Developmental Trajectories and Predictors of Incident Dementia among Elderly Taiwanese People: A 14-Year Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Population
2.3. Identification of Dementia
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Developmental Trajectories and Its Baseline Demographic Characteristics
3.2. Factors Associated with the Trajectory Groups
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javaid, S.F.; Giebel, C.; Khan, M.A.; Hashim, M.J. Epidemiology of Alzheimer’s disease and other dementias: Rising global burden and forecasted trends. F1000Research 2021, 10, 425. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.; Nichols, E.; Abd-Allah, F.; Abdoli, A.; Abosetugn, A.E.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.; Akinyemi, R.O.; Alahdab, F. Global mortality from dementia: Application of a new method and results from the Global Burden of Disease Study 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12200. [Google Scholar]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef]
- Olivari, B.S.; French, M.E.; McGuire, L.C. The public health road map to respond to the growing dementia crisis. Innov. Aging 2020, 4, igz043. [Google Scholar] [CrossRef] [PubMed]
- Clair, L.; Anderson, H.; Anderson, C.; Ekuma, O.; Prior, H.J. Cardiovascular disease and the risk of dementia: A survival analysis using administrative data from Manitoba. Can. J. Public Health Rev. Can. De Sante Publique 2022, 113, 455–464. [Google Scholar] [CrossRef]
- Juul Rasmussen, I.; Rasmussen, K.L.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Impact of cardiovascular risk factors and genetics on 10-year absolute risk of dementia: Risk charts for targeted prevention. Eur. Heart J. 2020, 41, 4024–4033. [Google Scholar] [CrossRef]
- Skogen, J.C.; Bergh, S.; Stewart, R.; Knudsen, A.K.; Bjerkeset, O. Midlife mental distress and risk for dementia up to 27 years later: The Nord-Trøndelag Health Study (HUNT) in linkage with a dementia registry in Norway. BMC Geriatr. 2015, 15, 23. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef]
- James, B.D.; Bennett, D.A. Causes and Patterns of Dementia: An Update in the Era of Redefining Alzheimer’s Disease. Annu. Rev. Public Health 2019, 40, 65–84. [Google Scholar] [CrossRef]
- Xiang, X.; Lai, P.H.L.; Bao, L.; Sun, Y.; Chen, J.; Dunkle, R.E.; Maust, D. Dual Trajectories of Social Isolation and Dementia in Older Adults: A Population-Based Longitudinal Study. J. Aging Health 2021, 33, 63–74. [Google Scholar] [CrossRef]
- Edwin, T.H.; Strand, B.H.; Persson, K.; Engedal, K.; Selbæk, G.; Knapskog, A.B. Trajectories and risk factors of dementia progression: A memory clinic cohort followed up to 3 years from diagnosis. Int. Psychogeriatr. IPA 2021, 33, 779–789. [Google Scholar] [CrossRef]
- Ferraro, O.E.; Guaita, A.; Villani, S. Cognitive, physical and disability trajectories in community-dwelling elderly people. Aging Clin. Exp. Res. 2021, 33, 2671–2677. [Google Scholar] [CrossRef]
- Ding, X.; Charnigo, R.J.; Schmitt, F.A.; Kryscio, R.J.; Abner, E.L. Evaluating trajectories of episodic memory in normal cognition and mild cognitive impairment: Results from ADNI. PLoS ONE 2019, 14, e0212435. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S.; Roeder, K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 2001, 29, 374–393. [Google Scholar] [CrossRef]
- Wu, Z.; Woods, R.L.; Wolfe, R.; Storey, E.; Chong, T.T.; Shah, R.C.; Orchard, S.G.; McNeil, J.J.; Murray, A.M.; Ryan, J. Trajectories of cognitive function in community-dwelling older adults: A longitudinal study of population heterogeneity. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12180. [Google Scholar] [CrossRef]
- Cheng, C.L.; Kao, Y.H.Y.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Chen, C.-H.; Li, C.-Y.; Lai, M.-L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J. Formos. Med. Assoc. 2015, 114, 254–259. [Google Scholar] [CrossRef]
- Lin, C.F.; Wu, F.L.; Lin, S.W.; Bai, C.H.; Chan, D.C.; Gau, C.S.; Hsiao, F.Y.; Shen, L.J. Age, dementia and care patterns after admission for acute coronary syndrome: An analysis from a nationwide cohort under the National Health Insurance coverage. Drugs Aging 2012, 29, 819–828. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Messinger-Rapport, B.J.; Sprecher, D. Prevention of cardiovascular diseases: Coronary artery disease, congestive heart failure, and stroke. Clin. Geriatr. Med. 2002, 18, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Tzourio, C.; Laurent, S.; Debette, S. Is hypertension associated with an accelerated aging of the brain? Hypertension 2014, 63, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.; Marseglia, A.; Shang, Y.; Grande, G.; Vetrano, D.L.; Laukka, E.J.; Fratiglioni, L.; Xu, W. Cardiometabolic multimorbidity accelerates cognitive decline and dementia progression. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Bahorik, A.L.; Hoang, T.D.; Forrester, S.; Jacobs, D.R.; Lewis, C.E.; Lloyd-Jones, D.M.; Sidney, S.; Reis, J.P. Cardiovascular risk factors and accelerated cognitive decline in midlife: The CARDIA Study. Neurology 2020, 95, e839–e846. [Google Scholar] [CrossRef]
- Lee, P.C.; Liu, L.L.; Sun, Y.; Chen, Y.A.; Liu, C.C.; Li, C.Y.; Yu, H.L.; Ritz, B. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ. Int. 2016, 96, 75–81. [Google Scholar] [CrossRef]
- Choi, D.; Choi, S.; Park, S.M. Effect of smoking cessation on the risk of dementia: A longitudinal study. Ann. Clin. Transl. Neurol. 2018, 5, 1192–1199. [Google Scholar] [CrossRef]
- Chen, T.-B.; Yiao, S.-Y.; Sun, Y.; Lee, H.-J.; Yang, S.-C.; Chiu, M.-J.; Chen, T.-F.; Lin, K.-N.; Tang, L.-Y.; Lin, C.-C. Comorbidity and dementia: A nationwide survey in Taiwan. PLoS ONE 2017, 12, e0175475. [Google Scholar] [CrossRef]
- Abbott, A. Dementia: A problem for our age. Nature 2011, 475, S2–S4. [Google Scholar] [CrossRef]
- Fan, Z.-Y.; Yang, Y.; Zhang, C.-H.; Yin, R.-Y.; Tang, L.; Zhang, F. Prevalence and patterns of comorbidity among middle-aged and elderly people in China: A cross-sectional study based on CHARLS data. Int. J. Gen. Med. 2021, 14, 1449. [Google Scholar] [CrossRef]
- Zuo, W.; Wu, J. The interaction and pathogenesis between cognitive impairment and common cardiovascular diseases in the elderly. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063020. [Google Scholar] [CrossRef]
- Obisesan, T.O.; Gillum, R.F.; Johnson, S.; Umar, N.; Williams, D.; Bond, V.; Kwagyan, J. Neuroprotection and neurodegeneration in Alzheimer’s disease: Role of cardiovascular disease risk factors, implications for dementia rates, and prevention with aerobic exercise in African Americans. Int. J. Alzheimer’s Disease 2012, 2012, 568382. [Google Scholar] [CrossRef]
- Monnier, A.; Prigent-Tessier, A.; Quirié, A.; Bertrand, N.; Savary, S.; Gondcaille, C.; Garnier, P.; Demougeot, C.; Marie, C. Brain-derived neurotrophic factor of the cerebral microvasculature: A forgotten and nitric oxide-dependent contributor of brain-derived neurotrophic factor in the brain. Acta Physiol. 2017, 219, 790–802. [Google Scholar] [CrossRef]
- Marchant, N.L.; Reed, B.R.; DeCarli, C.S.; Madison, C.M.; Weiner, M.W.; Chui, H.C.; Jagust, W.J. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol. Aging 2012, 33, 1006.e25–1006.e36. [Google Scholar] [CrossRef]
- Qiu, C.; Fratiglioni, L. A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 2015, 12, 267–277. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling (nearly) two decades later. J. Quant. Criminol. 2010, 26, 445–453. [Google Scholar] [CrossRef]

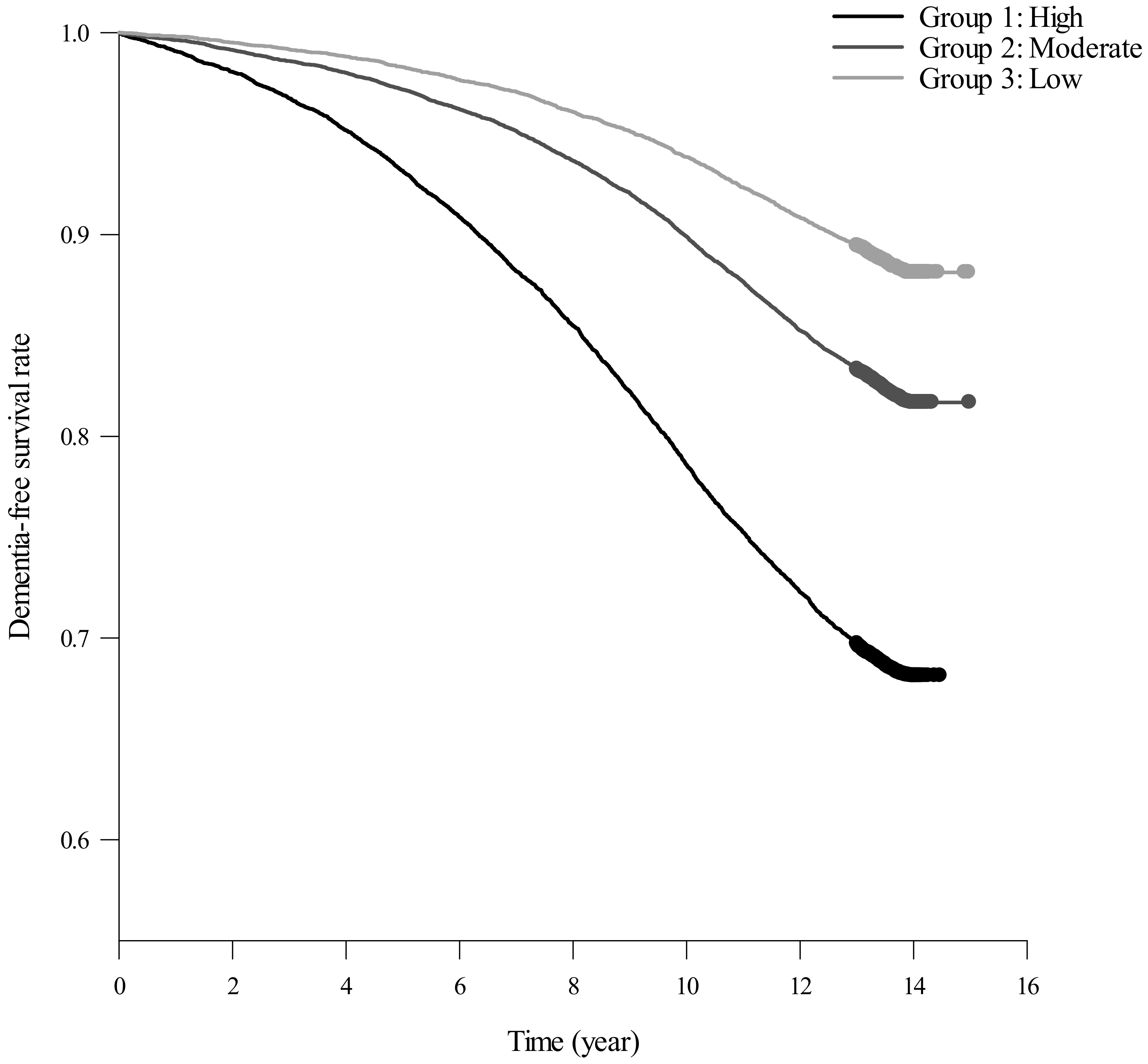
| Dementia Incidence by Trajectory Groups | p Value a | ||||
|---|---|---|---|---|---|
| Total (n = 42,407) | High (n = 11,637) | Moderate (n = 19,036) | Low (n = 11,734) | ||
| Age, mean (s.d.) | 70.99 (4.76) | 77.25 (3.31) | 70.17 (1.94) | 66.1 (1.05) | <0.001 |
| Sex, n (%) | <0.001 | ||||
| Man | 20,726 (48.87) | 4578 (39.3) | 8880 (46.6) | 7268 (61.9) | |
| Women | 21,681 (51.13) | 7059 (60.7) | 10,156 (53.4) | 4466 (38.1) | |
| Diabetes mellitus, n (%) | <0.001 | ||||
| Yes | 5441 (12.83) | 1439 (12.4) | 2608 (13.7) | 1394 (11.9) | |
| No | 36,966 (87.17) | 10,198 (87.6) | 16,428 (86.3) | 10,340 (88.1) | |
| Hypertension, n (%) | <0.001 | ||||
| Yes | 15,105 (35.62) | 4644 (39.9) | 6955 (36.5) | 3506 (29.9) | |
| No | 27,302 (64.38) | 6993 (60.1) | 12,081 (63.5) | 8228 (70.1) | |
| Hyperlipidemia, n (%) | 0.012 | ||||
| Yes | 4579 (10.8) | 1197 (10.3) | 2148 (11.3) | 1234 (10.5) | |
| No | 37,828 (89.2) | 10,440 (89.7) | 16,888 (88.7) | 10,500 (89.5) | |
| Stroke, n (%) | <0.001 | ||||
| Yes | 3373 (7.95) | 1172 (10.1) | 1478 (7.8) | 723 (6.2) | |
| No | 39,034 (92.05) | 10,465 (89.9) | 17,558 (92.2) | 11,011 (93.8) | |
| Coronary heart disease, n (%) | <0.001 | ||||
| Yes | 6065 (14.3) | 1958 (16.8) | 2766 (14.5) | 1341 (11.4) | |
| No | 36,342 (85.7) | 9679 (83.2) | 16,270 (85.5) | 10,393 (88.6) | |
| Kidney disease, n (%) | 0.004 | ||||
| Yes | 1624 (3.83) | 465 (4) | 768 (4) | 391 (3.3) | |
| No | 40,783 (96.17) | 11,172 (96) | 18,268 (96) | 11,343 (96.7) | |
| Atrial fibrillation, n (%) | 0.005 | ||||
| Yes | 349 (0.82) | 122 (1) | 146 (0.8) | 81 (0.7) | |
| No | 42,058 (99.18) | 11,515 (99) | 18,890 (99.2) | 11,653 (99.3) | |
| Depression, n (%) | 0.387 | ||||
| Yes | 835 (1.97) | 246 (2.1) | 369 (1.9) | 220 (1.9) | |
| No | 41,572 (98.03) | 11,391 (97.9) | 18,667 (98.1) | 11,514 (98.1) | |
| Anxiety, n (%) | 0.009 | ||||
| Yes | 1568 (3.7) | 465 (4) | 720 (3.8) | 383 (3.3) | |
| No | 40,839 (96.3) | 11,172 (96) | 18,316 (96.2) | 11,351 (96.7) | |
| Heart failure, n (%) | <0.001 | ||||
| Yes | 1078 (2.54) | 412 (3.5) | 462 (2.4) | 204 (1.7) | |
| No | 41,329 (97.46) | 11,225 (96.5) | 18,574 (97.6) | 11,530 (98.3) | |
| Alcoholism, n (%) | 0.462 | ||||
| Yes | 19 (0.04) | 3 (0) | 9 (0) | 7 (0.1) | |
| No | 42,388 (99.96) | 11,634 (100) | 19,027 (100) | 11,727 (99.9) | |
| COPD, n (%) | <0.001 | ||||
| Yes | 6882 (16.23) | 2017 (17.3) | 3178 (16.7) | 1687 (14.4) | |
| No | 35,525 (83.77) | 9620 (82.7) | 15,858 (83.3) | 10,047 (85.6) | |
| Obesity, n (%) | 0.024 | ||||
| Yes | 41 (0.1) | 8 (0.1) | 27 (0.1) | 6 (0.1) | |
| No | 42,366 (99.9) | 11,629 (99.9) | 19,009 (99.9) | 11,728 (99.9) | |
| Baseline Characteristics | High Group vs. Low Group | Moderate Group vs. Low Group | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value a | OR | 95% CI | p Value a | |
| Diabetes mellitus | 0.93 | 0.86–1.01 | 0.086 | 1.08 | 1.00–1.16 | 0.039 |
| Hypertension | 1.43 | 1.35–1.52 | <0.001 | 1.27 | 1.20–1.34 | <0.001 |
| Hyperlipidemia | 0.81 | 0.74–0.89 | <0.001 | 0.94 | 0.86–1.01 | 0.097 |
| Stroke | 1.45 | 1.31–1.60 | <0.001 | 1.14 | 1.03–1.25 | 0.008 |
| Coronary heart disease | 1.29 | 1.19–1.39 | <0.001 | 1.16 | 1.07–1.24 | <0.001 |
| Kidney disease | 1.07 | 0.93–1.23 | 0.349 | 1.11 | 0.98–1.26 | 0.103 |
| Atrial fibrillation | 1.07 | 0.80–1.43 | 0.653 | 0.92 | 0.70–1.21 | 0.552 |
| Depression | 0.97 | 0.81–1.17 | 0.756 | 0.95 | 0.80–1.12 | 0.526 |
| Anxiety | 1.05 | 0.92–1.21 | 0.461 | 1.06 | 0.93–1.20 | 0.380 |
| Heart failure | 1.62 | 1.36–1.93 | <0.001 | 1.19 | 1.00–1.41 | 0.048 |
| Alcoholism | 0.32 | 0.08–1.27 | 0.105 | 0.66 | 0.25–1.79 | 0.416 |
| COPD | 1.10 | 1.02–1.18 | 0.009 | 1.11 | 1.04–1.19 | 0.002 |
| Obesity | 1.23 | 0.42–3.56 | 0.705 | 2.50 | 1.03–6.06 | 0.043 |
| Baseline Characteristics | HR | 95% CI | p Value a |
|---|---|---|---|
| Age | 1.08 | 1.08–1.09 | <0.001 |
| Sex (Female) | 1.20 | 1.15–1.25 | <0.001 |
| Diabetes mellitus | 1.26 | 1.18–1.34 | <0.001 |
| Hypertension | 1.01 | 0.96–1.06 | 0.668 |
| Hyperlipidemia | 1.00 | 0.94–1.07 | 0.950 |
| Stroke | 1.61 | 1.51–1.72 | <0.001 |
| CHD | 1.13 | 1.06–1.20 | <0.001 |
| Kidney disease | 1.17 | 1.06–1.30 | 0.002 |
| Atrial fibrillation | 1.05 | 0.85–1.30 | 0.671 |
| Depression | 1.96 | 1.75–2.20 | <0.001 |
| Anxiety | 1.17 | 1.06–1.29 | 0.002 |
| Heart failure | 0.94 | 0.83–1.07 | 0.362 |
| Alcoholism | 2.44 | 1.22–4.88 | 0.012 |
| COPD | 1.08 | 1.02–1.14 | 0.010 |
| Obesity | 1.55 | 0.88–2.74 | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-C.; Lin, S.-F.; Chou, C.-C.; Bai, C.-H. Developmental Trajectories and Predictors of Incident Dementia among Elderly Taiwanese People: A 14-Year Longitudinal Study. Int. J. Environ. Res. Public Health 2023, 20, 3065. https://doi.org/10.3390/ijerph20043065
Fan Y-C, Lin S-F, Chou C-C, Bai C-H. Developmental Trajectories and Predictors of Incident Dementia among Elderly Taiwanese People: A 14-Year Longitudinal Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3065. https://doi.org/10.3390/ijerph20043065
Chicago/Turabian StyleFan, Yen-Chun, Sheng-Feng Lin, Chia-Chi Chou, and Chyi-Huey Bai. 2023. "Developmental Trajectories and Predictors of Incident Dementia among Elderly Taiwanese People: A 14-Year Longitudinal Study" International Journal of Environmental Research and Public Health 20, no. 4: 3065. https://doi.org/10.3390/ijerph20043065
APA StyleFan, Y.-C., Lin, S.-F., Chou, C.-C., & Bai, C.-H. (2023). Developmental Trajectories and Predictors of Incident Dementia among Elderly Taiwanese People: A 14-Year Longitudinal Study. International Journal of Environmental Research and Public Health, 20(4), 3065. https://doi.org/10.3390/ijerph20043065






