Damage Effect of Amorphous Carbon Black Nanoparticle Aggregates on Model Phospholipid Membranes: Surface Charge, Exposure Concentration and Time Dependence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification, Dispersion and Characterization of CBNs
2.3. Preparation of Giant Unilamellar Vesicles (GUVs)
2.4. Exposure of NMs to GUVs
3. Results and Discussion
3.1. Characterization of CBNs
3.2. Effect of CB and MCB on GUVs+ and GUVs−
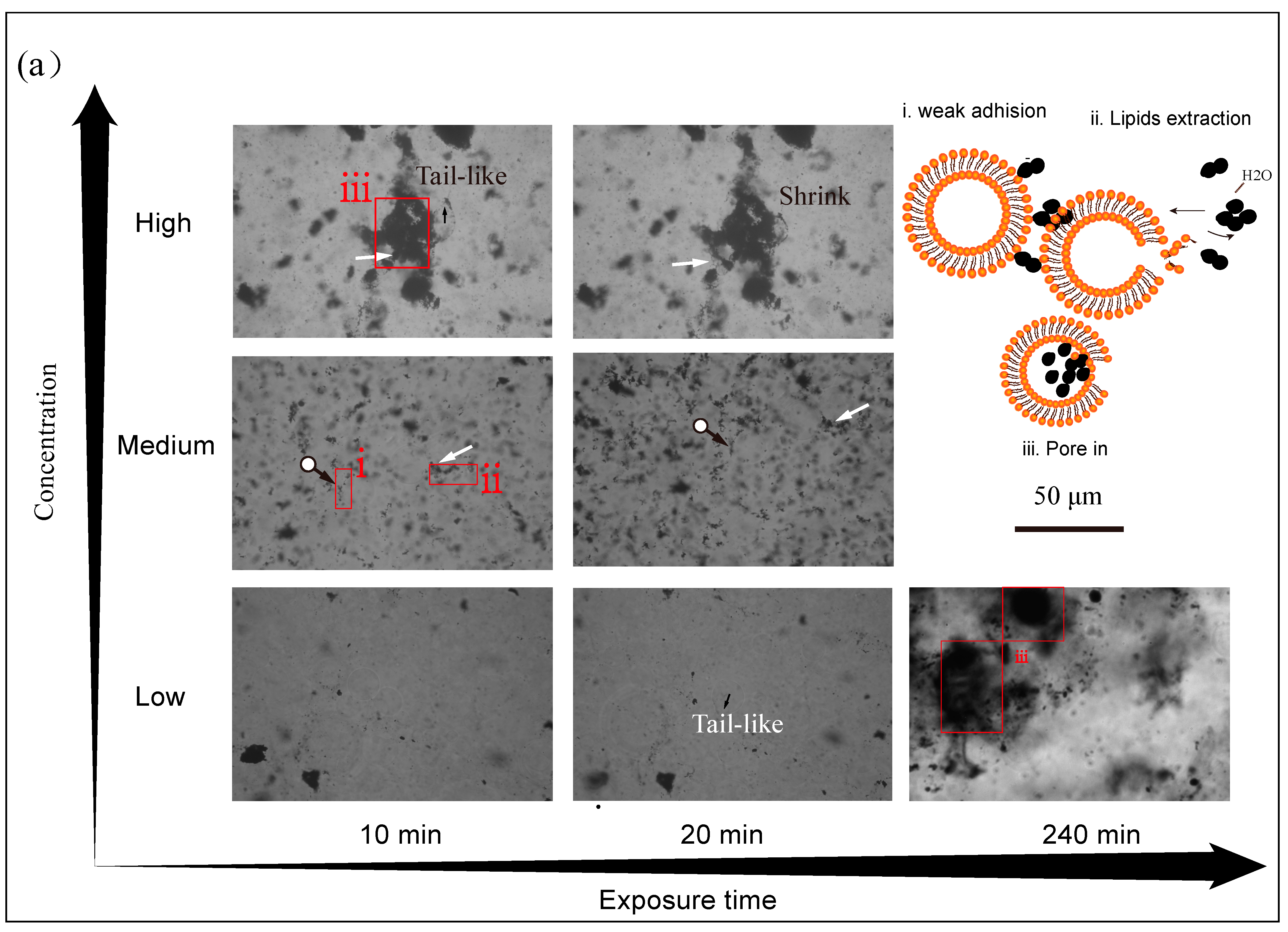
3.3. Effect of Concentration Level of CBNs on GUVs+ Damage
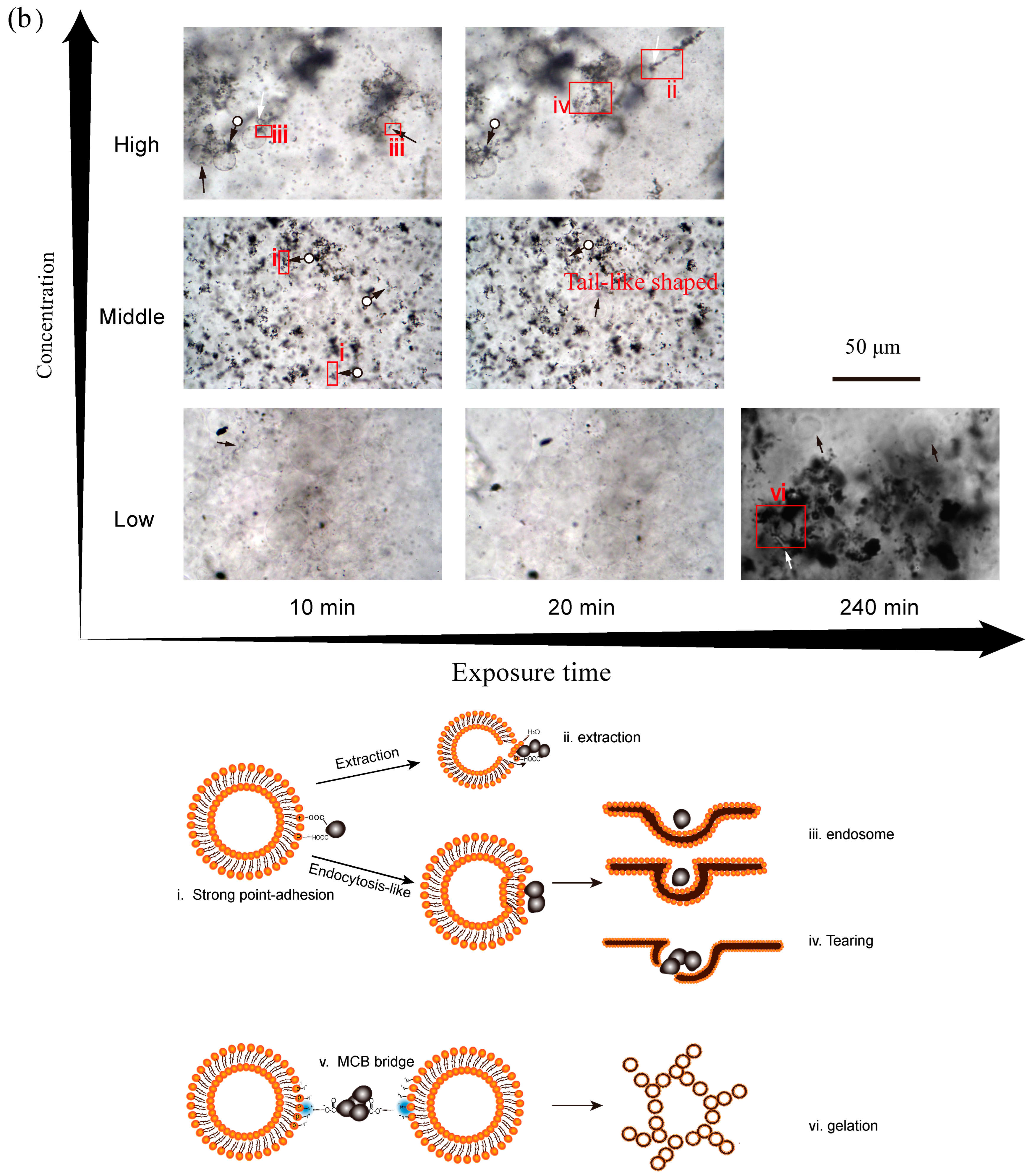
3.4. Effect of Exposure Time on Membrane Disruption
3.5. Proposed Interaction Mechanisms of CBNs and GUVs
3.6. Limitations of This Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masciangioli, T.; Zhang, W. Environmental technologies at the nanoscale. Environ. Sci. Technol. 2003, 37, 102A–108A. [Google Scholar] [CrossRef]
- Ban, M.; Langonné, I.; Huguet, N.; Goutet, M. Effect of submicron and nano-iron oxide particles on pulmonary immunity in mice. Toxicol. Lett. 2012, 210, 267–275. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef]
- Wan, R.; Mo, Y.; Zhang, Z.; Jiang, M.; Tang, S.; Zhang, Q. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part. Fibre Toxicol. 2017, 14, 38. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Du, X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 2009, 34, 559–567. [Google Scholar] [CrossRef]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2015, 13, 12. [Google Scholar] [CrossRef]
- Donaldson, K.; Poland, C.A. Nanotoxicity: Challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 2013, 24, 724–734. [Google Scholar] [CrossRef]
- Wiesner, M.; Lowry, G.; Pedro, A.; Dianysios, D.; Pratim, B. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006, 40, 4336–4345. [Google Scholar] [CrossRef]
- Li, K.; Xia, C.; Wang, B.; Chen, H.; Wang, T.; He, Q.; Cao, H.; Wang, Y. Effects of quantum dots on the ROS amount of liver cancer stem cells. Colloids Surf. B Biointerfaces 2017, 155, 193–199. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Mu, Q.; Broughton, D.L.; Yan, B. Endosomal Leakage and Nuclear Translocation of Multiwalled Carbon Nanotubes: Developing a Model for Cell Uptake. Nano Lett. 2009, 9, 4370–4375. [Google Scholar] [CrossRef]
- Li, S.-Q.; Zhu, R.-R.; Zhu, H.; Xue, M.; Sun, X.-Y.; Yao, S.-D.; Wang, S.-L. Nanotoxicity of TiO2 nanoparticles to erythrocyte in vitro. Food Chem. Toxicol. 2008, 46, 3626–3631. [Google Scholar] [CrossRef]
- Pajnic, M.; Drasler, B.; Sustar, V.; Krek, J.L.; Stukelj, R.; Simundic, M.; Kononenko, V.; Makovec, D.; Hägerstrand, H.; Drobne, D.; et al. Effect of carbon black nanomaterial on biological membranes revealed by shape of human erythrocytes, platelets and phospholipid vesicles. J. Nanobiotechnol. 2015, 13, 28. [Google Scholar] [CrossRef]
- Omid, A.; Elham, G. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar]
- Seoktae, K.; Moshe, H.; Rodrigues, D.; Menachem, E. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.-H.; Yun, K.; Kim, S.J. Antibacterial Efficiency of Graphene Nanosheets against Pathogenic Bacteria via Lipid Peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Guo, Q.; Cong, H.; Min, L.; Yong, S.; Hua, S.; Jian, L.; Feng, X. Advances in the understanding of nanomaterial-biomembrane interactions and their mathematical and numerical modeling. Nanomedicine 2013, 8, 995–1011. [Google Scholar]
- Wei, X.; Jiang, W.; Yu, J.; Ding, L.; Hu, J.; Jiang, G. Effects of SiO2 nanoparticles on phospholipid membrane integrity and fluidity. J. Hazard. Mater. 2015, 287, 217–224. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Cao, Z.; Zhou, X.; Yang, F.; Fu, P.; Wang, Z.; Hu, J.; Ding, L.; Jiang, W. Dispersion of atmospheric fine particulate matters in simulated lung fluid and their effects on model cell membranes. Sci. Total Environ. 2016, 542, 36–43. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Q.; Qu, X.; Wang, L.; Wei, X.; Zhu, D.; Yang, K. Effects of charge and surface defects of multi-walled carbon nanotubes on the disruption of model cell membranes. Sci. Total Environ. 2017, 574, 771–780. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Surya, I.; Ismail, H.; Azura, A. The effect of alkanolamide loading on properties of carbon black-filled natural rubber (SMR-L), epoxidised natural rubber (ENR), and styrene-butadiene rubber (SBR) compounds. Polym. Test. 2015, 42, 208–214. [Google Scholar] [CrossRef]
- Cheng, J.-M.; Liu, Y.-Z.; Wang, H.-W. Effects of Surface-Modified Nano-Scale Carbon Black on Cu and Zn Fractionations in Contaminated Soil. Int. J. Phytoremediat. 2013, 16, 86–94. [Google Scholar] [CrossRef]
- Lyu, Y.; Yu, Y.; Li, T.; Cheng, J. Rhizosphere effects of Loliumperenne L. and Beta vulgaris var. cicla L. on the immobilization of Cd by modified nanoscale black carbon in contaminated soil. J. Soils Sediments 2017, 18, 1–11. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Wang, Y.; Wang, H.-W.; Wang, S.-Q.; Cheng, J.-M. Surface-modified nanoscale carbon black used as sorbents for Cu(II) and Cd(II). J. Hazard. Mater. 2010, 174, 34–39. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Z.; Yu, Y.; Li, X.; Li, T. Effects of modified carbon black nanoparticles on plant-microbe remediation of petroleum and heavy metal co-contaminated soils. Int. J. Phytoremediat. 2019, 21, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.A.; BeruBe, K.A.; Richards, R.J. Bioreactivity of carbon black and diesel exhaust particles to primary Clara and type II epithelial cell cultures. Occup. Environ. Med. 1999, 56, 813–819. [Google Scholar] [CrossRef]
- Tong, H.; McGee, J.K.; Saxena, R.K.; Kodavanti, U.P.; Devlin, R.B.; Gilmour, M.I. Influence of acid functionalization on the cardiopulmonary toxicity of carbon nanotubes and carbon black particles in mice. Toxicol. Appl. Pharmacol. 2009, 239, 224–232. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Carmo, M.; Linardi, M.; Poco, J.G.R. Characterization of nitric acid functionalized carbon black and its evaluation as electrocatalyst support for direct methanol fuel cell applications. Appl. Catal. A Gen. 2009, 355, 132–138. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Cheng, J. A Comparison Study of Mechanism: Cu2+ Adsorption on Different Adsorbents and Their Surface-Modified Adsorbents. J. Chem. 2016, 2016, 7936258. [Google Scholar] [CrossRef]
- Smith, K.A.; Jasnow, D.; Balazs, A. Designing synthetic vesicles that engulf nanoscopic particles. J. Chem. Phys. 2007, 127, 084703. [Google Scholar] [CrossRef]
- Akashi, K.; Miyata, H.; Itoh, H.; Kinosita, K. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 1996, 71, 3242–3250. [Google Scholar] [CrossRef]
- Geng, J.; Kim, K.; Zhang, J.; Escalada, A.; Tunuguntla, R.; Comolli, L.R.; Allen, F.I.; Shnyrova, A.V.; Cho, K.R.; Munoz, D.; et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature 2014, 514, 612–615. [Google Scholar] [CrossRef]
- Titov, A.; Petr, K.; Ryan, P. Sandwiched graphene–membrane superstructures. ACS Nano 2010, 4, 229–234. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Tsai, P.-J.; Chen, C.-W.; Chen, D.-R.; Hsu, D.-J. Using a Modified Electrical Aerosol Detector To Predict Nanoparticle Exposures to Different Regions of the Respiratory Tract for Workers in a Carbon Black Manufacturing Industry. Environ. Sci. Technol. 2010, 44, 6767–6774. [Google Scholar] [CrossRef]
- Churg, A.; Brauer, M. Ambient atmospheric particles in the airways of human lungs. Ultrastruct. Pathol. 2000, 24, 353–361. [Google Scholar]
- Roiter, Y.; Ornatska, M.; Rammohan, A.R.; Balakrishnan, J.; Heine, D.R.; Minko, S. Interaction of Lipid Membrane with Nanostructured Surfaces. Langmuir 2009, 25, 6287–6299. [Google Scholar] [CrossRef]

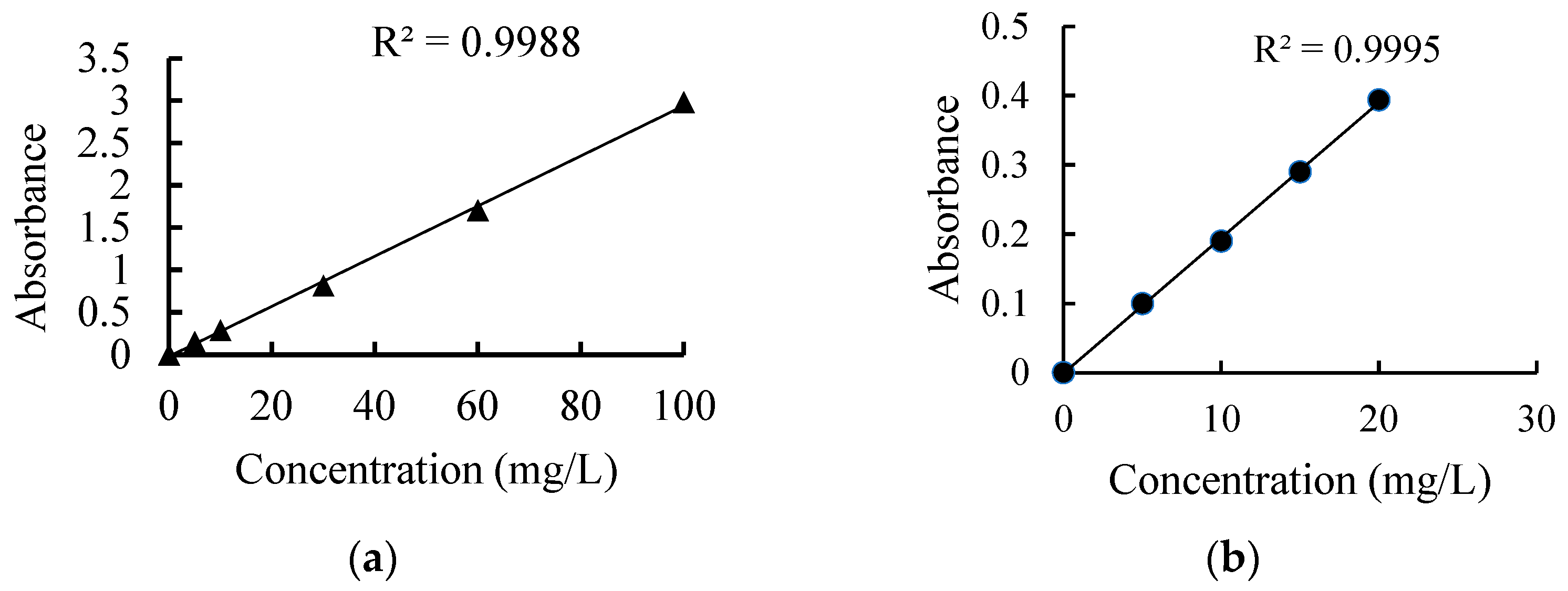


| CBN | pH | Primary Size (nm) | SSA (m2/g) | DH (nm) | UE (μm·cm/(V·s)) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| CB | 6.5 | 47.35 | 635.96 | 537 ± 28 | −0.80 ± 0.09 | −10.20 ± 1.22 |
| MCB | 6.5 | 44.15 | 603.38 | 337 ± 13 | −3.01 ± 0.33 | −38.33 ± 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-F.; Xu, K.; Li, X.-R.; Liu, Y.-X.; Cheng, J.-M. Damage Effect of Amorphous Carbon Black Nanoparticle Aggregates on Model Phospholipid Membranes: Surface Charge, Exposure Concentration and Time Dependence. Int. J. Environ. Res. Public Health 2023, 20, 2999. https://doi.org/10.3390/ijerph20042999
Wang X-F, Xu K, Li X-R, Liu Y-X, Cheng J-M. Damage Effect of Amorphous Carbon Black Nanoparticle Aggregates on Model Phospholipid Membranes: Surface Charge, Exposure Concentration and Time Dependence. International Journal of Environmental Research and Public Health. 2023; 20(4):2999. https://doi.org/10.3390/ijerph20042999
Chicago/Turabian StyleWang, Xiao-Feng, Kun Xu, Xin-Rui Li, Ya-Xin Liu, and Jie-Min Cheng. 2023. "Damage Effect of Amorphous Carbon Black Nanoparticle Aggregates on Model Phospholipid Membranes: Surface Charge, Exposure Concentration and Time Dependence" International Journal of Environmental Research and Public Health 20, no. 4: 2999. https://doi.org/10.3390/ijerph20042999




