Organic Compounds and Antibiotic-Resistant Bacteria Behavior in Greywater Treated by a Constructed Wetland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the System and Operating Characteristics
2.2. Analytic Methods
2.2.1. Water Quality Parameters
2.2.2. Determination of Biological Contaminants
2.2.3. Molecular Weight Distribution
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Characterization of Laundry Greywater
4.2. Behavior of Organic Compounds in a CW
4.3. Behavior of Microbiological Compounds in a CW and Their Relationship with Organic Compounds Present in Laundry Greywater
5. Conclusions
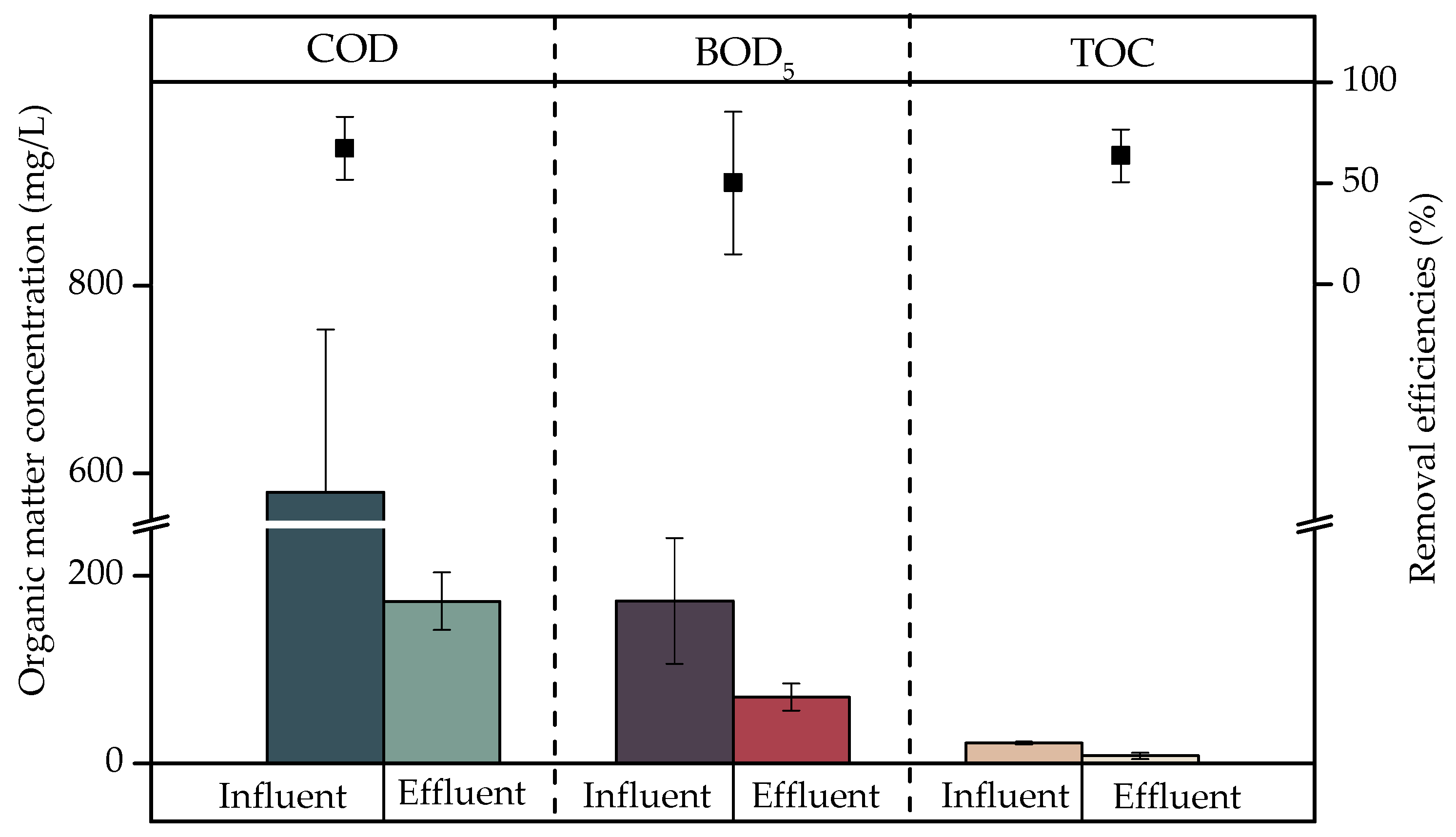
- The organic matter removal efficiencies using a CW were 67.19%, 50.15%, and 63.57% for COD, BOD5, and TOC, respectively. These efficiencies were not significant (p > 0.05); therefore, further studies on the XOCs present in laundry greywater are suggested to evaluate their effect on biodegradation.
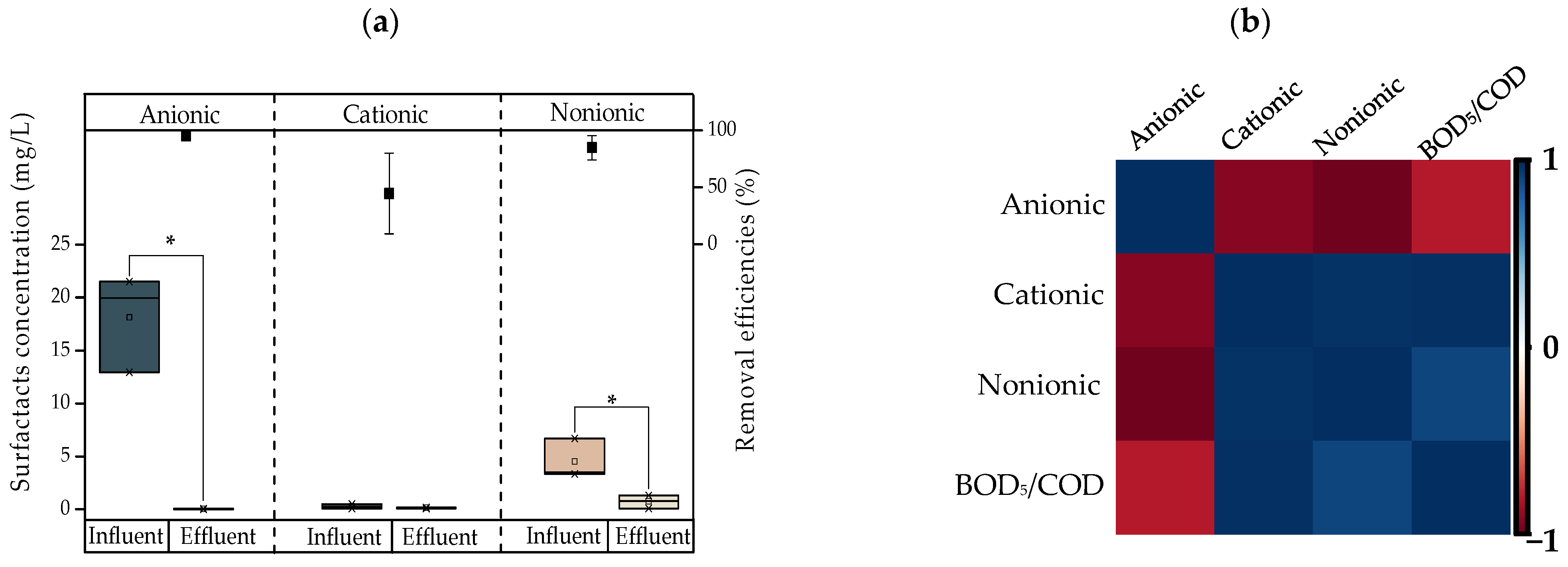
- The assessment of molecular weight distribution showed that the TOC percentage increased by 5.4% in the fraction below 1000 Da in the effluents. The same behavior was found for the ionic compounds NH4+, Na+, and EC, with increases of 36.44%, 2.71%, and 12.51%, respectively. Thus, the CW reduces the molecular weight of organic compounds, making them simpler, and therefore more mineralizable.
- The TC, FC, and ARB removal efficiencies were 82.38%, 82.38%, and 1.78%, respectively; these efficiencies were not significant (p > 0.05). There were also increases in the bacteria resistant to the antibiotics CIP and CTX of 36.34% and 40.79%, respectively. A strong association between ARB and CTX, CIP, cationic and non-ionic surfactants was found according to PCA. This behavior indicates the role of surfactants in resistance selection. Due to the results obtained, the use of disinfection systems is suggested to decrease the impact of treated laundry greywater on the environment and health.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.; Fazal, D.B.; Ahmad, F.; Fazal, A.B.; Abdullah, A.Z.; Ahmed, M.; Qamar, M.; Rafatullah, M. Photocatalytic degradation of recalcitrant pollutants of greywater. Catalysts 2022, 12, 557. [Google Scholar] [CrossRef]
- Glover, C.M.; Liu, Y.; Liu, J. Assessing the risk from trace organic contaminants released via greywater irrigation to the aquatic environment. Water Res. 2021, 205, 117664. [Google Scholar] [CrossRef] [PubMed]
- Organización de la Naciones Unidas para la Educación, Ciencia y Cultura (UNESCO). World Water Development Report; Richard Connor: Paris, France, 2016; pp. 1–164. [Google Scholar]
- Radingoana, M.P.; Dube, T.; Mazvimavi, D. An assessment of irrigation water quality and potential of reusing greywater in home gardens in water-limited environments. Phys. Chem. Earth 2020, 116, 102857. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Impact on human health of climate changes. Eur. J. Intern. Med. 2015, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, S.; Nakhaee, N.; Khankeh, H.; Haghdoost, A.A. Impacts of drought on health: A qualitative case study from Iran. Int. J. Disaster Risk Reduct. 2022, 76, 103007. [Google Scholar] [CrossRef]
- Ghaitidak, D.M.; Yadav, K.D. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef]
- Braga, J.K.; Varesche, M.B.A. Commercial Laundry Water Characterisation. J. Anal. Chem. 2014, 5, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Oteng-Peprah, M.; Acheampong, M.A.; deVries, N.K. Greywater characteristics, treatment systems, reuse strategies and user perception—A review. Water Air Soil Poll. 2018, 229, 255. [Google Scholar] [CrossRef] [Green Version]
- Ramprasad, C.; Philip, L. Contributions of various processes to the removal of surfactants and personal care products in constructed wetland. Chem. Eng. J. 2018, 334, 322–333. [Google Scholar] [CrossRef]
- Maimon, A.; Gross, A.; Arye, G. Greywater-induced soil hydrophobicity. Chemosphere 2017, 184, 1012–1019. [Google Scholar] [CrossRef]
- Khalil, M.; Liu, Y. Greywater biodegradability and biological treatment technologies: A critical review. Int. Biodeterior. Biodegrad. 2021, 161, 105211. [Google Scholar] [CrossRef]
- Carrillo, V.; Fuentes, B.; Gómez, G.; Vidal, G. Characterization and recovery of phosphorus from wastewater by combined technologies. Rev. Environ. Sci. Biotechnol. 2020, 19, 389–418. [Google Scholar] [CrossRef]
- Porob, S.; Craddock, H.A.; Motro, Y.; Sagi, O.; Gdalevich, M.; Ezery, Z.; Davidovitch, N.; Ronen, Z.; Moran-Gilad, J. Quantification and characterization of antimicrobial resistance in greywater discharged to the environment. Water 2020, 12, 1460. [Google Scholar] [CrossRef]
- Chamorro, S.; Hernandez, V.; Matamoros, V.; Dominguez, C.; Becerra, J.; Vidal, G.; Piña, B.; Bayona, J.M. Chemical characterization of organic microcontaminant sources and biological effects in riverine sediments impacted by urban sewage and pulp mill discharges. Chemosphere 2013, 90, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Some, S.; Mondal, R.; Mitra, D.; Jain, D.; Verma, D.; Das, S. Microbial pollution of water with special reference to coliform bacteria and their nexus with environment. Energy Nexus 2021, 1, 100008. [Google Scholar] [CrossRef]
- Monsalves, N.; Leiva, A.M.; Gómez, G.; Vidal, G. Antibiotic-resistant gene behavior in constructed wetlands treating sewage: A critical review. Sustainability 2022, 14, 8524. [Google Scholar] [CrossRef]
- López, D.; Fuenzalida, D.; Vera, I.; Rojas, K.; Vidal, G. Relationship between the removal of organic matter and the production of methane in subsurface flow constructed wetlands designed for wastewater treatment. Ecol. Eng. 2015, 83, 296–304. [Google Scholar] [CrossRef]
- Burgos, V.; Araya, F.; Reyes-Contreras, C.; Vera, I.; Vidal, G. Performance of ornamental plants in mesocosm subsurface constructed wetlands under different organic sewage loading. Ecol. Eng. 2017, 99, 246–255. [Google Scholar] [CrossRef]
- Leiva, A.M.; Gutierrez, E.; Arias, C.A.; Vidal, G. Influence of water quality parameters on the removal of triclosan and ibuprofen in vertical subsurface flow constructed wetlands using multivariate analysis. Environ. Technol. Innov. 2021, 24, 101846. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef]
- Hernández Leal, L.; Temmink, H.; Zeeman, G.; Buisman, C.J.N. Characterization and anaerobic biodegradability of grey water. Desalination 2011, 270, 111–115. [Google Scholar] [CrossRef]
- Halalsheh, M.; Dalahmeh, S.; Sayed, M.; Suleiman, W.; Shareef, M.; Mansour, M.; Safi, M. Grey water characteristics and treatment options for rural areas in Jordan. Bioresour. Technol. 2008, 99, 6635–6641. [Google Scholar] [CrossRef] [PubMed]
- Ramprasad, C.; Philip, L. Surfactants and personal care products removal in pilot scale horizontal and vertical flow constructed wetlands while treating greywater. Chem. Eng. J. 2016, 284, 458–468. [Google Scholar] [CrossRef]
- Arden, S.; Ma, X. Constructed wetlands for greywater recycle and reuse: A review. Sci. Total Environ. 2018, 630, 587–599. [Google Scholar] [CrossRef]
- González, Y.; Salgado, P.; Vidal, G. Disinfection behavior of a UV-treated wastewater system using constructed wetlands and the rate of reactivation of pathogenic microorganisms. Water Sci. Technol. 2019, 80, 1870–1879. [Google Scholar] [CrossRef]
- Salgado, P.; Melín, V.; Albornoz, M.; Mansilla, H.; Vidal, G.; Contreras, D. Effect of pH and substituted 1,2-dihydroxybenzenes on reaction pathway of Fenton-like systems. Appl. Catal. B Environ. 2018, 226, 93–102. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater (APHA); American Public Health Association: Washington, DC, USA, 2005.
- Bavestrello, L.; Cabello, A. Community consumption of antimicrobials in Chile, 2000–2008. Rev. Chil. Infect. 2011, 28, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Wayne, P.A. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2014; pp. 100–122. [Google Scholar]
- Vidal, G.; Videla, S.; Diez, M.C. Molecular weight distribution of pinus radiata kraft mill wastewater treated by anaerobic digestion. Bioresour. Technol. 2001, 77, 183–191. [Google Scholar] [CrossRef]
- Gómez, G.; Salinas, M.; Ruiz-Tagle, N.; Sossa, K.; Vidal, G. Molecular weight distribution of the recalcitrant organic matter contained in kraft mill effluents and the identification of microbial consortia responsible for an anaerobic biodegradable fraction. Environ. Sci. Health A 2020, 55, 281–291. [Google Scholar] [CrossRef]
- Leiva, A.M.; Piña, B.; Vidal, G. Antibiotic resistance dissemination in wastewater treatment plants: A challenge for the reuse of treated wastewater in agriculture. Rev. Environ. Sci. Biotechnol. 2021, 20, 1043–1072. [Google Scholar] [CrossRef]
- Shreya; Verma, A.K.; Dash, A.K.; Bhunia, P.; Dash, R.R. Removal of surfactants in greywater using low-cost natural adsorbents: A review. Surf. Interfaces 2021, 27, 101532. [Google Scholar] [CrossRef]
- De Boer, M.A.; Hammerton, M.; Slootweg, J.C. Uptake of pharmaceuticals by sorbent-amended struvite fertilisers recovered from human urine and their bioaccumulation in tomato fruit. Water Res. 2018, 133, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.; Ergas, S.J.; Ghebremichael, K.; Gross, A.; Ronen, Z. Occurrence of antibiotic-resistant genes and bacteria in household greywater treated in constructed wetlands. Water 2022, 14, 758. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Ren, J.; Zhou, Z.; Li, X.; Liu, Y.; Feng, J. Fate of organic fractions of greywater in combined process of vacuum-ultraviolet (VUV/UV)/ozone pre-oxidation with enhanced coagulation. Environ. Chem. Eng. 2022, 10, 107417. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, M.; Wang, H.; Dai, X.; Chen, H. Removal of personal care products in greywater using membrane bioreactor and constructed wetland methods. Sci. Total Environ. 2021, 797, 148773. [Google Scholar] [CrossRef] [PubMed]
- Khanam, K.; Patidar, S.K. Greywater characteristics in developed and developing countries. Mater. Today Proc. 2022, 57, 1494–1499. [Google Scholar] [CrossRef]
- Fedeila, M.; Hachaïchi-Sadouk, Z.; Bautista, L.F.; Simarro, R.; Nateche, F. Biodegradation of anionic surfactants by Alcaligenes faecalis, Enterobacter cloacae and Serratia marcescens strains isolated from industrial wastewater. Ecotoxicol. Environ. Saf. 2018, 163, 629–635. [Google Scholar] [CrossRef]
- Finklea, H.; Lin, L.-S.; Khajouei, G. Electrodialysis of softened produced water from shale gas development. Water Process. Eng. 2022, 45, 102486. [Google Scholar] [CrossRef]
- Jia, S.; Shi, P.; Hu, Q.; Li, B.; Zhang, T.; Zhang, X. Bacterial Community Shift Drives Antibiotic Resistance Promotion during Drinking Water Chlorination. Environ. Scie. Technol. 2015, 49, 12271–12279. [Google Scholar] [CrossRef]
- Craddock, H.A.; Chattopadhyay, S.; Rjoub, Y.; Rosen, D.; Greif, J.; Lipchin, C.; Mongodin, E.F.; Sapkota, A.R. Antibiotic-resistant Escherichia coli and Klebsiella spp. in greywater reuse systems and pond water used for agricultural irrigation in the West Bank, Palestinian Territories. Environ. Res. 2020, 188, 109777. [Google Scholar] [CrossRef]
- Noman, E.A.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.; Al-Shaibani, M.M.; Al-Wrafy, F.A.; Al-Maqtari, Q.A.; Vo, D.-V.N. Antibiotics and antibiotic-resistant bacteria in greywater: Challenges of the current treatment situation and predictions of future scenario. Environ. Res. 2022, 212, 113380. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lu, H.; Zhu, L. Molecular mechanism of antibiotic resistance induced by mono- and twin-chained quaternary ammonium compounds. Sci. Total Environ. 2022, 832, 155090. [Google Scholar] [CrossRef] [PubMed]
- Oron, G.; Adel, M.; Agmon, V.; Friedler, E.; Halperin, R.; Leshem, E.; Weinberg, D. Greywater use in Israel and worldwide: Standards and prospects. Water Res. 2014, 58, 92–101. [Google Scholar] [CrossRef]
- Nagarkar, M.; Keely, S.P.; Brinkman, N.E.; Garland, J.L. Human-and infrastructure-associated bacteria in greywater. J. Appl. Microbiol. 2021, 131, 2178–2192. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, M.; Stefanatou, A.; Schiza, S.; Petousi, I.; Stasinakis, A.S.; Fountoulakis, M.S. Removal of microfiber in vertical flow constructed wetlands treating greywater. Sci. Total Environ. 2023, 858, 159723. [Google Scholar] [CrossRef]
- Kotsia, D.; Deligianni, A.; Fyllas, N.M.; Stasinakis, A.S.; Fountoulakis, M.S. Converting treatment wetlands into “treatment gardens”: Use of ornamental plants for greywater treatment. Sci. Total Environ. 2020, 744, 140889. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.; de Oliveira Vaz, L.; Peres, M.; Merlo, S.S. Microbiological risk from non-potable reuse of greywater treated by anaerobic filters associated to vertical constructed wetlands. Water Process. Eng. 2021, 39, 101751. [Google Scholar] [CrossRef]
- Maucieri, C.; Barbera, A.C.; Vymazal, J.; Borin, M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Oh, K.S.; Leong, J.Y.C.; Poh, P.E.; Chong, M.N.; Lau, E.V. A review of greywater recycling related issues: Challenges and future prospects in Malaysia. J. Clean. Prod. 2018, 171, 17–29. [Google Scholar] [CrossRef]
- Dordio, A.V.; Carvalho, A.J.P. Organic xenobiotics removal in constructed wetlands, with emphasis on the importance of the support matrix. J. Hazard. Mater. 2013, 252, 272–292. [Google Scholar] [CrossRef]
- Contreras, J.; López, D.; Gómez, G.; Vidal, G. Seasonal enhancement of nitrogen removal on domestic wastewater treatment performance by partially saturated and saturated hybrid constructed wetland. Water 2022, 14, 1089. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef]
- Imfeld, G.; Braeckevelt, M.; Kuschk, P.; Richnow, H.H. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere 2009, 74, 349–362. [Google Scholar] [CrossRef]
- Su, Z.; Liu, T.; Yu, W.; Li, X.; Graham, N.J.D. Coagulation of surface water: Observations on the significance of biopolymers. Water Res. 2017, 126, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.W.; Wang, C.W.; Jiang, S.C. Quantitative microbial risk assessment of Greywater on-site reuse. Sci. Total Environ. 2018, 635, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Shingare, R.P.; Thawale, P.R.; Raghunathan, K.; Mishra, A.; Kumar, S. Constructed wetland for wastewater reuse: Role and efficiency in removing enteric pathogens. J. Environ. Manag. 2019, 246, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Zhu, G.; Gong, T.; Lu, Y. Dissolved organic matter derived from aquatic plants in constructed wetlands: Characteristics and disinfection by products formation. Environ. Chem. Eng. 2022, 10, 107991. [Google Scholar] [CrossRef]
- Dires, S.; Birhanu, T.; Ambelu, A.; Sahilu, G. Antibiotic resistant bacteria removal of subsurface flow constructed wetlands from hospital wastewater. J. Environ. Chem. Eng. 2018, 6, 4265–4272. [Google Scholar] [CrossRef]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Benami, M.; Gillor, O.; Gross, A. Potential microbial hazards from graywater reuse and associated matrices: A review. Water Res. 2016, 106, 183–195. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, Y.; Zhang, Y.; Song, B.; Li, H.; Chen, Z. Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 2016, 92, 243–250. [Google Scholar] [CrossRef]
- He, Y.; Nurul, S.; Schmitt, H.; Sutton, N.B.; Murk, T.A.; Blokland, M.H.; Rijnaarts, H.H.; Langenhoff, A.A. Evaluation of attenuation of pharmaceuticals, toxic potency, and antibiotic resistance genes in constructed wetlands treating wastewater effluents. Sci. Total Environ. 2018, 631, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, C.; Li, K.; Su, J.; Zhu, G.; Liu, L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015, 70, 109–117. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.; Forde, B.; Kidd, T.; Harris, P.; Schembri, M.; Beatson, S.; Paterson, D.; Walker, M. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Liu, Y.; Lu, S.; Qin, P.; Guo, X.; Bi, B.; Wang, L.; Xi, B.; Wu, F.; et al. Zhang. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef]
- Du, L.; Zhao, Y.; Wang, C.; Zhang, H.; Chen, Q.; Zhang, X.; Zhang, L.; Wu, J.; Wu, Z.; Zhou, Q. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total Environ. 2020, 721, 137765. [Google Scholar] [CrossRef] [PubMed]
- Samardžić, M.; Sak-Bosnar, M.; Madunić-Čačić, D. Simultaneous potentiometric determination of cationic and ethoxylated nonionic surfactants in liquid cleaners and disinfectants. Talanta 2011, 83, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Zaky, M.F.; Aiad, I.A.; Tawfik, S.M. Synthesis, characterization, surface and biocidal effect of some germinate nonionic surfactants. J. Ind. Eng. Chem. 2015, 21, 1174–1182. [Google Scholar] [CrossRef]

 : <1000 Da;
: <1000 Da;  : 5000–1000 Da;
: 5000–1000 Da;  : 1000–10,000 Da;
: 1000–10,000 Da;  : >10,000 Da.
: >10,000 Da.
 : <1000 Da;
: <1000 Da;  : 5000–1000 Da;
: 5000–1000 Da;  : 1000–10,000 Da;
: 1000–10,000 Da;  : >10,000 Da.
: >10,000 Da.
 : ARB;
: ARB;  : antibiotic-susceptible bacteria.
: antibiotic-susceptible bacteria.
 : ARB;
: ARB;  : antibiotic-susceptible bacteria.
: antibiotic-susceptible bacteria.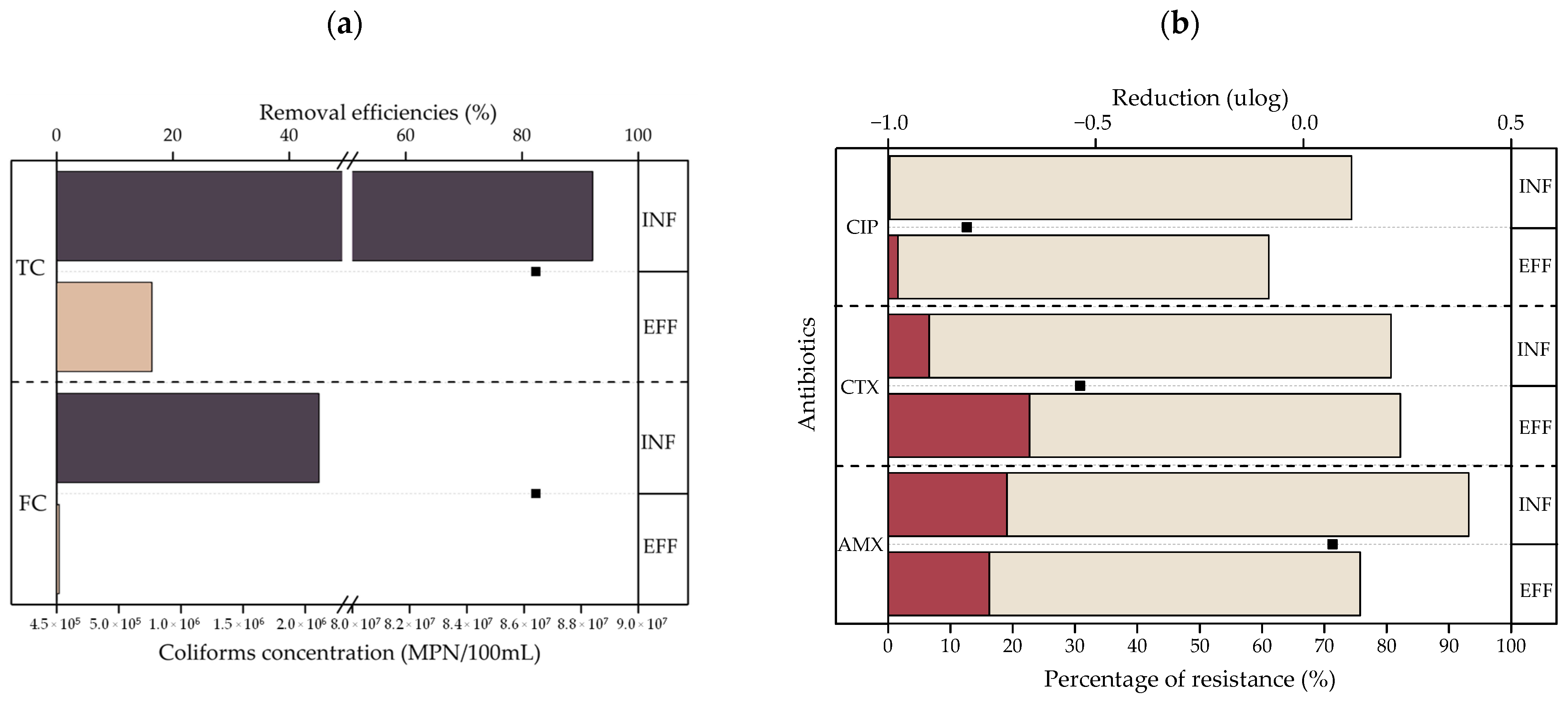

| Parameter | Unit | Average | Range | |
|---|---|---|---|---|
| In situ | T | °C | 15.7 ± 1.4 | 13.3–16.8 |
| pH | - | 6.84 ± 0.35 | 6.58–7.54 | |
| ORP | mV | 70.28 ± 79.18 | −119.70–66.60 | |
| EC | µS/cm | 660.7 ± 599.2 | 177.1–1704.0 | |
| DO | mg/L | 2.5 ± 1.2 | 1.0–4.9 | |
| Turbidity | NTU | 99.7 ± 62.2 | 3.1–144.0 | |
| Nutrients | NH4+-N | mg/L | 0.62 ± 0.38 | 0.13–0.99 |
| NO2--N | mg/L | 0.04 ± 0.04 | 0.01–0.13 | |
| NO3--N | mg/L | 1.27 ± 0.92 | 0.06–2.39 | |
| TKN-N | mg/L | 5.93 ± 4.03 | 3.29–10.56 | |
| PO43--P | mg/L | 0.280 ± 0.350 | 0.003–0.915 | |
| TP | mg/L | 0.6 ± 0.4 | 0.1–1.4 | |
| TN | mg/L | 16.2 ± 27.5 | 0.8–78.0 | |
| Organic matter | COD | mg/L | 562.3 ± 279.4 | 67.0–902.9 |
| BOD5 | mg/L | 296.8 ± 387.9 | 3.8–940.0 | |
| TOC | mg/L | 7.23 ± 0.55 | 6.69–7.78 | |
| Surfactants | Anionic | mg/L | 18.30 ± 4.57 | 12.93–32.52 |
| Cationic | mg/L | 0.23 ± 0.18 | 0.11–0.49 | |
| Non-ionic | mg/L | 5.26 ± 2.14 | 3.34–7.50 | |
| Cations | NH4+ | mg/L | 19.59 ± 25.03 | <LD–48.50 |
| K+ | mg/L | 5.62 ± 4.51 | <LD–9.42 | |
| Na+ | mg/L | 111.34 ± 106.13 | <LD–211.36 | |
| Li+ | mg/L | <LD | <LD | |
| Anions | NO2− | mg/L | 0.14 ± 0.20 | <LD–0.28 |
| NO3− | mg/L | 0.18 ± 0.04 | 0.13–0.21 | |
| F− | mg/L | 3.17 ± 5.42 | <LD–9.42 | |
| SO43− | mg/L | 68.03 ± 61.81 | 26.06–139.02 | |
| PO43− | mg/L | 0.27 ± 0.15 | 0.11–0.39 | |
| Cl− | mg/L | 398.20 ± 354.46 | 97.82–789.15 | |
| Br− | mg/L | <LD | <LD | |
| Parameter | Unit | Average (×106) | Range (×106) | |
|---|---|---|---|---|
| Microbiological | TC | MPN/100 mL | 70.4 ± 71.0 | 0.7–160.0 |
| FC | MPN/100 mL | 2.15 ± 1.63 | 0.017–4.0 | |
| ARB | CFU/100 mL | 0.65 ± 0.92 | <LD–1.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monsalves, N.; Leiva, A.M.; Gómez, G.; Vidal, G. Organic Compounds and Antibiotic-Resistant Bacteria Behavior in Greywater Treated by a Constructed Wetland. Int. J. Environ. Res. Public Health 2023, 20, 2305. https://doi.org/10.3390/ijerph20032305
Monsalves N, Leiva AM, Gómez G, Vidal G. Organic Compounds and Antibiotic-Resistant Bacteria Behavior in Greywater Treated by a Constructed Wetland. International Journal of Environmental Research and Public Health. 2023; 20(3):2305. https://doi.org/10.3390/ijerph20032305
Chicago/Turabian StyleMonsalves, Naomi, Ana María Leiva, Gloria Gómez, and Gladys Vidal. 2023. "Organic Compounds and Antibiotic-Resistant Bacteria Behavior in Greywater Treated by a Constructed Wetland" International Journal of Environmental Research and Public Health 20, no. 3: 2305. https://doi.org/10.3390/ijerph20032305
APA StyleMonsalves, N., Leiva, A. M., Gómez, G., & Vidal, G. (2023). Organic Compounds and Antibiotic-Resistant Bacteria Behavior in Greywater Treated by a Constructed Wetland. International Journal of Environmental Research and Public Health, 20(3), 2305. https://doi.org/10.3390/ijerph20032305






