1207–1222. [Google Scholar] [CrossRef]
- Jia, R.L.; Zhou, J.L.; Zhou, Y.Z.; Li, Q.; Gao, Y.X. A Vulnerability Evaluation of the Phreatic Water in the Plain Area of the Junggar Basin, Xinjiang Based on the VDEAL Model. Sustainability 2014, 6, 8604–8617. [Google Scholar] [CrossRef] [Green Version]
- Kooy, M.; Walter, C.T.; Prabaharyaka, I. Inclusive development of urban water services in Jakarta: The role of groundwater. Habitat Int. 2018, 73, 109–118. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Wang, L.; Wang, H.W.; Zhu, X.; Wang, L.J. Spatio-Temporal Variation of Groundwater Quality and Source Apportionment Using Multivariate Statistical Techniques for the Hutuo River Alluvial-Pluvial Fan, China. Int. J. Environ. Res. Public Health 2020, 17, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.D.; Chen, X.G.; Wang, W.K.; Liu, B. Investigation and Assessment of Groundwater Resources and Its Environmental Issues in the Junggar Basin; Geological Press: Beijing, China, 2009; pp. 25–61. [Google Scholar]
- Zhao, J.M.; Deng, G.; Xu, Q.; Shao, X.Z.; Zhang, X.K.; Chen, X.F.; Ma, Z.J. Basement structure and properties of the southern Junggar Basin. J. Geodyn. 2018, 121, 26–35. [Google Scholar] [CrossRef]
- Dong, X.G.; Deng, M.J. Xinjiang Groundwater Resources; Xinjiang Science and Technology Press: Xinjiang, China, 2005; pp. 14–22. [Google Scholar]
- Xinjiang Ecological Environment Department. Basic Information Table of Pollution Sources in Xinjiang. Available online: http://sthjt.xinjiang.gov.cn/xjepd/zhywczdwry/201607/aceaab594c364bf78992da36d0c72691.shtml (accessed on 1 June 2015).
- De Laender, F.; Van Sprang, P.; Janssen, C.R. A re-evaluation of fifteen years of european risk assessment using effect models. Environ. Toxicol. Chem. 2013, 32, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Topaz, T.; Boxall, A.; Suari, Y.; Egozi, R.; Sade, T.; Chefetz, B. Ecological Risk Dynamics of Pharmaceuticals in Micro-Estuary Environments. Environ. Sci. Technol. 2020, 54, 11182–11190. [Google Scholar] [CrossRef]
- Sailaukhanuly, Y.; Zhakupbekova, A.; Amutova, F.; Carlsen, L. On the ranking of chemicals based on their PBT characteristics: Comparison of different ranking methodologies using selected POPs as an illustrative example. Chemosphere 2013, 90, 112–117. [Google Scholar] [CrossRef]
- Acharya, K.; Werner, D.; Dolfing, J.; Barycki, M.; Meynet, P.; Mrozik, W.; Komolafe, O.; Puzyn, T.; Davenport, R.J. A quantitative structure-biodegradation relationship (QSBR) approach to predict biodegradation rates of aromatic chemicals. Water Res. 2019, 157, 181–190. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Nedyalkova, M.; Madurga, S.; Pena-Pereira, F.; Namieśnik, J.; Simeonov, V. Pre-selection and assessment of green organic solvents by clustering chemometric tools. Ecotoxicol. Environ. Saf. 2018, 147, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.M.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klánová, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- Tang, J.F.; Sun, J.; Wang, W.D.; Yang, L.; Xu, Y.Y. Pharmaceuticals in two watersheds in Eastern China and their ecological risks. Environ. Pollut. 2021, 277, 116773. [Google Scholar] [CrossRef]
- EPA. Human Health Evaluation Manual, Supplemental Guidance: Update of Standard Default Exposure Factor; Office of Emergency and Remedial Response: Washington, DC, USA, 2014; pp. 1–120.
- Wang, F.; Song, K.; He, X.L.; Peng, Y.; Liu, D.; Liu, J. Identification of Groundwater Pollution Characteristics and Health Risk Assessment of a Landfill in a Low Permeability Area. Int. J. Environ. Res. Public Health 2021, 18, 7690. [Google Scholar] [CrossRef]
- EPA. Integrated Risk Information System. Available online: https://www.epa.gov/iris (accessed on 20 May 2022).
- Huang, X.; Cheng, J.P.; Bo, D.D.; Betha, R.H.; Balasubramanian, R. Bioaccessibility of Airborne Particulate-Bound Trace Elements in Shanghai and Health Risk Assessment. Front. Environ. Sci. 2016, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wen, X. Performance and kinetics of benzo(a)pyrene biodegradation in contaminated water and soil and improvement of soil properties by biosurfactant amendment. Ecotoxicol. Environ. Saf. 2021, 207, 111292. [Google Scholar] [CrossRef]
- Cui, X.H.; Li, B.H.; Chen, H.H. Contamination characteristics and pollutant sources analysis on PAHs in shallow groundwater in suburb of Taihu Plain. Environ. Sci. 2008, 29, 1806–1810. Available online: https://en.cnki.com.cn/Article_en/CJFDTOTAL-CCDZ200704021.htm (accessed on 20 May 2022). (In Chinese).
- Krzyszczak, A.; Dybowski, M.P.; Kończak, M.; Czech, B. Low bioavailability of derivatives of polycyclic aromatic hydrocarbons in biochar obtained from different feedstock. Environ. Res. 2022, 214, 113787. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Dybowski, M.P.; Czech, B. Formation of polycyclic aromatic hydrocarbons and their derivatives in biochars: The effect of feedstock and pyrolysis conditions. J. Anal. Appl. Pyrolysis 2021, 160, 105339. [Google Scholar] [CrossRef]
- Liang, M.; Liang, H.; Rao, Z.; Xu, D. Occurrence of polycyclic aromatic hydrocarbons in groundwater from rural areas in eastern China: Spatial distribution, source apportionment and health cancer risk assessment. Chemosphere 2020, 259, 127534. [Google Scholar] [CrossRef]
- Wu, J.; Bian, J.; Wan, H.; Sun, X.; Li, Y. Probabilistic human health-risk assessment and influencing factors of aromatic hydrocarbon in groundwater near urban industrial complexes in Northeast China. Sci. Total Environ. 2021, 800, 149484. [Google Scholar] [CrossRef]
- Perini, B.L.B.; Bitencourt, R.L.; Daronch, N.A.; dos Santos Schneider, A.L.; de Oliveira, D. Surfactant-enhanced in-situ enzymatic oxidation: A bioremediation strategy for oxidation of polycyclic aromatic hydrocarbons in contaminated soils and aquifers. J. Environ. Chem. Eng. 2020, 8, 104013. [Google Scholar] [CrossRef]
- Zheng, H.; Qu, C.; Zhang, J.; Talpur, S.A.; Ding, Y.; Xing, X.; Qi, S. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soils from Ningde, China: Levels, sources, and human health risk assessment. Environ. Geochem. Health 2019, 41, 907–919. [Google Scholar] [CrossRef]
- Desalme, D.; Binet, P.; Chiapusio, G. Challenges in tracing the fate and effects of atmospheric polycyclic aromatic hydrocarbon deposition in vascular plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef]
- Gao, S.X.; Sun, C.; Zhang, A.Q. Chapter 5 Pollution of Polycyclic Aromatic Hydrocarbons in China. In Developments in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2007; Volume 7, pp. 237–287. [Google Scholar]
- Li, Z. Correlation between Aromatic Hydrocarbons and Benzo[a]pyrene in Coke Plant Emissions. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2012. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201501&filename=1015569611.nh&v=MzA0MTFyRnkvbVU3elBWRjI2RzdhK0Y5Zk5ycEViUElSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI3aWVaK2Q= (accessed on 20 May 2022).
- Rhead, M.M.; Hardy, S.A. The sources of polycyclic aromatic compounds in diesel engine emissions. Fuel 2003, 82, 385–393. [Google Scholar] [CrossRef]
- Begeman, C.R.; Colucci, J.M. Benzo[a]pyrene in Gasoline Partially Persists in Automobile Exhaust. Science 1968, 161, 271. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Distribuion and Degradation of PAHs from Soils under Different Environments. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2009. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201301&filename=1012490477.nh&v=MjA0Njl0WExxSkViUElSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI3aWVaK2RyRnk3aFZMck1WRjI2SExleEg= (accessed on 20 May 2022).
- Mo, C.-H.; Cai, Q.-Y.; Li, Y.-H.; Zeng, Q.-Y. Occurrence of priority organic pollutants in the fertilizers, China. J. Hazard. Mater. 2008, 152, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ma, Y.; Ma, L. Utilization of straw in biomass energy in China. Renew. Sustain. Energy Rev. 2007, 11, 976–987. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, H.; Chang, B.; Wei, Z.; Qiu, W.; Liu, S.; Liu, W.; Tao, S. Emission of polycyclic aromatic hydrocarbons from indoor straw burning and emission inventory updating in China. Ann. N. Y. Acad. Sci. 2008, 1140, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Li, L.; Zhang, Y. Analysis and Assessment Research on Organic Compounds from fuel of Vehicle. Environ. Sci. Manag. 2010, 35, 192–194. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD2010&filename=BFHJ201009050&v=MTEyNzdydktKeXZEWkxHNEg5SE1wbzlBWklSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI3aWVaK2RyRkNqbFc= (accessed on 20 May 2022).
- Feng, J.; Li, X.; Zhao, J.H.; Sun, J.H. Distribution, transfer, and health risks of polycyclic aromatic hydrocarbons (PAHs) in soil-wheat systems of Henan Province, a typical agriculture province of China. Environ. Sci. Pollut. Res. Int. 2017, 24, 18195–18203. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; Lopez, F.; Becerra, S.; Jamhoury, H.; Le Menach, K.; Devier, M.H.; Budzinski, H.; Prunier, J.; Juteau-Martineau, G.; Ochoa-Herrera, V.; et al. Drinking water quality in areas impacted by oil activities in Ecuador: Associated health risks and social perception of human exposure. Sci. Total Environ. 2019, 690, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.G.; Chernikova, N.; Minkina, T.; Sushkova, S.; Dudnikova, T.; Antonenko, E.; Fedorenko, G.; Bauer, T.; Mandzhieva, S.; Barbashev, A. Effects of benzo[a]pyrene toxicity on morphology and ultrastructure of Hordeum sativum. Environ. Geochem. Health 2021, 43, 1551–1562. [Google Scholar] [CrossRef]
- Yu, R.; Peethambaram, H.S.; Falta, R.W.; Verce, M.F.; Henderson, J.K.; Bagwell, C.E.; Brigmon, R.L.; Freedman, D.L. Kinetics of 1,2-Dichloroethane and 1,2-Dibromoethane Biodegradation in Anaerobic Enrichment Cultures. Appl. Environ. Microbiol. 2013, 79, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Frascari, D.; Zanaroli, G.; Danko, A.S. In situ aerobic cometabolism of chlorinated solvents: A review. J. Hazard. Mater. 2015, 283, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.G.; Kim, J.G.; Baek, K. Removal of 1,2-dichloroethane in groundwater using Fenton oxidation. J. Hazard. Mater. 2022, 428, 128253. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, M.R.; Johns, D.O.; Bateson, T.F.; Guyton, K.Z. A review of the genotoxicity of 1,2-dichloroethane (EDC). Mutat. Res. 2011, 727, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, Y.P. Toxic effects of combined treatment of 1,2-dichloroethane and ethanol on mouse brain and the related mechanisms. J. Biochem. Mol. Toxicol. 2019, 33, e22294. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.S.; Rashid, N.; Basheer, S. Chapter 37—Physiological and molecular basis of plants tolerance to linear halogenated hydrocarbons. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 591–602. [Google Scholar]
- Zhao, L. Research on the Removal Process of 1,2-Dichloroethane in Wastewater in Chemical Industry Park. Master’s Thesis, East China University of Science and Technology, Shanghai, China, 2021. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD202102&filename=1021056144.nh&v=MTM0MzFyRkN2a1ZidlBWRjI1SDdPOUdORElxNUViUElSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI3aWVaK2Q= (accessed on 25 May 2022).
- Golfinopoulos, S.K. The occurrence of trihalomethanes in the drinking water in Greece. Chemosphere 2000, 41, 1761–1767. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhang, Z.J.; Fei, Y.H.; Wang, Z.; Zhang, F.E. Occurrence features and leaching migration of chloroform in shallow groundwater. Environ. Sci. 2011, 33, 36–42. (In Chinese) [Google Scholar] [CrossRef]
- Zamani, I.; Bouzari, M.; Emtiazi, G.; Fanaei, M. Rapid quantitative estimation of chlorinated methane utilizing bacteria in drinking water and the effect of nanosilver on biodegradation of the trichloromethane in the environment. Jundishapur J. Microbiol. 2015, 8, e14965. [Google Scholar] [CrossRef] [Green Version]
- Jayaweera, D.; Islam, S.; Gunja, N.; Cowie, C.; Broska, J.; Poojara, L.; Roberts, M.S.; Isbister, G.K. Chloroform ingestion causing severe gastrointestinal injury, hepatotoxicity and dermatitis confirmed with plasma chloroform concentrations. Clin. Toxicol. (Phila) 2017, 55, 147–150. [Google Scholar] [CrossRef]
- Zhang, H.F. Material Safety Data Sheet, 2nd ed.; Chemical Industry Press: Beijing, China, 2007; pp. 1063–1064. [Google Scholar]
- Li, Y. Study on the Adsorption Treatment of Chloroform in Water By Macroporous Adsorption Resin. Master’s Thesis, Sichuan University, Chengdu, China, 2006. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD0506&filename=2005130870.nh&uniplatform=NZKPT&v=ijR3HipHsZ4R5l1NQm_1bKys7_YiBGfAiAhJF5jRQyTZQ24_2fcXgd5AAKrkCwnm (accessed on 25 May 2022).
- Rebelo, A.; Ferra, I.; Marques, A.; Silva, M.M. Wastewater reuse: Modeling chloroform formation. Environ. Sci. Pollut. Res. Int. 2016, 23, 24560–24566. [Google Scholar] [CrossRef] [PubMed]
- Okoya, A.A.; Olaiya, O.O.; Akinyele, A.B.; Ochor, N.O. Efficacy of Moringa oleifera Seed Husk as Adsorptive Agent for Trihalomethanes from a Water Treatment Plant in Southwestern, Nigeria. J. Chem. 2020, 2020, 3450954. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.T.; Enfield, C.G.; Dunlap, W.J.; Cosby, R.L.; Foster, D.A.; Baskin, L.B. Transport and Fate of Selected Organic Pollutants in a Sandy Soil. J. Environ. Qual. 1981, 10, 501–506. [Google Scholar] [CrossRef]
- Zhao, X.D.; Chen, Z.G.; Che, M.D.; Qiu, S.; Huang, R.L.; Qi, W.; He, Z.M.; Su, R.X. Tannic acid enhances the removal of chloroform from water using NaOH-activated persulfate. Environ. Chem. Lett. 2020, 18, 1441–1446. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Zou, S.; Liu, L. Halogenated hydrocarbon contaminants in shallow groundwater. Geol. China 2002, 29, 326–329. Available online: https://geochina.cgs.gov.cn/geochina/article/abstract/20020317?st=search (accessed on 25 May 2022).
- Li, Y.; Fei, Y.; Wang, Z.; Qian, Y. Occurrence features and leaching of chloroform in shallow groundwater. Environ. Pollut. Control 2011, 33, 36–42. Available online: https://10.15985/j.cnki.1001-3865.2011.07.016 (accessed on 25 May 2022).
- Dang, H.V.; Nguyen, L.T.; Tran, H.T.; Nguyen, H.T.; Dang, A.K.; Viet, D.; Frazzoli, C. Risk Factors for Non-communicable Diseases in Vietnam: A Focus on Pesticides. Front. Environ. Sci. 2017, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.W.; Yang, Z.F.; Shen, Z.Y.; Niu, J.F.; Cai, Y.P. Residues of organochlorine pesticides in water and suspended particulate matter from the Yangtze River catchment of Wuhan, China. Environ. Monit. Assess. 2007, 137, 427. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.X.; Shen, Z.Y.; Yang, Z.F.; Mo, L.; Kong, Y.H.; Lou, I. Occurrence and possible sources of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the Chao River, China. Chemosphere 2014, 114, 136–143. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, M.; Gao, H.; Zheng, J.; Jia, L. Atomic elucidation of the cyclodextrin effects on DDT solubility and biodegradation. Phys. Chem. Chem. Phys. 2016, 18, 17380–17388. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, Y.Z.; Wang, J.; Lu, S.Y.; Cao, Y.J.; Tang, C.Y.; Yan, Z.G.; Zheng, L. History traces of HCHs and DDTs by groundwater dating and their behaviours and ecological risk in northeast China. Chemosphere 2020, 257, 127212. [Google Scholar] [CrossRef]
- Hacskaylo, J.; Scales, A.L. Some Effects of Guthion Alone and in Combination with DDT and of a Dieldrin-DDT Mixture on Growth and Fruiting of the Cotton Plant. J. Econ. Entomol. 1959, 52, 396–398. [Google Scholar] [CrossRef]
- Mekonen, S.; Lachat, C.; Ambelu, A.; Steurbaut, W.; Kolsteren, P.; Jacxsens, L.; Wondafrash, M.; Houbraken, M.; Spanoghe, P. Risk of DDT residue in maize consumed by infants as complementary diet in southwest Ethiopia. Sci. Total Environ. 2015, 511, 454–460. [Google Scholar] [CrossRef]
- Van den Berg, H. Global Status of DDT and Its Alternatives for Use in Vector Control to Prevent Disease. Environ. Health Perspect. 2009, 117, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Beard, J. DDT and human health. Sci. Total Environ. 2006, 355, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Wang, G.; Chen, Q.; Yao, Y. Investigating the role of vadose zone breathing in vapor intrusion from contaminated groundwater. J. Hazard. Mater. 2021, 416, 126272. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Zhou, J.L.; Zhou, Y.Z.; Jia, R.L. Assessment and Causes of Groundwater Organic Pollution in Typical Plain Areas in Xinjiang, China. Expo. Health 2016, 8, 401–417. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Obiri-Nyarko, F.; Duah, A.A.; Karikari, A.Y.; Agyekum, W.A.; Manu, E.; Tagoe, R. Assessment of heavy metal contamination in soils at the Kpone landfill site, Ghana: Implication for ecological and health risk assessment. Chemosphere 2021, 282, 131007. [Google Scholar] [CrossRef] [PubMed]




| Organic Pollutants | CAS a | Sample Quantity | Positive Detection Quantity | Detection Limit (μg/L) | Organic Pollutants | CAS a | Sample Quantity | Positive Detection Quantity | Detection Limit (μg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Benzene | 71-43-2 | 454 | 1 | 0.3 | Chlorodibromomethane | 124-48-1 | 455 | 3 | 0.15 |
| Ethylbenzene | 100-41-4 | 454 | 1 | 0.3 | Chloroethylene | 75-01-4 | 455 | 2 | 0.5 |
| Toluene | 108-88-3 | 454 | 2 | 0.3 | 1,1-Dichloroethylene | 75-35-4 | 455 | 3 | 0.2 |
| Bromodichloromethane | 75-27-4 | 455 | 3 | 0.15 | Bromoform | 75-25-2 | 454 | 0 | 0.5 |
| Dichloromethane | 75-09-2 | 454 | 0 | 0.5 | 1,2-Dichloroethylene | 540-59-0 | 455 | 9 | 0.2 |
| Trichloroethylene | 79-01-6 | 454 | 0 | 0.2 | Styrene | 100-42-5 | 454 | 0 | 0.25 |
| 1,2-Dichloroethane | 107-06-2 | 454 | 16 | 0.25 | 1,2-Dichlorobenzene | 95-50-1 | 454 | 0 | 0.1 |
| Chloroform | 67-66-3 | 454 | 10 | 0.2 | Chlorobenzene | 108-90-7 | 454 | 0 | 0.05 |
| Tetrachloroethylene | 127-18-4 | 454 | 1 | 0.2 | 1,3-Dichlorobenzene | 541-73-1 | 454 | 0 | 0.1 |
| 1,1,1-Trichloroethane | 2747-58-2 | 454 | 0 | 0.15 | 1,4-Dichlorobenzene | 106-46-7 | 454 | 0 | 0.1 |
| Carbon Tetrachloride | 56-23-5 | 454 | 1 | 0.2 | β-Hexachlorocyclohexane | 319-85-7 | 453 | 5 | 0.01 |
| 1,2-Dichloropropane | 78-87-5 | 455 | 3 | 0.15 | α-Hexachlorocyclohexane | 319-84-6 | 452 | 2 | 0.01 |
| 1,1,2-Trichloroethane | 79-00-5 | 454 | 1 | 0.15 | δ-Hexachlorocyclohexane | 319-86-8 | 454 | 2 | 0.01 |
| Benzo[a]pyrene | 50-32-8 | 453 | 38 | 0.002 | Lindane | 58-89-9 | 452 | 3 | 0.5 |
| Dichlorodiphenyltric-hloroethanes(DDTs) | 50-29-3 | 454 | 2 | 0.01 | 1,3-Dimethylbenzene | 108-38-3 | 454 | 1 | 0.5 |
| Hexachlorobenzene | 118-74-1 | 453 | 0 | 0.005 | 1,4-Dimethylbenzene | 106-42-3 | 454 | 1 | 0.5 |
| 1,2,4-Trichlorobenzene | 120-82-1 | 454 | 0 | 0.2 | 1,2-Dimethylbenzene | 95-47-6 | 454 | 1 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Z.; Zhou, Y.; Zhou, J.; Han, S.; Liu, J.; Liu, J.; Sun, Y.; Yang, F. Identification and Risk Assessment of Priority Control Organic Pollutants in Groundwater in the Junggar Basin in Xinjiang, P.R. China. Int. J. Environ. Res. Public Health 2023, 20, 2051. https://doi.org/10.3390/ijerph20032051
Tu Z, Zhou Y, Zhou J, Han S, Liu J, Liu J, Sun Y, Yang F. Identification and Risk Assessment of Priority Control Organic Pollutants in Groundwater in the Junggar Basin in Xinjiang, P.R. China. International Journal of Environmental Research and Public Health. 2023; 20(3):2051. https://doi.org/10.3390/ijerph20032051
Chicago/Turabian StyleTu, Zhi, Yinzhu Zhou, Jinlong Zhou, Shuangbao Han, Jinwei Liu, Jiangtao Liu, Ying Sun, and Fangyuan Yang. 2023. "Identification and Risk Assessment of Priority Control Organic Pollutants in Groundwater in the Junggar Basin in Xinjiang, P.R. China" International Journal of Environmental Research and Public Health 20, no. 3: 2051. https://doi.org/10.3390/ijerph20032051





