In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: rodents of both sexes, of all ages and species and of all genetic back-grounds (wild type, transgenic and tumor-prone animal models);
- Comparison: the “sham” sample, i.e., animals treated under conditions similar to those of exposed ones except for RF-EMF exposure, with particular reference to restraint conditions and stressing manipulations; papers describing experiments with cage control only or using the group exposed at the lowest dose level as a comparison were excluded;
- Outcome: the onset of neoplasms in laboratory animals exposed to RF, in terms of incidence of primary tumors; tumor incidence and survival were the main endpoints (outcome measures) on which this systematic review was focused;
- Articles reporting exclusively tumor-related parameters (i.e., genotoxicity, oxidative stress, etc.) were excluded from the analysis. Papers not written in English language and were not peer-reviewed and were not original (review, letters and comments) were excluded too.
2.2. Search Strategy
- the list of references of the selected papers;
- No limits were set on the year of publication;
- The query used for PubMed search and the criteria adopted on EMF Portal are attached to the protocol as supplement (suppl_1) [7].
2.3. Selection Process
2.4. Data extraction and Data Extraction Format
- Study design (number of experimental groups, control group(s), number of animals per group, randomization and blinding);
- Animal model: species, strain, sex and genotype of animals (wild type (WT)/transgenic);
- Exposure duration (LTE: long-term exposure, MTE: medium-term exposure, STE: short-term exposure);
- Timing of treatment (i.e., hours per day, days per week and total period);
- Exposure details (i.e., frequency, modulation, dose, exposure modalities in terms of whole body vs. localized exposure and restrained vs. freely moving animals type of exposure system);
- Primary outcome(s): all tumor-related outcome measures (incidence, tumor multiplicity, tumor volume, progression and survival) and numerical data were extracted from text, tables and figures (by using digital rulers) of each article, even if not all of this information was reported by all articles;
- Method to assess the endpoints;
- Data analysis and statistical evaluation;
- Authors, year of publication, title, journal;
- Information on animals died spontaneously of sacrificed for ethical reasons before the end of the exposure period.Three separate sheets were prepared in the file with the following data:
- General information on the experimental protocol: exposure characteristics, animal population, experimental protocol and endpoints (incidence, survival);
- Results;
- Risk of Bias (RoB).
2.5. Classification of Tumors
2.6. Risk of Bias (RoB) Evaluation
- Adequate randomization of administered dose or level of exposure, evaluating whether each animal had an equal chance of being assigned to a control or a treatment group;
- Allocation of animals to treatment groups unknown to operators;
- Evaluation of the experimental protocol or analysis of possible confounding variables not adequately identified and characterized;
- Blinded treatment and analysis of groups of animals (blind or double-blind);
- Evaluation of the exposure conditions, which had to be well defined and documented;
- Use of standardized methods for determining the results (effects): specific and reliable tests and adequate statistical methodology;
- Reporting of all expected outcomes;
- Calculation of animal losses (attrition bias), due to death, during the experimental period, for reasons other than those foreseen by the experimental protocol;
- Considering the relevance of the topic and knowing that many studies were funded by companies with significant commercial interests in the mobile telecommunications sector, it was decided to include in the Reporting Domain of the RoB, the possible Conflict of Interest as item 9.
2.7. Meta-Analysis: Strategy
- the SAR value (without uncertainty);
- the type of exposure duration (LTE-longer than 52 weeks, MTE-longer than 9 weeks, STE);
- the number of exposed animals with tumor (treated incidence), the total number of animals in the exposed sample, the number of sham animals with tumor (sham incidence), the total number of the animals in the sham sample; in the papers employing both sexes, the incidence data in males and females were added together, so analysis by sex was not performed;
- the animal type and the genetic background (WT/prone).
2.8. Quality Assessment (Confidence Ratings and Evidence of Health Effects)
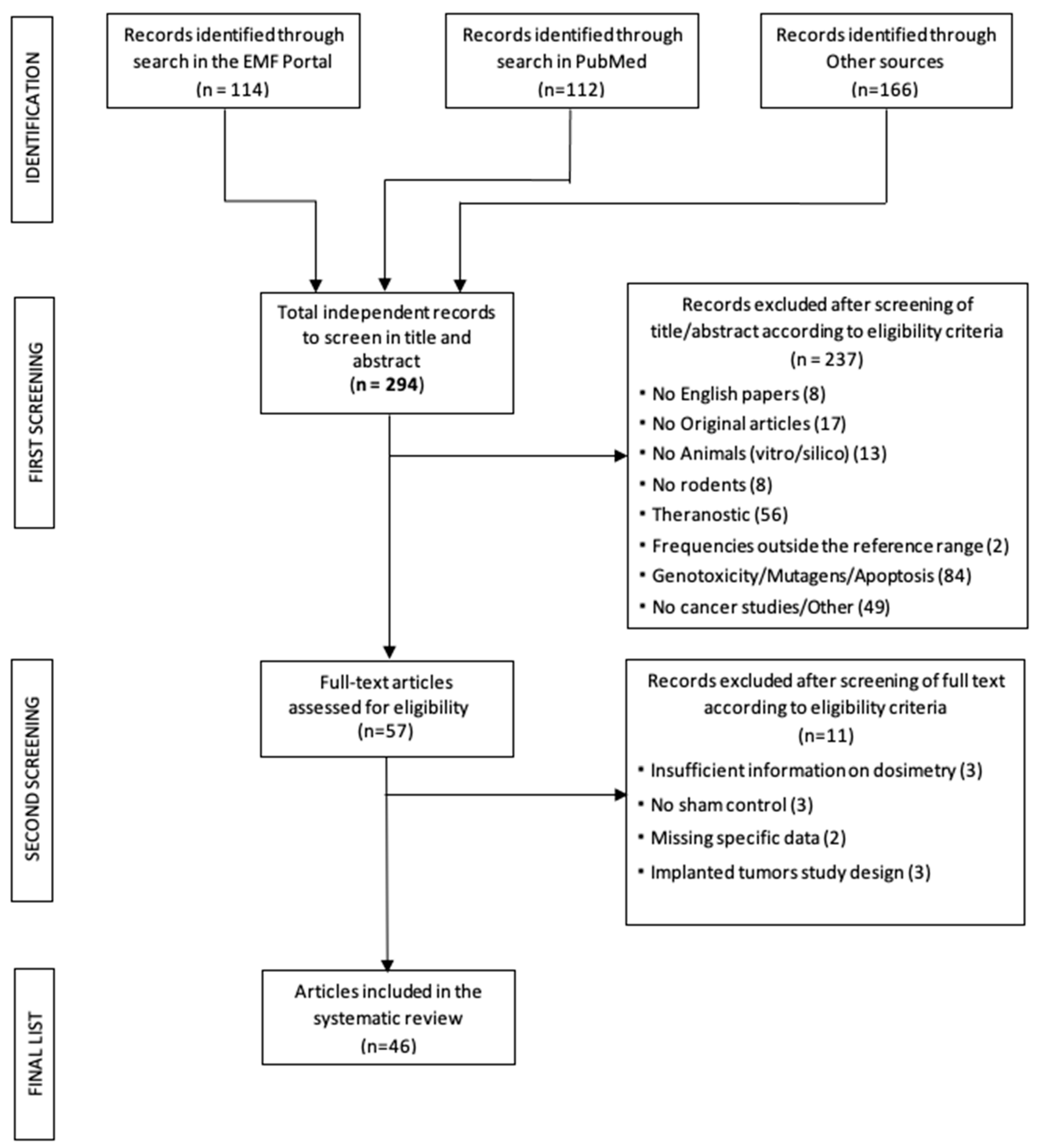
3. Results
3.1. General Description of the Selected Carcinogenicity studies
- Absence of specific data (n = 2; [42,43]). In particular, [42] examines the onset and growth of neoplasms exclusively by palpation and the data are only given in terms of cumulative tumor appearance without specifying the organ of onset and [43] provides data on various histological parameters not strictly related to the onset of neoplasms, where only the lack of onset of leucosis (a tumor process affecting the progenitor cells of leukocytes) is observed, and tumor incidence is not reported.
3.2. RoB of the Selected Papers
3.3. Incidence Analyses
- de Seze et al. [64]: 3.7 GHz pulsed signal administered for two 8-min intervals per day, 5 times per week for a total of 8 weeks;
- Jauchem et al. [67]: UWB signal administered for 12 min/week for a total of 12 weeks;
- Saran et al. [77]: 900 MHz GSM modulation signal, administered for two 30 min/day for 5 days.
- Furthermore, the article by Jin et al. [68] was also excluded from the meta-analysis as it only reports inflammatory phenomena and does not detect the onset of tumors.
- A qualitative descriptive analysis of these papers is separately reported.
3.3.1. Heart and CNS/Brain Analysis
3.3.2. Subgroup Analysis
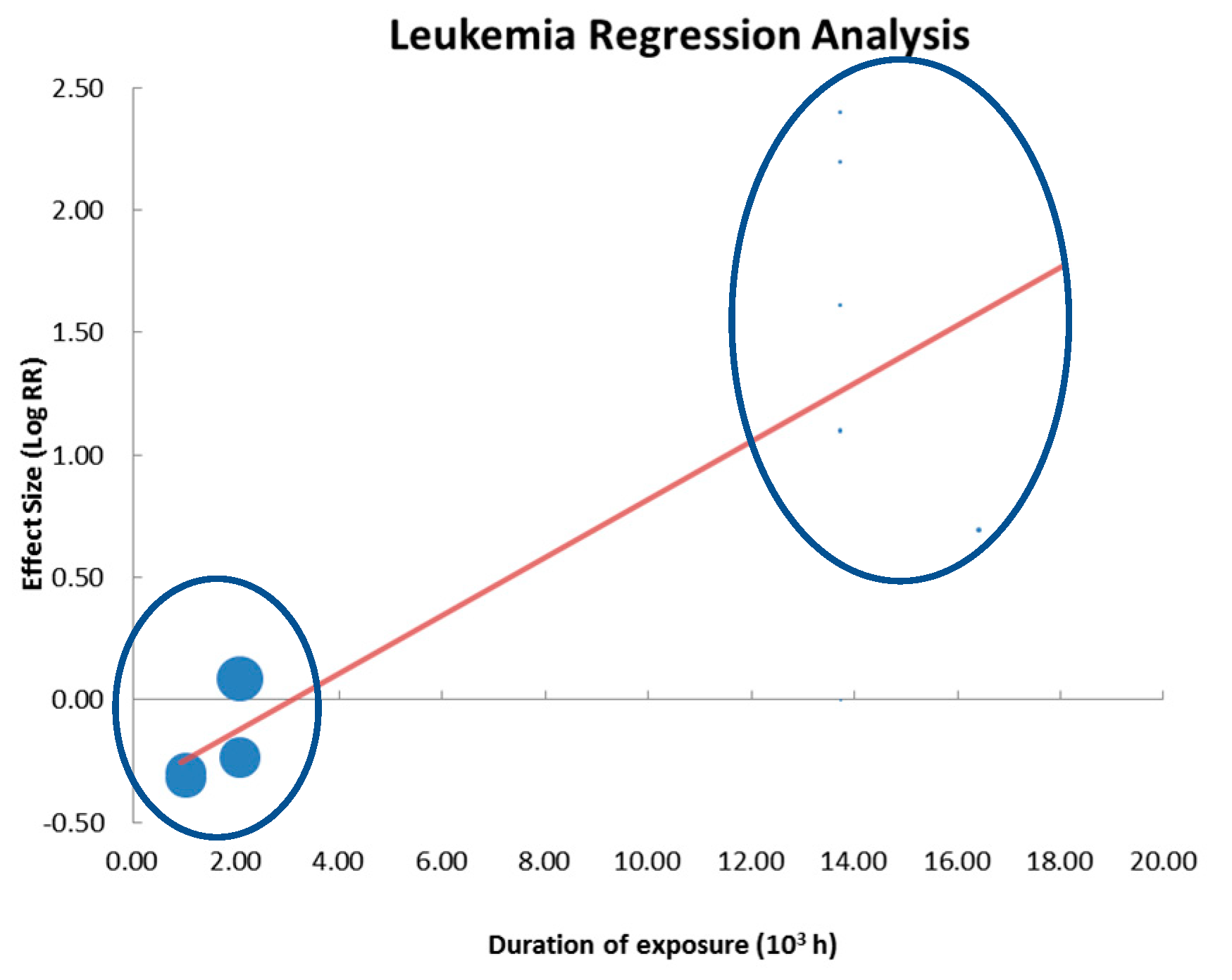
3.3.3. Regression Analysis
3.4. Survival Analysis
3.5. Qualitative Summary of the Excluded Works from the Meta-Analysis
3.6. Quality Assessment (Confidence Ratings and Evidence of Health Effects)
4. Discussion
- The studies of [31,32] are not peer-reviewed and [80], by admission of the authors themselves, published only the data relating to heart to support the results of [31], stating to publish the complete data (on the other organs) at a later time. For these reasons the heart sample results affected by publication bias;
- In this sample, a dose-effect response is not demonstrated, despite a very wide range of variability in SAR levels (from 0.001 W/kg to 6 W/kg), (see Table 8);
- The Authors of [31], regarding the increase of malignant tumor onset in the heart of male rats, declares: “In many cases isolated non-neoplastic or neoplastic lesion increases occurred in single or lower exposure groups, lacked a clear exposure response, or incidences were similar to incidences seen in control groups in past NTP studies. This reduced the confidence that these lesion increases were attributable to the cell phone RFR exposure.”
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID Paper [ref] | ID Treatment Group | Author Year (Treat-Group Number) | RoB (Quality Category) | Species | Strain | Prone/ WT | Sex | Exposure Starts in Utero | Number of Animals per Group | Frequency | Modulation/ Platform | SAR (W/Kg) | wb SAR/ Local SAR | Duration (w) | Timing (h/d, d/w) | Organ | Type of Tumor (Mal/Ben) | Outcome Measure | Note |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [62] | 1 | Adey 1999 | 2 | Rats | F344 | WT | M+F | Y | 60 | 836 MHz | TDMA | 1–1.60 | local | LTE (94) | 2 h/d 4d/w | CNS | Malignant tumors | Incidence/ survival | SAR values related to growth |
| 2 [61] | 2 | Adey 2000 | 2 | Rats | F344 | WT | M+F | Y | 90 | 836 MHz | TDMA | 0.74–1.60 | local | LTE (96) | 3 h/d 4d/w | CNS | Malignant tumors | Incidence/ survival | SAR values related to growth |
| 3 [75] | 3 | Anderson 2004 (1) | 2 | Rats | F344 | WT | M+F | Y | 180 | 1600 MHz | Iridium (QPSK) | 0.16 | local | LTE (104) | 4 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | There is a cage control group, sham is shared |
| 4 | Anderson 2004 (2) | 2 | Rats | F344 | WT | M+F | Y | 180 | 1600 MHz | Iridium (QPSK) | 1.6 | local | LTE (104) | 5 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 4 [82] | 5 | Bartsch 2010 (1) | 2 | Rats | SD | WT | F | N | 12 | 900 MHz | GSM | 0.038–0.08 | wb | LTE (101) | 24 h/d 7d/w | Pituitary | Malignant tumors | Incidence/ survival | SAR values related to growth |
| 6 | Bartsch 2010 (2) | 2 | Rats | SD | WT | F | N | 12 | 900 MHz | GSM | 0.038–0.08 | wb | LTE (75) | 24 h/d 7d/w | Pituitary | Malignant tumors | Incidence/ survival | SAR values related to growth | |
| 7 | Bartsch 2010 (3) | 2 | Rats | SD | WT | F | N | 30 | 900 MHz | GSM | 0.038–0.08 | wb | LTE (150) | 24 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | SAR values related to growth | |
| 8 | Bartsch 2010 (4) | 2 | Rats | SD | WT | F | N | 30 | 900 MHz | GSM | 0.038–0.08 | wb | LTE (151) | 24 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | SAR values related to growth | |
| 5 [63] | 9 | Chou 1992 | 1 | Rats | SD | WT | M | N | 100 | 2450 MHz | pulse: 10 μs; 800 pps | 0.15–0.4 | wb | LTE (109) | 21,5 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | SAR values related to growth |
| 6 [64] | 10 | De Seze 2020 | 3 | Rats | SD | WT | M | N | 24 | 3700 MHz | pulse: 2,5 ns; 100 pps | 0.83 | wb | STE (8) | 2 x 8 min 5d/w | Multiple | Malignant and benign tumors | Incidence/ survival | |
| 7 [80] | 11 | Falcioni 2018 (1) | 1 | Rats | SD | WT | M+F | N | 811/817 | 1800 MHz | DCS | 0.001 | wb | LTE (152) | 19 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | reported heart and brain only, sham is shared |
| 12 | Falcioni 2018 (2) | 1 | Rats | SD | WT | M+F | N | 411/817 | 1800 MHz | DCS | 0.03 | wb | LTE (152) | 20 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 13 | Falcioni 2018 (3) | 1 | Rats | SD | WT | M+F | N | 409/817 | 1800 MHz | DCS | 0.1 | wb | LTE (152) | 21 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 8 [65] | 14 | Frei et al 1998 a | 1 | Mice | C3H/HeJ | Prone | F | N | 100 | 2450 MHz | CW | 0.3 | wb | LTE (78) | 20 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | |
| 9 [66] | 15 | Frei et al 1998 b | 1 | Mice | C3H/HeJ | Prone | F | N | 100 | 2450 MHz | CW | 1 | wb | LTE (78) | 20 h/d 7d/w | Multiple | Malignant and benign tumors | Incidence/ survival | |
| 10 [67] | 16 | Jauchem 2001 | 1 | Mice | C3H/HeJ | Prone | F | N | 100 | UWB | pulse width 2.5 ns; PRF: 1KHz | 0.01 | wb | MTE (12) | 2 m 1d/w | Multiple | Malignant and benign tumors | Incidence/ survival | duration: 12 m/w × 12 w (short duration and single pulse signal) |
| 11 [68] | 17 | Jin 2010 | 1 | Rats | SD | WT | M+F | N | 40 | 849 MHz 1950 MHz | CDMA+WCDMA | 4 | wb | LTE (52) | 45 m 5d/w | Multiple | Benign tumors | Incidence/ survival | No tumors found, except 2 benign and some non-neoplastic lesions |
| 12 [76] | 18 | La Regina 2003 (1) | 2 | Rats | F344 | WT | M+F | N | 160 | 835.62 MHz | FDMA | 1.3 ± 0.5 (in brain) | local | LTE (104) | 4 h/d 5d/w | Multiple | Malignant and benign tumors | Incidence/ survival | sham is shared |
| 19 | La Regina 2003 (2) | 2 | Rats | F344 | WT | M+F | N | 160 | 847.74 MHz | CDMA | 1.3 ± 0.5 (in brain) | local | LTE (104) | 4 h/d 5d/w | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 13 [69] | 20 | Lee 2011 | 1 | Mice | AKR/J mice | Prone | M+F | N | 80 | 849 MHz 1950 MHz | CDMA+WCDMA | 6 | wb | MTE (42) | 45 min 5d/w | Lymphoma | Malignant tumors | Incidence/ survival | |
| 14 [31] | 21 | NTP 2018 (1) | 2 | Rats | SD | WT | M+F | Y | 180 | 900 MHz | GSM | 1.5 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | only 1 sham vs 6 exposed groups; data about "historical" control groups reported in Annexes |
| 22 | NTP 2018 (2) | 2 | Rats | SD | WT | M+F | Y | 180 | 900 MHz | GSM | 3 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 23 | NTP 2018 (3) | 2 | Rats | SD | WT | M+F | Y | 180 | 900 MHz | GSM | 6 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 24 | NTP 2018 (4) | 2 | Rats | SD | WT | M+F | Y | 180 | 900 MHz | CDMA | 1.5 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 25 | NTP 2018 (5) | 2 | Rats | SD | WT | M+F | Y | 180 | 900 MHz | CDMA | 3 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 26 | NTP 2018 (6) | 2 | Rats | SD | WT | M+F | Y | 180 | 900 MHz | CDMA | 6 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 15 [32] | 27 | NTP 2018^ (1) (*) | 2 | Mice | B6C3F1/N | WT | M+F | N | 180 | 1900 MHz | DCS | 2.5 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | only 1 sham vs 6 exposed groups; data about "historical" control groups reported in Annexes |
| 28 | NTP 2018^ (2) | 2 | Mice | B6C3F1/N | WT | M+F | N | 180 | 1900 MHz | DCS | 5 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 29 | NTP 2018^ (3) | 2 | Mice | B6C3F1/N | WT | M+F | N | 180 | 1900 MHz | DCS | 10 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 30 | NTP 2018^ (4) | 2 | Mice | B6C3F1/N | WT | M+F | N | 180 | 1900 MHz | CDMA | 2.5 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 31 | NTP 2018^ (5) | 2 | Mice | B6C3F1/N | WT | M+F | N | 180 | 1900 MHz | CDMA | 5 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 32 | NTP 2018^ (6) | 2 | Mice | B6C3F1/N | WT | M+F | N | 180 | 1900 MHz | CDMA | 10 | wb | LTE (107) | 18h 20’/7 (10’ ON / 10’ OFF) | Multiple | Malignant and benign tumors | Incidence/ survival | ||
| 16 [81] | 33 | Oberto 2007 (1) | 1 | Mice | Eu- Pim1 | Prone | M+F | N | 100 | 900 MHz | GSM | 0.5 | wb | LTE (78) | 1 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | The study is a replica of Repacholi1997, but the exposure system is different shared sham |
| 34 | Oberto 2007 (2) | 1 | Mice | Eu- Pim1 | Prone | M+F | N | 100 | 900 MHz | GSM | 1.4 | wb | LTE (78) | 1 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 35 | Oberto 2007 (3) | 1 | Mice | Eu- Pim1 | Prone | M+F | N | 100 | 900 MHz | GSM | 4 | wb | LTE (78) | 1 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 17 [70] | 36 | Rapacholi 1997 | 2 | Mice | Eu- Pim1 | Prone | F | N | 100 | 900 MHz | GSM | 0.13–1.4 | wb | LTE (78) | 2 x 30 min 7d/w | Lymphoma | Malignant tumors | Incidence | |
| 18 [77] | 37 | Saran 2007 (1) | 1 | Mice | Patch ++ | WT | M+F | N | 50 | 900 MHz | GSM | 0.4 | wb | STE (>1=5d) | 30 min x2/d 5d/w | CNS | Malignant tumors | Incidence/ survival | |
| 38 | Saran 2007 (2) | 1 | Mice | Patch +- | Prone | M+F | N | 50 | 900 MHz | GSM | 0.4 | wb | STE (>1=5d) | 30 min x2/d 5d/w | CNS | Malignant tumors | Incidence/ survival | ||
| 19 [83] | 39 | Smith 2007 (1) | 1 | Rats | Wistar | WT | M+F | N | 100 | 902 MHz | GSM | 0.27 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | incidence | There are 2 sham groups for 6 exposed ones. |
| 40 | Smith 2007 (2) | 1 | Rats | Wistar | WT | M+F | N | 100 | 902 MHz | GSM | 0.8 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | incidence | ||
| 41 | Smith 2007 (3) | 1 | Rats | Wistar | WT | M+F | N | 100 | 902 MHz | GSM | 2.42 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | incidence | ||
| 42 | Smith 2007 (4) | 1 | Rats | Wistar | WT | M+F | N | 100 | 1747 MHz | DCS | 0.29 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | incidence | ||
| 43 | Smith 2007 (5) | 1 | Rats | Wistar | WT | M+F | N | 100 | 1747 MHz | DCS | 0.87 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | incidence | ||
| 44 | Smith 2007 (6) | 1 | Rats | Wistar | WT | M+F | N | 100 | 1747 MHz | DCS | 2.61 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | incidence | ||
| 20 [72] | 45 | Sommer 2004 | 1 | Mice | AKR/J mice | with retrovirus AKV | F | N | 80 | 900 MHz | GSM | 0.4 | wb | MTE (42) | 24 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | |
| 21 [71] | 46 | Sommer 2007 | 1 | Mice | AKR/J mice | with retrovirus AKV | F | N | 80 | 1966 MHz | UMTS | 0.4 | wb | MTE (42) | 24 h/d 7d/w | Multiple | Malignant tumors | Incidence/ survival | |
| 22 [78] | 47 | Szmigielski 1982 (1) | 3 | Mice | C3H/HeJ | Prone | F | N | 40 | 2450 MHz | CW | 2–3 | wb | MTE (46) | 2 h/d 6d/w | Breast cancer | Undefined | Incidence | It doesn’t discriminate between malignant and benign because tumors are detected by palpation only |
| 48 | Szmigielski 1982 (2) | 3 | Mice | C3H/HeJ | Prone | F | N | 40 | 2450 MHz | CW | 6–8 | wb | MTE (46) | 2 h/d 6d/w | Breast cancer | Undefined | Incidence | ||
| 23 [84] | 49 | Tillmann 2007 (1) | 1 | Mice | B6C3F1 | WT | M+F | N | 100 | 902 MHz | GSM | 0.29 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | Incidence | there are 2 sham for 6 exposed groups. |
| 50 | Tillmann 2007 (2) | 1 | Mice | B6C3F1 | WT | M+F | N | 100 | 902 MHz | GSM | 0.86 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | Incidence | ||
| 51 | Tillmann 2007 (3) | 1 | Mice | B6C3F1 | WT | M+F | N | 100 | 902 MHz | GSM | 2.6 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | Incidence | ||
| 52 | Tillmann 2007 (4) | 1 | Mice | B6C3F1 | WT | M+F | N | 100 | 1747 MHz | DCS | 0.29 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | Incidence | ||
| 53 | Tillmann 2007 (5) | 1 | Mice | B6C3F1 | WT | M+F | N | 100 | 1747 MHz | DCS | 0.86 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | Incidence | ||
| 54 | Tillmann 2007 (6) | 1 | Mice | B6C3F1 | WT | M+F | N | 100 | 1747 MHz | DCS | 2.6 | wb | LTE (104) | 2 h/d 5d/w | Multiple | Malignant/ benign tumors | Incidence | ||
| 24 [73] | 55 | Tillmann 2010 (1) | 1 | Mice | B6C3F1 | WT | F | N | 60 | 1966 MHz | UMTS | 1.5-5 | wb | LTE (104) | 20 h/d 7d/w | Multiple | Malignant/ benign tumors | Incidence | SAR values related to growth |
| 25 [74] | 56 | Toler 1997 | 1 | Mice | C3H/HeJ | Prone | F | N | 200 | 435 MHz | pulse: 1 μs; PRF: 1KHz | 0.32 | wb | LTE (91) | 22 h/d 7d/w | Multiple | Malignant/ benign tumors | Incidence/ survival | |
| 26 [85] | 57 | Utteridge 2002 (1) | 1 | Mice | Eμ- Pim 1 ++ | WT | F | N | 120 | 898.4 MHz | GSM | 0.25 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | the study is a replica of Repacholi1997, but the exposure system is different Lymphoma and CNS only there are 2 sham for 8 exposed groups |
| 58 | Utteridge 2002 (2) | 1 | Mice | Eμ- Pim 1 ++ | WT | F | N | 120 | 898.4 MHz | GSM | 1 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 59 | Utteridge 2002 (3) | 1 | Mice | Eμ- Pim 1 ++ | WT | F | N | 120 | 898.4 MHz | GSM | 2 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 60 | Utteridge 2002 (4) | 1 | Mice | Eμ- Pim 1 ++ | WT | F | N | 120 | 898.4 MHz | GSM | 4 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 61 | Utteridge 2002 (5) | 1 | Mice | Eμ- Pim 1 + - | Prone | F | N | 120 | 898.4 MHz | GSM | 0.25 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 62 | Utteridge 2002 (6) | 1 | Mice | Eμ- Pim 1 + - | Prone | F | N | 120 | 898.4 MHz | GSM | 1 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 63 | Utteridge 2002 (7) | 1 | Mice | Eμ- Pim 1 + - | Prone | F | N | 120 | 898.4 MHz | GSM | 2 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 64 | Utteridge 2002 (8) | 1 | Mice | Eμ- Pim 1 + - | Prone | F | N | 120 | 898.4 MHz | GSM | 4 | wb | LTE (104) | 1 h/d 5d/w | Multiple | Malignant tumors | Incidence/ survival | ||
| 27 [79] | 65 | Zook 2001 (1) | 2 | Rats | SD | WT | M+F | Y | 60 | 860 MHz | digital modulation | 1 | local | LTE (95) | 6 h/d 5d/w | CNS | Malignant tumors | Incidence/ survival | shared sham |
| 66 | Zook 2001 (2) | 2 | Rats | SD | WT | M+F | Y | 60 | 860 MHz | CW | 1 | local | LTE (95) | 6 h/d 5d/w | CNS | Malignant tumors | Incidence/ survival |
References
- ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- IARC. Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Durney, C.H.; Massoudi, H.; Iskander, M.F. Radiofrequency Radiation Dosimetry Handbook, 4th ed.; Ft. Belvoir Defense Technical Information Center: Fort Belvoir, VA, USA, 1986. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- NTP-OHAT (Ed.). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Toxicology Program—Office of Health Assessment and Translation: Bethesda, Maryland, US, 2019. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef]
- Pinto, R.; Ardoino, L.; Giardullo, P.; Villani, P.; Marino, C. Protocol for a systematic review of the in vivo studies on radiofrequency (100 kHz–300 GHz) electromagnetic field exposure and cancer. Syst. Rev. 2022, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kuster, N.; Schönborn, F. Recommended minimal requirements and development guidelines for exposure setups of bio-experiments addressing the health risk concern of wireless communications. Bioelectromagnetics 2000, 21, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Paffi, A.; Merla, C.; Pinto, R.; Lovisolo, G.A.; Liberti, M.; Marino, C.; Repacholi, M.; Apollonio, F. Microwave exposure systems for in vivo biological experiments: A systematic review. IEEE Trans. Microw. Theory Tech. 2013, 61, 1980–1993. [Google Scholar] [CrossRef]
- AGNIR. Report of the Independent Advisory Group on Non-ionising Radiation: Health Effects from Radiofrequency Electromagnetic Fields; Advisory Group on Non-Ionising Radiation: London, UK, 2012. [Google Scholar]
- ANSES. Radiofréquences et Santé. Mise à jour de l’Expertise; Agence Nationale de Sécurité sanitaire de l’Alimentation de l’Environnement et du Travail: Maisons-Alfort Cedex, France, 2013. [Google Scholar]
- ARPANSA. Radiofrequency Expert Panel. Review of Radiofrequency Health Effects Research—Scientific Literature 2000–2012; Australian Radiation Protection and Nuclear Safety Agency: Yallambie, Australia, 2014. [Google Scholar]
- HCN. Mobile Phones and Cancer Part 2. Animal Studies on Carcinogenesis; Health Council of The Netherlands: The Hague, The Netherlands, 2014. [Google Scholar]
- Demers, P.; Findlay, R.; Foster, K.R.; Kolb, B.; Moulder, J.; Nicol, A.-M.; Prato, F.; Stam, R. Expert Panel Report on A Review of Safety Code 6 (2013): Health Canada’s Safety Limits for Exposure to Radiofrequency Fields; The Royal Society of Canada: Ottawa, Canada, 2014; ISBN 978-1-928140-00-9. [Google Scholar]
- SCENIHR. Potential Health Effects of Exposure to Electromagnetic Fields (EMF); Scientific Committee on Emerging and Newly Identified Health Risks, European Commission: Luxembourg, 2015. [Google Scholar]
- SSM. Eleventh Report from SSM’s Scientific Council on Electromagnetic Fields; SSM’s Scientific Council on Electromagnetic Fields, Swedish Radiation Safety Authority: Stockholm, Sweden, 2016. [Google Scholar]
- HCN. Mobile Phones and Cancer Part 3. Update and Overall Conclusions from Epidemiological and Animal Studies; Health Council of The Netherlands: The Hague, The Netherlands, 2016. [Google Scholar]
- CCARS. Informe Sobre Radiofrecuencia y Salud (2013–2016) Madrid: Colegio Oficial de Ingenieros de Telecomunicación (COIT); Comité Científico Asesor en Radiofrecuencias y Salud: Madrid, Spain, 2017. [Google Scholar]
- ICHENF. Interagency Committee on the Health Effects of Non-Ionising Fields: Report to Ministers 2018; Ministry of Health: Wellington, New Zealand, 2018. [Google Scholar]
- SSM. Recent Research on EMF and Health Risk: Thirteenth report from SSM’s Scientific Council on Electromagnetic Fields; Swedish Radiation Safety Authority: Stockholm, Sweden, 2019. [Google Scholar]
- FDA. Review of Published Literature between 2008 and 2018 of Relevance to Radiofrequency Radiation and Cancer; Food and Drug Administration, Center for Devices & Radiological Health: Silver Spring, MA, USA, 2020.
- Hooijmans, C.; Rovers, M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M. SYRCLE’s risk of bias tool for animal studies. Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta Analysis; John Wiley & Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- Bonett, D.; Price, R. Meta-analysis methods for risk differences. Br. J. Math. Stat. Psychol. 2013, 67, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.; Inthout, J.; Ritskes-Hoitinga, M.; Rovers, M. Meta-Analyses of Animal Studies: An Introduction of a Valuable Instrument to Further Improve Healthcare. ILAR J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 2014, 55, 418–426. [Google Scholar] [CrossRef]
- Vesterinen, H.; Sena, E.; Egan, K.; Hirst, T.C.; Churolov, L.; Currie, G.; Antonic, A.; Howells, D.; Macleod, M. Meta-analysis of data from animal studies: A practical guide. J. Neurosci. Methods 2013, 221, 92–102. [Google Scholar] [CrossRef]
- Warn, D.; Thompson, S.; Spiegelhalter, D. Bayesian Random Effects Meta-Analysis of Trials with Binary Outcomes: Methods for the absolute risk difference and relative risk scales. Stat. Med. 2002, 21, 1601–1623. [Google Scholar] [CrossRef]
- van Rhee, H.; Suurmond, R.; Hak, T. User Manual for Meta-Essentials: Workbooks for Meta-Analysis. Erasmus Res. Inst. Manag. 2015. [Google Scholar] [CrossRef]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef]
- NTP. Technical Report on the Toxicology and Carcinogenesis Studies in Hsd:Sprague Dawley SD Rats Exposed to Whole-Body Radio Frequency Radiation at a Frequency (900 MHz) and Modulations (GSM and CDMA) Used by Cell Phones; National Toxicology Program: Bethesda, MA, USA, 2018. [Google Scholar]
- NTP. Technical Report on the Toxicology and Carcinogenesis Studies in P6C3F1/N Mice Exposed to Full Body Radiofrequency Radiation at a Frequency (1900 MHz); National Toxicology Program: Bethesda, MA, USA, 2018. [Google Scholar]
- Ivanov, V.B.; Subbotina, T.I.; Khadartsev, A.A.; Yashin, M.A.; Yashin, A.A. Exposure to low-intensive superhigh frequency electromagnetic field as a factor of carcinogenesis in experimental animals. Bull. Exp. Biol. Med. 2005, 139, 241–244. [Google Scholar] [CrossRef]
- Salford, L.; Brun, A.; Persson, B.R.R. Brain tumour development in rats exposed to electromagnetic fields used in wireless cellular communication. Wirel. Netw. 1997, 3, 463–469. [Google Scholar] [CrossRef]
- Salford, L.G.; Brun, A.; Persson, B.R.; Eberhardt, J. Experimental studies of brain tumour development during exposure to continuous and pulsed 915 MHz radiofrequency radiation. Bioelectrochem. Bioenerg. 1993, 30, 313–318. [Google Scholar] [CrossRef]
- Bantysh, B.B.; Krylov, A.Y.; Subbotina, T.I.; Khadartsev, A.A.; Ivanov, D.V.; Yashin, A.A. Peculiar Effects of Electromagnetic Millimeter Waves on Tumor Development in BALB/c Mice. Bull. Exp. Biol. Med. 2018, 165, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, R.; Behari, J. Effects of low level microwave radiation on carcinogenesis in Swiss Albino mice. Mol. Cell. Biochem. 2011, 348, 191–197. [Google Scholar] [CrossRef]
- Zook, B.C.; Simmens, S.J. Neurogenic tumors in rats induced by ethylnitrosourea. Exp. Toxicol. Pathol. 2005, 57, 7–14. [Google Scholar] [CrossRef]
- Higashikubo, R.; Culbreth, V.O.; Spitz, D.R.; LaRegina, M.C.; Pickard, W.F.; Straube, W.L.; Moros, E.G.; Roti, J.L. Radiofrequency electromagnetic fields have no effect on the in vivo proliferation of the 9L brain tumor. Radiat. Res. 1999, 152, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H.; Edwards, W.D.; Justesen, D.R. Retarded tumor growth and greater longevity in mice after fetal irradiation by 2450-MHz microwaves. J. Surg. Oncol. 1978, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Santini, R.; Hosni, M.; Deschaux, P.; Pacheco, H. B16 melanoma development in black mice exposed to low-level microwave radiation. Bioelectromagnetics 1988, 9, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Chagnaud, J.L.; Moreau, J.M.; Veyret, B. No effect of short-term exposure to GSM-modulated low-power microwaves on benzo(a)pyrene-induced tumours in rat. Int. J. Radiat. Biol. 1999, 75, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, S.S.C. Effects of chronic microwave irradiation on mice. IRE Trans. Bio-Med. Electron. 1962, 9, 104–108. [Google Scholar] [CrossRef]
- Anane, R.; Dulou, P.E.; Taxile, M.; Geffard, M.; Crespeau, F.L.; Veyret, B. Effects of GSM-900 microwaves on DMBA-induced mammary gland tumors in female Sprague-Dawley rats. Radiat. Res. 2003, 160, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Bartsch, C.; Seebald, E.; Deerberg, F.; Dietz, K.; Vollrath, L.; Mecke, D. Chronic exposure to a GSM-like signal (mobile phone) does not stimulate the development of DMBA-induced mammary tumors in rats: Results of three consecutive studies. Radiat. Res. 2002, 157, 183–190. [Google Scholar] [CrossRef]
- Heikkinen, P.; Ernst, H.; Huuskonen, H.; Komulainen, H.; Kumlin, T.; Mäki-Paakkanen, J.; Puranen, L.; Juutilainen, J. No effects of radiofrequency radiation on 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone-induced tumorigenesis in female Wistar rats. Radiat. Res. 2006, 166, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, P.; Kosma, V.M.; Alhonen, L.; Huuskonen, H.; Komulainen, H.; Kumlin, T.; Laitinen, J.T.; Lang, S.; Puranen, L.; Juutilainen, J. Effects of mobile phone radiation on UV-induced skin tumourigenesis in ornithine decarboxylase transgenic and non-transgenic mice. Int. J. Radiat. Biol. 2003, 79, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, P.; Kosma, V.M.; Hongisto, T.; Huuskonen, H.; Hyysalo, P.; Komulainen, H.; Kumlin, T.; Lahtinen, T.; Lang, S.; Puranen, L.; et al. Effects of mobile phone radiation on X-ray-induced tumorigenesis in mice. Radiat. Res. 2001, 156, 775–785. [Google Scholar] [CrossRef]
- Hruby, R.; Neubauer, G.; Kuster, N.; Frauscher, M. Study on potential effects of “902-MHz GSM-type Wireless Communication Signals” on DMBA-induced mammary tumours in Sprague-Dawley rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 649, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Imaida, K.; Taki, M.; Watanabe, S.; Kamimura, Y.; Ito, T.; Yamaguchi, T.; Ito, N.; Shirai, T. The 1.5 GHz electromagnetic near-field used for cellular phones does not promote rat liver carcinogenesis in a medium-term liver bioassay. Jpn. J. Cancer Res. 1998, 89, 995–1002. [Google Scholar] [CrossRef]
- Imaida, K.; Taki, M.; Yamaguchi, T.; Ito, T.; Watanabe, S.; Wake, K.; Aimoto, A.; Kamimura, Y.; Ito, N.; Shirai, T. Lack of promoting effects of the electromagnetic near-field used for cellular phones (929.2 MHz) on rat liver carcinogenesis in a medium-term liver bioassay. Carcinogenesis 1998, 19, 311–314. [Google Scholar] [CrossRef]
- Lerchl, A.; Klose, M.; Grote, K.; Wilhelm, A.F.; Spathmann, O.; Fiedler, T.; Streckert, J.; Hansen, V.; Clemens, M. Tumor promotion by exposure to radiofrequency electromagnetic fields below exposure limits for humans. Biochem. Biophys. Res. Commun. 2015, 459, 585–590. [Google Scholar] [CrossRef]
- Mason, P.A.; Walters, T.J.; DiGiovanni, J.; Beason, C.W.; Jauchem, J.R.; Dick Jr, E.J.; Mahajan, K.; Dusch, S.J.; Shields, B.A.; Merritt, J.H.; et al. Lack of effect of 94 GHz radio frequency radiation exposure in an animal model of skin carcinogenesis. Carcinogenesis 2001, 22, 1701–1708. [Google Scholar] [CrossRef]
- Shirai, T.; Ichihara, T.; Wake, K.; Watanabe, S.; Yamanaka, Y.; Kawabe, M.; Taki, M.; Fujiwara, O.; Wang, J.; Takahashi, S.; et al. Lack of promoting effects of chronic exposure to 1.95-GHz W-CDMA signals for IMT-2000 cellular system on development of N-ethylnitrosourea-induced central nervous system tumors in F344 rats. Bioelectromagnetics 2007, 28, 562–572. [Google Scholar] [CrossRef]
- Shirai, T.; Kawabe, M.; Ichihara, T.; Fujiwara, O.; Taki, M.; Watanabe, S.; Wake, K.; Yamanaka, Y.; Imaida, K.; Asamoto, M.; et al. Chronic exposure to a 1.439 GHz electromagnetic field used for cellular phones does not promote N-ethylnitrosourea induced central nervous system tumors in F344 rats. Bioelectromagnetics 2005, 26, 59–68. [Google Scholar] [CrossRef]
- Szudziński, A.; Pietraszek, A.; Janiak, M.; Wrembel, J.; Kałczak, M.; Szmigielski, S. Acceleration of the development of benzopyrene-induced skin cancer in mice by microwave radiation. Arch. Derm. Res. 1982, 274, 303–312. [Google Scholar] [CrossRef]
- Wu, R.Y.; Chiang, H.; Shao, B.J.; Li, N.G.; Fu, Y.D. Effects of 2.45-GHz microwave radiation and phorbol ester 12-O-tetradecanoylphorbol-13-acetate on dimethylhydrazine-induced colon cancer in mice. Bioelectromagnetics 1994, 15, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shen, Y.; Kuster, N.; Fu, Y.; Chiang, H. Effects of 900 MHz GSM wireless communication signals on DMBA-induced mammary tumors in rats. Radiat. Res. 2006, 165, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zook, B.C.; Simmens, S.J. Effects of a cell phone radiofrequency (860 MHz) on the latency of brain tumors in rats. Int. Congr. Ser. 2002, 1236, 137–139. [Google Scholar] [CrossRef]
- Zook, B.C.; Simmens, S.J. The effects of pulsed 860 MHz radiofrequency radiation on the promotion of neurogenic tumors in rats. Radiat. Res. 2006, 165, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Adey, W.R.; Byus, C.V.; Cain, C.D.; Higgins, R.J.; Jones, R.A.; Kean, C.J.; Kuster, N.; MacMurray, A.; Stagg, R.B.; Zimmerman, G. Spontaneous and nitrosourea-induced primary tumors of the central nervous system in Fischer 344 rats exposed to frequency-modulated microwave fields. Cancer Res. 2000, 60, 1857–1863. [Google Scholar]
- Adey, W.R.; Byus, C.V.; Cain, C.D.; Higgins, R.J.; Jones, R.A.; Kean, C.J.; Kuster, N.; MacMurray, A.; Stagg, R.B.; Zimmerman, G.; et al. Spontaneous and nitrosourea-induced primary tumors of the central nervous system in Fischer 344 rats chronically exposed to 836 MHz modulated microwaves. Radiat. Res. 1999, 152, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.K.; Guy, A.W.; Kunz, L.L.; Johnson, R.B.; Crowley, J.J.; Krupp, J.H. Long-term, low-level microwave irradiation of rats. Bioelectromagnetics 1992, 13, 469–496. [Google Scholar] [CrossRef]
- de Seze, R.; Poutriquet, C.; Gamez, C.; Maillot-Maréchal, E.; Robidel, F.; Lecomte, A.; Fonta, C. Repeated exposure to nanosecond high power pulsed microwaves increases cancer incidence in rat. PLoS ONE 2020, 15, e0226858. [Google Scholar] [CrossRef] [PubMed]
- Frei, M.R.; Berger, R.E.; Dusch, S.J.; Guel, V.; Jauchem, J.R.; Merritt, J.H.; Stedham, M.A. Chronic exposure of cancer-prone mice to low-level 2450 MHz radiofrequency radiation. Bioelectromagnetics 1998, 19, 20–31. [Google Scholar] [CrossRef]
- Frei, M.R.; Jauchem, J.R.; Dusch, S.J.; Merritt, J.H.; Berger, R.E.; Stedham, M.A. Chronic, low-level (1.0 W/kg) exposure of mice prone to mammary cancer to 2450 MHz microwaves. Radiat. Res. 1998, 150, 568–576. [Google Scholar] [CrossRef]
- Jauchem, J.R.; Ryan, K.L.; Frei, M.R.; Dusch, S.J.; Lehnert, H.M.; Kovatch, R.M. Repeated exposure of C3H/HeJ mice to ultra-wideband electromagnetic pulses: Lack of effects on mammary tumors. Radiat. Res. 2001, 155, 369–377. [Google Scholar] [CrossRef]
- Jin, Y.B.; Lee, H.J.; Seon Lee, J.; Pack, J.K.; Kim, N.; Lee, Y.S. One-year, simultaneous combined exposure of CDMA and WCDMA radiofrequency electromagnetic fields to rats. Int. J. Radiat. Biol. 2011, 87, 416–423. [Google Scholar] [CrossRef]
- Lee, H.J.; Jin, Y.B.; Lee, J.S.; Choi, S.Y.; Kim, T.H.; Pack, J.K.; Choi, H.D.; Kim, N.; Lee, Y.S. Lymphoma development of simultaneously combined exposure to two radiofrequency signals in AKR/J mice. Bioelectromagnetics 2011, 32, 485–492. [Google Scholar] [CrossRef]
- Repacholi, M.H.; Basten, A.; Gebski, V.; Noonan, D.; Finnie, J.; Harris, A.W. Lymphomas in E mu-Pim1 transgenic mice exposed to pulsed 900 MHZ electromagnetic fields. Radiat. Res. 1997, 147, 631–640. [Google Scholar] [CrossRef]
- Sommer, A.M.; Bitz, A.K.; Streckert, J.; Hansen, V.W.; Lerchl, A. Lymphoma development in mice chronically exposed to UMTS-modulated radiofrequency electromagnetic fields. Radiat. Res. 2007, 168, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.M.; Streckert, J.; Bitz, A.K.; Hansen, V.W.; Lerchl, A. No effects of GSM-modulated 900 MHz electromagnetic fields on survival rate and spontaneous development of lymphoma in female AKR/J mice. BMC Cancer 2004, 4, 77. [Google Scholar] [CrossRef]
- Tillmann, T.; Ernst, H.; Streckert, J.; Zhou, Y.; Taugner, F.; Hansen, V.; Dasenbrock, C. Indication of cocarcinogenic potential of chronic UMTS-modulated radiofrequency exposure in an ethylnitrosourea mouse model. Int. J. Radiat. Biol. 2010, 86, 529–541. [Google Scholar] [CrossRef]
- Toler, J.C.; Shelton, W.W.; Frei, M.R.; Merritt, J.H.; Stedham, M.A. Long-term, low-level exposure of mice prone to mammary tumors to 435 MHz radiofrequency radiation. Radiat. Res. 1997, 148, 227–234. [Google Scholar] [CrossRef]
- Anderson, L.E.; Sheen, D.M.; Wilson, B.W.; Grumbein, S.L.; Creim, J.A.; Sasser, L.B. Two-year chronic bioassay study of rats exposed to a 1.6 GHz radiofrequency signal. Radiat. Res. 2004, 162, 201–210. [Google Scholar] [CrossRef] [PubMed]
- La Regina, M.; Moros, E.G.; Pickard, W.F.; Straube, W.L.; Baty, J.; Roti Roti, J.L. The effect of chronic exposure to 835.62 MHz FDMA or 847.74 MHz CDMA radiofrequency radiation on the incidence of spontaneous tumors in rats. Radiat. Res. 2003, 160, 143–151. [Google Scholar] [CrossRef]
- Saran, A.; Pazzaglia, S.; Mancuso, M.; Rebessi, S.; Di Majo, V.; Tanori, M.; Lovisolo, G.A.; Pinto, R.; Marino, C. Effects of exposure of newborn patched1 heterozygous mice to GSM, 900 MHz. Radiat. Res. 2007, 168, 733–740. [Google Scholar] [CrossRef]
- Szmigielski, S.; Szudzinski, A.; Pietraszek, A.; Bielec, M.; Janiak, M.; Wrembel, J.K. Accelerated development of spontaneous and benzopyrene-induced skin cancer in mice exposed to 2450-MHz microwave radiation. Bioelectromagnetics 1982, 3, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Zook, B.C.; Simmens, S.J. The effects of 860 MHz radiofrequency radiation on the induction or promotion of brain tumors and other neoplasms in rats. Radiat. Res. 2001, 155, 572–583. [Google Scholar] [CrossRef]
- Falcioni, L.; Bua, L.; Tibaldi, E.; Lauriola, M.; De Angelis, L.; Gnudi, F.; Mandrioli, D.; Manservigi, M.; Manservisi, F.; Manzoli, I.; et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8GHz GSM base station environmental emission. Environ. Res 2018, 165, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Oberto, G.; Rolfo, K.; Yu, P.; Carbonatto, M.; Peano, S.; Kuster, N.; Ebert, S.; Tofani, S. Carcinogenicity Study of 217 Hz Pulsed 900 MHz Electromagnetic Fields in Pim1 Transgenic Mice. Radiat. Res. 2007, 168, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Küpper, H.; Scheurlen, U.; Deerberg, F.; Seebald, E.; Dietz, K.; Mecke, D.; Probst, H.; Stehle, T.; Bartsch, C. Effect of chronic exposure to a GSM-like signal (mobile phone) on survival of female Sprague-Dawley rats: Modulatory effects by month of birth and possibly stage of the solar cycle. Neuro Endocrinol. Lett. 2010, 31, 457–473. [Google Scholar]
- Smith, P.; Kuster, N.; Ebert, S.; Chevalier, H.J. GSM and DCS Wireless Communication Signals: Combined Chronic Toxicity/Carcinogenicity Study in the Wistar Rat. Radiat. Res. 2007, 168, 480–492. [Google Scholar] [CrossRef]
- Tillmann, T.; Ernst, H.; Ebert, S.; Kuster, N.; Behnke, W.; Rittinghausen, S.; Dasenbrock, C. Carcinogenicity study of GSM and DCS wireless communication signals in B6C3F1 mice. Bioelectromagnetics 2007, 28, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Utteridge, T.D.; Gebski, V.; Finnie, J.W.; Vernon-Roberts, B.; Kuchel, T.R. Long-term exposure of E-mu-Pim1 transgenic mice to 898.4 MHz microwaves does not increase lymphoma incidence. Radiat. Res. 2002, 158, 357–364. [Google Scholar] [CrossRef]
- Pazzaglia, S. Ptc1 heterozygous knockout mice as a model of multi-organ tumorigenesis. Cancer Lett. 2006, 234, 124–134. [Google Scholar] [CrossRef]






| Tumors Always Classified as Benign |
| Teratoma (ovarian), Stromal Polyp, Neurilemmoma, Cholangioma, Keratoacanthoma, Squamous Cell Papilloma, Pilomatrixoma, Cystadenoma, Leiomyoma, Hibernoma, Fibroma, Fibroadenoma, Lipoma. |
| Tumors (Malignant or Benign) Classified as Malignant by Precautionary Approach, Unless the Authors Have not Indicated Otherwise |
| Hemangioma, Granular Cell Tumor, Renal Mesenchymal Tumor, Hepatoblastoma, Nephroblastoma. |
| Tumors (Rare with Low Percentage of Cases of Malignancy) Classified as Benign, Unless the Authors Have not Indicated Otherwise |
| Pheochromocytoma, Interstitial Cell Tumor. |
| Paper | Item Score (−, −−, +, ++) | Quality Assessment (1–3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Adey 1999 [62] | + | + | + | + | − | ++ | ++ | ++ | − | 2 |
| Adey 2000 [61] | + | + | + | + | − | ++ | ++ | ++ | − | 2 |
| Anderson 2004 [75] | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | −− | 2 |
| Bartsch 2010 [45] | + | + | + | ++ | ++ | + | ++ | ++ | −− | 2 |
| Chou 1992 [63] | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| De Seze 2020 [64] | − | − | + | − | + | ++ | ++ | −− | − | 3 |
| Falcioni 2018 [80] | + | ++ | ++ | ++ | + | ++ | ++ | + | ++ | 1 |
| Frei 1998 a [65] | ++ | ++ | ++ | + | ++ | ++ | ++ | + | + | 1 |
| Frei 1998 b [66] | ++ | ++ | ++ | + | ++ | ++ | ++ | + | + | 1 |
| Jauchem 2001 [67] | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | 1 |
| Jin 2010 [68] | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| La Regina 2003 [76] | + | + | + | ++ | ++ | ++ | ++ | ++ | −− | 2 |
| Lee 2011 [69] | + | ++ | ++ | + | ++ | ++ | ++ | ++ | − | 1 |
| NTP Rats 2018 [31] | + | ++ | −− | ++ | ++ | ++ | ++ | ++ | ++ | 2 |
| NTP Topi 2018 [32] | + | ++ | −− | ++ | ++ | ++ | ++ | ++ | ++ | 2 |
| Oberto 2007 [81] | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| Repacholi 1997 [70] | + | − | −− | ++ | + | ++ | ++ | ++ | + | 2 |
| Saran 2007 [77] | ++ | ++ | + | ++ | ++ | ++ | + | − | ++ | 1 |
| Smith 2007 [83] | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | − | 1 |
| Sommer 2004 [72] | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| Sommer 2007 [71] | ++ | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| Szimigielski 1982 [78] | −− | −− | ++ | −− | + | ++ | ++ | − | ++ | 3 |
| Tillmann 2007 [84] | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | − | 1 |
| Tillmann 2010 [73] | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| Toler 1993 [74] | ++ | − | + | ++ | ++ | ++ | ++ | + | ++ | 1 |
| Utteridge 2002 [85] | ++ | ++ | − | ++ | ++ | ++ | + | ++ | ++ | 1 |
| Zook 2001 [79] | + | −− | + | ++ | ++ | ++ | ++ | ++ | −− | 2 |
| Malignant Tumors | Number of Included ’Treated-Sham Comparisons’ | Number of Papers | (*1) | Number of Exposed Animals/Number of sham Animals | Risk Ratio (RR) | Risk Difference (RD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ or Tumor | Summary Effect Size (RR) | Lower Limit RR | Upper Limit RR | Two tailed p Value RR | Summary Effect Size (RD) | Lower Limit RD | Upper Limit RD | Two tailed p Value RD | ||||
| Adrenal Glands | 24 | 8 | 4/6 | 3538/1166 | 1.016 | 0.684 | 1.510 | 0.93200 | −0.004 | −0.010 | 0.001 | 0.07940 |
| Bladder | 15 | 4 | 2/6 | 2495/770 | 0.904 | 0.540 | 1.512 | 0.67200 | −0.001 | −0.004 | 0.002 | 0.36310 |
| Histiocytic Sarcoma | 26 | 8 | 4/6 | 3724/1155 | 0.979 | 0.735 | 1.305 | 0.88000 | 0.003 | −0.001 | 0.010 | 0.17330 |
| Bone Marrow | 7 | 2 | 1/6 | 1175/279 | 0.558 | 0.304 | 1.024 | 0.01900 | −0.007 | −0.012 | −0.003 | 0.00004 |
| CNS (brain and spinal cord) | 26 | 9 | 4/6 | 4779/2007 | 1.405 | 1.070 | 1.840 | 0.00900 | 0.009 | 0.004 | 0.013 | 0.00020 |
| Brain | 26 | 9 | 4/6 | 4779/2007 | 1.392 | 1.072 | 1.807 | 0.00900 | 0.008 | 0.003 | 0.012 | 0.00020 |
| Sensorial System | 20 | 4 | 4/6 | 3034/712 | 1.028 | 0.681 | 1.552 | 0.89000 | 0.001 | −0.002 | 0.010 | 0.46240 |
| Male Uro-Genital System | 10 | 3 | 1/6 | 880/260 | 1.756 | 1.034 | 2.982 | 0.01600 | 0.006 | −0.020 | 0.010 | 0.08390 |
| Female Uro-Genital System | 32 | 9 | 5/6 | 2354/822 | 0.882 | 0.720 | 1.070 | 0.20000 | 0.001 | −0.010 | 0.010 | 0.84000 |
| Heart | 15 | 3 | 3/6 | 3790/1117 | 3.238 | 2.105 | 4.983 | 0.00000 | 0.008 | 0.003 | 0.010 | 0.00080 |
| Intestine | 14 | 3 | 2/6 | 2376/505 | 0.585 | 0.399 | 0.857 | 0.00200 | −0.008 | −0.012 | −0.004 | 0.00000 |
| Kidneys (Renal System) | 14 | 3 | 2/6 | 2460/519 | 0.949 | 0.572 | 1.573 | 0.82100 | −0.004 | −0.010 | 0.000 | 0.02050 |
| Leukemia | 17 | 5 | 2/6 | 2939/800 | 0.884 | 0.738 | 1.059 | 0.14700 | 0.006 | 0.002 | 0.010 | 0.00270 |
| Liver | 25 | 8 | 5/6 | 3497/1086 | 0.958 | 0.861 | 1.066 | 0.40300 | 0.002 | −0.002 | 0.010 | 0.39480 |
| Lung | 23 | 7 | 4/6 | 3394/1031 | 0.886 | 0.769 | 1.021 | 0.07700 | 0.000 | −0.003 | 0.004 | 0.88440 |
| Lymphoma | 41 | 15 | 7/6 | 5645/2184 | 1.003 | 0.969 | 1.038 | 0.86800 | −0.002 | −0.008 | 0.003 | 0.35600 |
| Mammary Tumors | 29 | 10 | 4/6 | 3362/1274 | 1.034 | 0.839 | 1.275 | 0.74200 | −0.001 | −0.010 | 0.002 | 0.92080 |
| Mesenteric Lymph Nodes | 16 | 4 | 4/6 | 2241/631 | 0.822 | 0.436 | 1.551 | 0.51000 | −0.002 | −0.010 | −0.006 | 0.31490 |
| Pancreas | 18 | 3 | 4/6 | 2713/555 | 1.167 | 0.941 | 1.448 | 0.13100 | −0.001 | −0.010 | −0.006 | 0.48810 |
| Pituitary Gland | 25 | 7 | 4/6 | 3193/887 | 0.981 | 0.774 | 1.243 | 0.86600 | 0.002 | −0.001 | 0.005 | 0.08840 |
| Skin | 15 | 5 | 2/6 | 2456/658 | 0.755 | 0.566 | 1.008 | 0.03700 | −0.009 | −0.020 | 0.002 | 0.07520 |
| Spleen | 14 | 4 | 2/6 | 2282/506 | 1.067 | 0.354 | 3.223 | 0.89900 | 0.001 | −0.012 | 0.013 | 0.91300 |
| Stomach | 7 | 2 | 1/6 | 1179/280 | 0.777 | 0.321 | 1.881 | 0.48500 | −0.003 | −0.008 | 0.001 | 0.07100 |
| Thymus | 14 | 3 | 3/6 | 1938/527 | 0.912 | 0.582 | 1.429 | 0.65800 | 0.000 | −0.003 | 0.004 | 0.97940 |
| Thyroid | 26 | 7 | 5/6 | 3790/1094 | 1.229 | 0.963 | 1.567 | 0.08100 | −0.001 | −0.005 | 0.002 | 0.45800 |
| Benign Tumors | Number of Included ’Treated-Sham Comparisons’ | Number of Papers | (*1) | Number of Exposed Animals/Number of Sham Animals | Risk Ratio (RR) | Risk Difference (RD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ or Tumor | Summary Effect Size (RR) | Lower Limit RR | Upper Limit RR | Two Tailed p Value RR | Summary Effect Size (RD) | Lower Limit RD | Upper Limit RD | Two Tailed p Value RD | ||||
| Adrenal Glands | 26 | 8 | 5/6 | 3656/1107 | 1.433 | 1.088 | 1.888 | 0.00700 | 0.010 | −0.004 | 0.025 | 0.14500 |
| Brain | 9 | 2 | 2/6 | 2711/997 | 2.163 | 1.371 | 3.411 | 0.00010 | 0.006 | 0.003 | 0.009 | 0.00001 |
| Sensorial System | 15 | 3 | 4/6 | 1976/479 | 1.007 | 0.754 | 1.345 | 0.95900 | 0.010 | −0.007 | 0.026 | 0.19600 |
| Male Uro-Genital System | 17 | 5 | 2/6 | 1523/451 | 0.951 | 0.915 | 0.987 | 0.00500 | −0.018 | −0.030 | −0.004 | 0.00770 |
| Female Uro-Genital System | 23 | 8 | 6/6 | 2219/822 | 1.021 | 0.888 | 1.174 | 0.76300 | 0.011 | −0.010 | 0.030 | 0.17790 |
| Intestine | 12 | 2 | 2/6 | 2055/345 | 1.137 | 0.488 | 2.651 | 0.73900 | 0.000 | −0.010 | 0.050 | 0.91840 |
| Kidneys (Renal System) | 14 | 4 | 2/6 | 2296/513 | 0.515 | 0.374 | 0.708 | 0.00001 | −0.010 | −0.015 | −0.005 | 0.00001 |
| Liver | 28 | 10 | 5/6 | 3915/1346 | 1.044 | 0.945 | 1.153 | 0.37500 | −0.004 | −0.010 | 0.010 | 0.38470 |
| Lung | 24 | 7 | 5/6 | 3314/911 | 0.994 | 0.870 | 1.135 | 0.92200 | −0.004 | −0.008 | 0.000 | 0.03000 |
| Mammary Tumors | 26 | 8 | 4/6 | 2975/929 | 0.993 | 0.930 | 1.060 | 0.82800 | 0.015 | −0.010 | 0.040 | 0.30250 |
| Pancreas | 23 | 7 | 4/6 | 3333/1007 | 1.082 | 0.906 | 1.293 | 0.35900 | 0.004 | −0.001 | 0.010 | 0.13170 |
| Pituitary Gland | 31 | 8 | 7/6 | 4105/1195 | 1.033 | 0.972 | 1.097 | 0.27800 | 0.005 | −0.004 | 0.013 | 0.26500 |
| Skin | 13 | 3 | 2/6 | 2260/460 | 1.140 | 0.797 | 1.630 | 0.42600 | 0.003 | −0.005 | 0.012 | 0.42600 |
| Stomach | 14 | 4 | 2/6 | 2330/548 | 0.719 | 0.525 | 0.985 | 0.02300 | −0.003 | −0.005 | −0.001 | 0.00030 |
| Thymus | 15 | 3 | 2/6 | 2104/527 | 0.830 | 0.645 | 1.067 | 0.11100 | −0.002 | −0.005 | 0.002 | 0.29070 |
| Thyroid | 24 | 7 | 4/6 | 3574/1080 | 1.139 | 0.979 | 1.327 | 0.07600 | 0.005 | 0.000 | 0.010 | 0.02680 |
| Brain Malignant Tumors | Risk Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organ or Tumor | Number of Included Studies/Number of papers | (*1) | Number of Exposed Animals/Number of Sham Animals | Summary Effect Size (RR) | Two Tailed p Value RR | Lower Limit RR | Upper Limit RR | Tau_square_value | I_square (%) |
| Glia tumors | 16/6 | 2/6 | 3601/1487 | 2.63 | 0.000004 | 1.69 | 4.11 | 0 | 0 |
| Meninges tumors | 15/6 | 2/6 | 3541/1487 | 1.60 | 0.018000 | 1.05 | 2.45 | 0 | 0 |
| Meninges benign tumors | 15/6 | 2/6 | 2711/997 | 2.16 | 0.000100 | 1.37 | 3.41 | 0 | 0 |
| Malignant Tumors | Mice vs Rats | Prone vs WT | ||||
|---|---|---|---|---|---|---|
| Organ or Tumor | Number of Studies with Mice/Number of Studies with Rats | Combined Summary Effect RR | p between | Number of Studies with Prone/Number of Studies with WT | Combined Summary Effect RR | p between |
| Adrenal Glands | 15/9 | 0.90 | 0.1130 | 3/21 | 1.02 | 0.9270 |
| Bladder | 6/9 | 1.03 | 0.4260 | WT only | - | - |
| Histiocytic Sarcoma | 17/9 | 1.48 | 0.0940 | 4/22 | 0.98 | 0.8370 |
| Bone Marrow | mice only | - | - | WT only | - | - |
| CNS (brain and spinal cord) | 9/17 | 1.37 | 0.2780 | 5/21 | 1.45 | 0.6720 |
| Brain | 9/17 | 1.35 | 0.3150 | 5/21 | 1.42 | 0.6420 |
| Sensorial System | 12/8 | 1.44 | 0.0810 | WT only | - | - |
| Male Uro-Genital System | rats only | - | - | WT only | - | - |
| Female Uro-Genital System | 18/14 | 0.85 | 0.0920 | WT only | - | - |
| Heart | 6/9 | 2.72 | 0.5850 | WT only | - | - |
| Intestine | 6/8 | 0.56 | 0.5940 | WT only | - | - |
| Kidneys (Renal System) | 6/8 | 0.66 | 0.0720 | WT only | - | - |
| Leukemia | 6/11 | 1.59 | 0.0810 | WT only | - | - |
| Liver | 18/7 | 1.20 | 0.3000 | 5/20 | 0.96 | 0.9280 |
| Lung | 15/8 | 0.89 | 0.9320 | 2/21 | 0.95 | 0.6960 |
| Lymphoma | 30/11 | 0.95 | 0.4010 | 13/28 | 1.03 | 0.7100 |
| Mammary Tumors | 11/18 | 1.01 | 0.0080 | 5/24 | 0.99 | 0.0270 |
| Mesenteric Lymph Nodes | 7/9 | 0.80 | 0.7900 | 1/15 | - | - |
| Pancreas | 6/12 | 1.16 | 0.8090 | WT only | - | - |
| Pituitary Gland | 9/16 | 1.43 | 0.1120 | WT only | - | - |
| Skin | 8/7 | 0.80 | 0.2310 | 2/13 | 0.69 | 0.7050 |
| Spleen | 8/6 | 0.96 | 0.0000 | 1/13 | - | - |
| Stomach | rats only | - | - | WT only | - | - |
| Thymus | rats only | - | - | WT only | - | - |
| Thyroid | 9/17 | 0.93 | 0.1750 | 3/23 | 1.30 | 0.9280 |
| Benign Tumors | Mice vs Rats | Prone vs WT | ||||
|---|---|---|---|---|---|---|
| Organ or Tumor | Number of Studies with Mice/Number of Studies with Rats | Combined Summary Effect RR | p between | Number of Studies with Mice/Number of Studies with Rats | Combined Summary Effect RR | p between |
| Adrenal Glands | 17/9 | 1.33 | 0.0550 | 5/21 | 1.07 | 0.0960 |
| Brain | Only Rats | - | - | Only WT | - | - |
| Sensorial System | Only mice | - | - | 3/12 | 2.43 | 0.0380 |
| Male Uro-Genital System | 6/11 | 0.65 | 0.0590 | Only WT | - | - |
| Female Uro-Genital System | 15/14 | 1.06 | 0.0800 | 3/26 | 1.02 | 0.9270 |
| Intestine | 6/6 | 1.12 | 0.0100 | Only WT | - | |
| Kidneys (Renal System) | 7/7 | 0.5 | 0.7370 | Only WT | - | - |
| Liver | 19/9 | 0.9 | 0.0650 | 6/22 | 1.02 | 0.9960 |
| Lung | 18/6 | 0.99 | 0.6470 | 5/19 | 0.95 | 0.3330 |
| Mammary Tumors | 7/17 | 1.26 | 0.3530 | 1/23 | - | - |
| Pancreas | 8/15 | 1.68 | 0.0750 | 2/21 | 1.54 | 0.4420 |
| Pituitary Gland | 16/15 | 1.12 | 0.0830 | 4/27 | 1.29 | 0.0730 |
| Skin | 6/7 | 0.66 | 0.0010 | Only WT | - | - |
| Stomach | Only Rats | - | - | Only WT | - | - |
| Thymus | 3/12 | 1.03 | 0.5390 | Only WT | - | - |
| Thyroid | 7/17 | 1.74 | 0.1330 | 1/23 | - | - |
| Malignant | SAR | Time of Exposure | ||||
|---|---|---|---|---|---|---|
| Organ or Tumor | β-Moderator | p Value | R2 % | β-Moderator | p Value | R2 % |
| Adrenal Glands | −0.45 | 0.0700 | 20.65 | 0.18 | 0.4600 | 3.36 |
| Bladder | −0.29 | 0.2880 | 8.62 | −0.24 | 0.6300 | 5.80 |
| Histiocytic Sarcoma | −0.09 | 0.7050 | 0.76 | −0.28 | 0.2300 | 7.60 |
| Bone Marrow | 0.05 | 0.9500 | 0.20 | - | - | - |
| CNS (brain and spinal cord) | −0.05 | 0.8220 | 0.22 | 0.53 | 0.0110 | 28.60 |
| Brain | −0.26 | 0.2250 | 6.97 | 0.55 | 0.0120 | 29.80 |
| Sensorial System | −0.09 | 0.7900 | 0.74 | 0.03 | 0.9200 | 0.10 |
| Male Uro-Genital System | 0.06 | 0.9200 | 0.35 | −0.13 | 0.8200 | 1.75 |
| Female Uro-Genital System | −0.16 | 0.4400 | 2.49 | −0.40 | 0.0600 | 14.90 |
| Heart | 0.29 | 0.4670 | 8.27 | −0.56 | 0.1530 | 31.90 |
| Intestine | −0.08 | 0.8630 | 0.57 | −0.03 | 0.9500 | 0.08 |
| Kidneys (Renal System) | −0.22 | 0.5710 | 5.00 | −0.36 | 0.3700 | 12.60 |
| Leukemia | 0.52 | 0.0350 | 26.79 | 0.84 | 0.0010 | 70.90 |
| Liver | −0.04 | 0.8720 | 0.18 | 0.13 | 0.6300 | 1.67 |
| Lung | 0.39 | 0.2490 | 15.03 | 0.54 | 0.1000 | 29.20 |
| Lymphoma | 0.25 | 0.1710 | 6.07 | −0.14 | 0.4400 | 1.90 |
| Mammary Tumors | 0.36 | 0.0600 | 13.00 | −0.68 | 0.0000 | 45.90 |
| Mesenteric Lymph Nodes | −0.07 | 0.8290 | 0.54 | 0.08 | 0.8000 | 0.70 |
| Pancreas | −0.31 | 0.3960 | 9.56 | 0.01 | 0.9700 | 0.01 |
| Pituitary Gland | 0.39 | 0.2150 | 15.18 | −0.50 | 0.1000 | 25.80 |
| Skin | −0.20 | 0.5040 | 3.95 | 0.21 | 0.4700 | 4.50 |
| Spleen | 0.24 | 0.3950 | 5.59 | −0.16 | 0.5560 | 2.70 |
| Stomach | 0.30 | 0.6400 | 9.00 | 0.57 | 0.3700 | 32.80 |
| Thymus | −0.28 | 0.3000 | 8.00 | −0.20 | 0.7000 | 4.20 |
| Thyroid | −0.10 | 0.7400 | 0.90 | −0.07 | 0.8000 | 0.50 |
| Benign | SAR | Time of Exposure | ||||
|---|---|---|---|---|---|---|
| Organ or Tumor | β-Moderator | p Value | R2 % | β-Moderator | p Value | R2 % |
| Adrenal Glands | 0.26 | 0.1950 | 6.60 | 0.64 | 0.0002 | 40.33 |
| Brain | −0.46 | 0.3930 | 20.81 | 0.73 | 0.1720 | 53.31 |
| Sensorial System | 0.17 | 0.5210 | 2.98 | 0.16 | 0.5470 | 2.63 |
| Male Uro-Genital System | −0.34 | 0.2200 | 11.39 | −0.31 | 0.2660 | 9.37 |
| Female Uro-Genital System | 0.32 | 0.1500 | 10.10 | 0.10 | 0.6520 | 0.99 |
| Intestine | 0.39 | 0.2900 | 15.16 | - | - | - |
| Kidneys (Renal System) | −0.45 | 0.3460 | 20.03 | 0.36 | 0.4540 | 12.60 |
| Liver | 0.02 | 0.9220 | 0.04 | 0.40 | 0.0260 | 15.94 |
| Lung | 0.27 | 0.3940 | 7.07 | 0.45 | 0.1460 | 20.57 |
| Mammary Tumors | −0.89 | 0.1000 | 23.70 | −0.45 | 0.1340 | 19.90 |
| Pancreas | −0.24 | 0.2920 | 5.69 | 0.48 | 0.0350 | 22.80 |
| Pituitary Gland | −0.25 | 0.3090 | 6.40 | −0.17 | 0.4870 | 3.00 |
| Skin | −0.44 | 0.0940 | 19.51 | 0.37 | 0.1670 | 13.70 |
| Stomach | 0.13 | 0.8420 | 1.80 | 0.16 | 0.8070 | 2.70 |
| Thymus | −0.08 | 0.8630 | 0.66 | 0.12 | 0.7110 | 1.50 |
| Thyroid | 0.52 | 0.0290 | 27.11 | 0.64 | 0.0080 | 40.30 |
| N. of studies (groups/papers) | Design | RoB | Inconsistency | Indirectness of Evidence | Imprecision | Publication Bias | Exposed | Comparison | Relative Effect RR (CI 95%) | Consistency between Species | Dose Response | Quality of Evidence | Health Evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adrenal Glands | 24/8 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3538 | 1166 | 1.01 (0.68–1.51) | Yes (+1) | No | Moderate | Inadequate |
| Bladder | 15/4 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2495 | 770 | 0.9 (0.54–1.512) | Yes (+1) | No | Moderate | Inadequate |
| Histiocytic Sarcoma | 26/8 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3724 | 1155 | 0.79 (0.41–1.53) | Yes (+1) | No | Moderate | Inadequate |
| Bone Marrow | 7/2 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 1175 | 279 | 0.56 (0.3–1.02) | No | No | Low | Inadequate |
| CNS (brain and spinal cord) | 26/9 | Very Serious (−2) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 4779 | 2007 | 1.40 (1.07–1.84) | Yes (+1) | No | Low | Low |
| Brain | 26/9 | Very Serious (−2) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 4779 | 2007 | 1.39 (1.07–1.81) | Yes (+1) | No | Low | Low |
| Sensorial system | 20/4 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3034 | 712 | 1.03 (0.68–1.55) | Yes (+1) | No | Moderate | Inadequate |
| Male Uro-Genital System | 10/3 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 880 | 260 | 1.76 (1.03-2.98) | No | No | Low | Inadequate |
| Female Uro-Genital System | 32/9 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2354 | 822 | 0.92 (0.72–1.17) | Yes (+1) | No | Moderate | Inadequate |
| Heart | 15/3 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | Yes (−2) | 3790 | 1117 | 3.24 (2.11–4.98) | Yes (+1) | No | Very Low | Inadequate |
| Intestine | 14/3 | Very Serious (−2) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2376 | 505 | 0.59 (0.4–0.86) | Yes (+1) | No | Low | Low |
| Kidneys (Renal System) | 14/3 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2460 | 519 | 0.95 (0.57–1.57) | Yes (+1) | No | Moderate | Inadequate |
| Leukemia | 17/5 | Serious (−1) | Some corcern (−1) | No (I2 = 3.9%) | No | No | No | 2939 | 800 | 0.88 (0.74–1.06) | Yes (+1) | No | Moderate | Inadequate |
| Liver | 25/8 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3497 | 1086 | 0.96 (0.86–1.07) | Yes (+1) | No | Moderate | Inadequate |
| Lung | 23/7 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3394 | 1031 | 0.89 (0.77–1.02) | Yes (+1) | No | Moderate | Inadequate |
| Lymphoma | 41/15 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 5645 | 2184 | 1.04 (1.03–1.05) | Yes (+1) | No | Moderate | Inadequate |
| Mammary Tumors | 29/10 | Serious (−1) | Some corcern (−1) | No (I2 = 43.9%) | No | No | No | 3362 | 1274 | 0.99 (0.55–1.80) | No | No | Low | Inadequate |
| Mesenteric Lymph Nodes | 16/4 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2241 | 631 | 0.82 (0.44–1.55) | Yes (+1) | No | Moderate | Inadequate |
| Pancreas | 18/3 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2713 | 555 | 1.17 (0.94–1.45) | Yes (+1) | No | Moderate | Inadequate |
| Pituitary Gland | 25/7 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3193 | 887 | 0.99 (0.77–1.24) | Yes (+1) | No | Moderate | Inadequate |
| Skin | 15/5 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 2456 | 658 | 0.75 (0.57–1.01) | Yes (+1) | No | Moderate | Inadequate |
| Spleen | 14/4 | Serious (−1) | Some corcern (−1) | No (I2 = 44.5%) | No | Yes (−1) | No | 2282 | 506 | 1.07 (0.35–3.22) | Yes (+1) | No | Low | Inadequate |
| Stomach | 7/2 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 1179 | 280 | 0.78 (0.32–1.88) | No | No | Low | Inadequate |
| Thymus | 14/3 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 1938 | 527 | 0.91 (0.58–1.43) | No | No | Low | Inadequate |
| Thyroid | 26/7 | Serious (−1) | Some corcern (−1) | No (I2 = 0%) | No | No | No | 3790 | 1094 | 1.23 (0.96–1.58) | Yes (+1) | No | Moderate | Inadequate |
| N. of Studies (Groups/Papers) | Design | RoB | Inconsistency | Indirectness of Evidence | Imprecision | Publication Bias | Exposed | Comparison | Relative Effect RR (CI 95%) | Consistency between Species | Dose Response | Quality of Evidence | Health Evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adrenal Glands | 26/8 | Serious (−1) | Some corcern (−1) | I2 = 28.08 | No | No | No | 3656 | 1107 | 1.43 (1.09–1.89) | Yes (+1) | No | Moderate | Inadequate |
| CNS/Brain | 9/2 | Very Serious (−2) | Some corcern (−1) | I2 = 0 | No | No | Yes (−1) | 2711 | 997 | 2.16 (1.37–3.41) | Yes (+1) | No | Very Low | Inadequate |
| Sensorial system | 15/3 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 1976 | 479 | 1.01 (0.75–1.35) | Yes (+1) | No | Moderate | Inadequate |
| Male Uro-Genital System | 17/5 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 1523 | 451 | 0.95 (0.92–0.99) | No | No | Low | Inadequate |
| Female Uro-Genital System | 23/8 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 2219 | 822 | 1.06 (0.98–1.15) | Yes (+1) | No | Moderate | Inadequate |
| Intestine | 12/2 | Very Serious (−2) | Some corcern (−1) | I2 = 0 | No | Yes (−1) | No | 2055 | 345 | 1.14 (0.48–2.65) | Yes (+1) | No | Low | Low |
| Kidneys (Renal System) | 14/4 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 2296 | 513 | 0.52 (0.37–0.71) | Yes (+1) | No | Moderate | Inadequate |
| Liver | 28/10 | Serious (−1) | Some corcern (−1) | I2 = 13.02 | No | No | No | 3915 | 1346 | 1.04 (0.95–1.15) | Yes (+1) | No | Moderate | Inadequate |
| Lung | 24/7 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 3314 | 911 | 0.95 (0.69–1.30) | Yes (+1) | No | Moderate | Inadequate |
| Mammary Tumors | 26/8 | Serious (−1) | Some corcern (−1) | I2 = 40 | No | No | No | 2975 | 929 | 1.13 (0.98–1.29) | No | No | Low | Inadequate |
| Pancreas | 23/7 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | Yes (−1) | No | 3333 | 1007 | 1.54 (0.71–3.35) | Yes (+1) | No | Moderate | Inadequate |
| Pituitary Gland | 31/8 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 4105 | 1195 | 1.03 (0.97–1.1) | Yes (+1) | No | Moderate | Inadequate |
| Skin | 13/3 | Serious (−1) | Some corcern (−1) | I2 = 21.32 | No | No | No | 2260 | 460 | 1.14 (0.8–1.63) | Yes (+1) | No | Moderate | Inadequate |
| Stomach | 14/4 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 2330 | 548 | 0.72 (0.52–0.98) | No | No | Low | Inadequate |
| Thymus | 15/3 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 2104 | 527 | 0.83 (0.65–1.1) | No | No | Low | Inadequate |
| Thyroid | 24/7 | Serious (−1) | Some corcern (−1) | I2 = 0 | No | No | No | 3574 | 1080 | 1.14 (0.98–1.33) | Yes (+1) | No | Moderate | Inadequate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, R.; Ardoino, L.; Villani, P.; Marino, C. In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Cancer: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 2071. https://doi.org/10.3390/ijerph20032071
Pinto R, Ardoino L, Villani P, Marino C. In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Cancer: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(3):2071. https://doi.org/10.3390/ijerph20032071
Chicago/Turabian StylePinto, Rosanna, Lucia Ardoino, Paola Villani, and Carmela Marino. 2023. "In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Cancer: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 3: 2071. https://doi.org/10.3390/ijerph20032071
APA StylePinto, R., Ardoino, L., Villani, P., & Marino, C. (2023). In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Cancer: A Systematic Review. International Journal of Environmental Research and Public Health, 20(3), 2071. https://doi.org/10.3390/ijerph20032071






