Effects of Non-Pharmacological Sleep Interventions in Older Adults: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Meta-Analysis
2.7. Ethical Considerations
3. Results
3.1. Literature Selection
3.2. Studies and Interventions
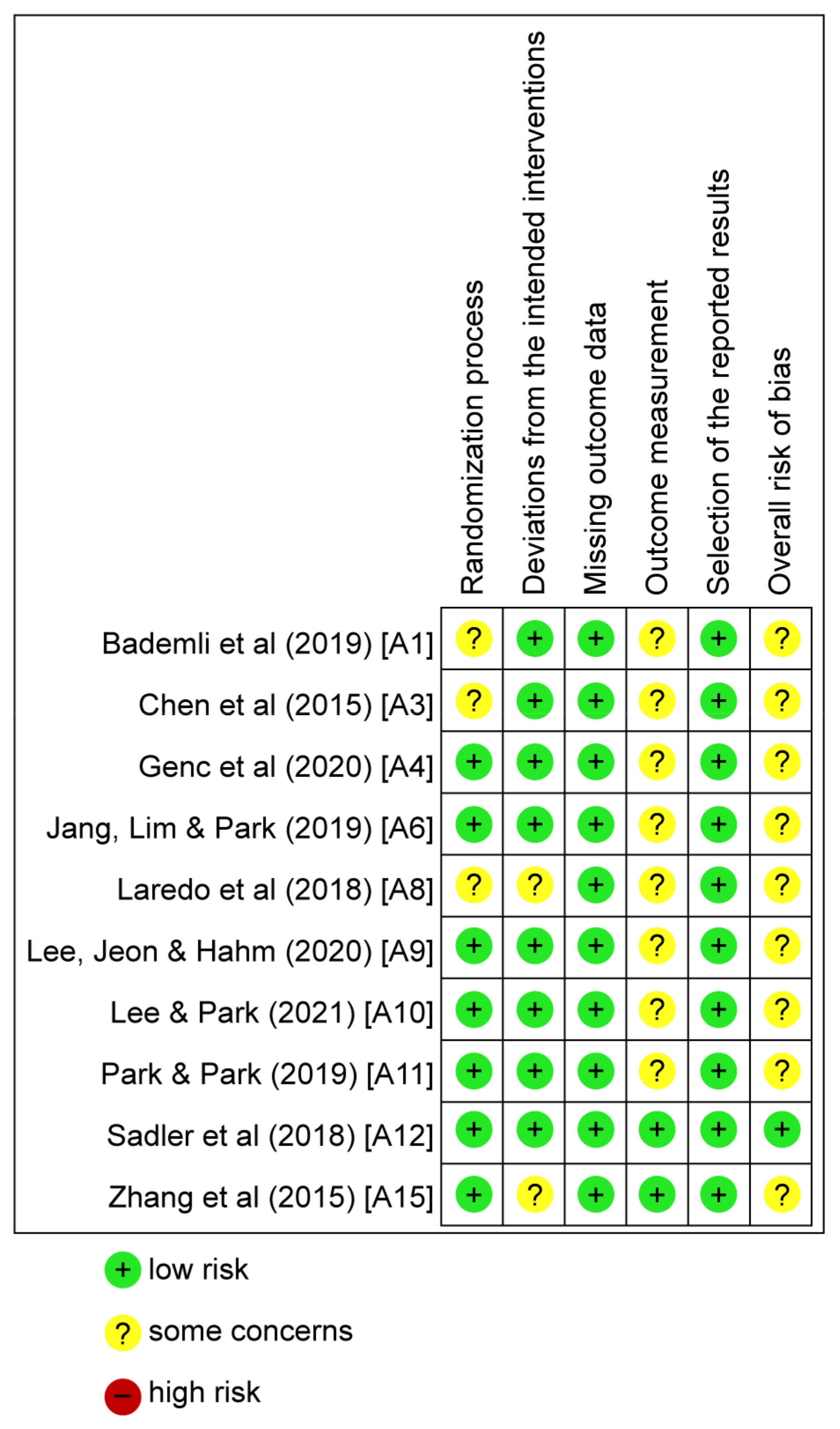
3.3. Literature Quality
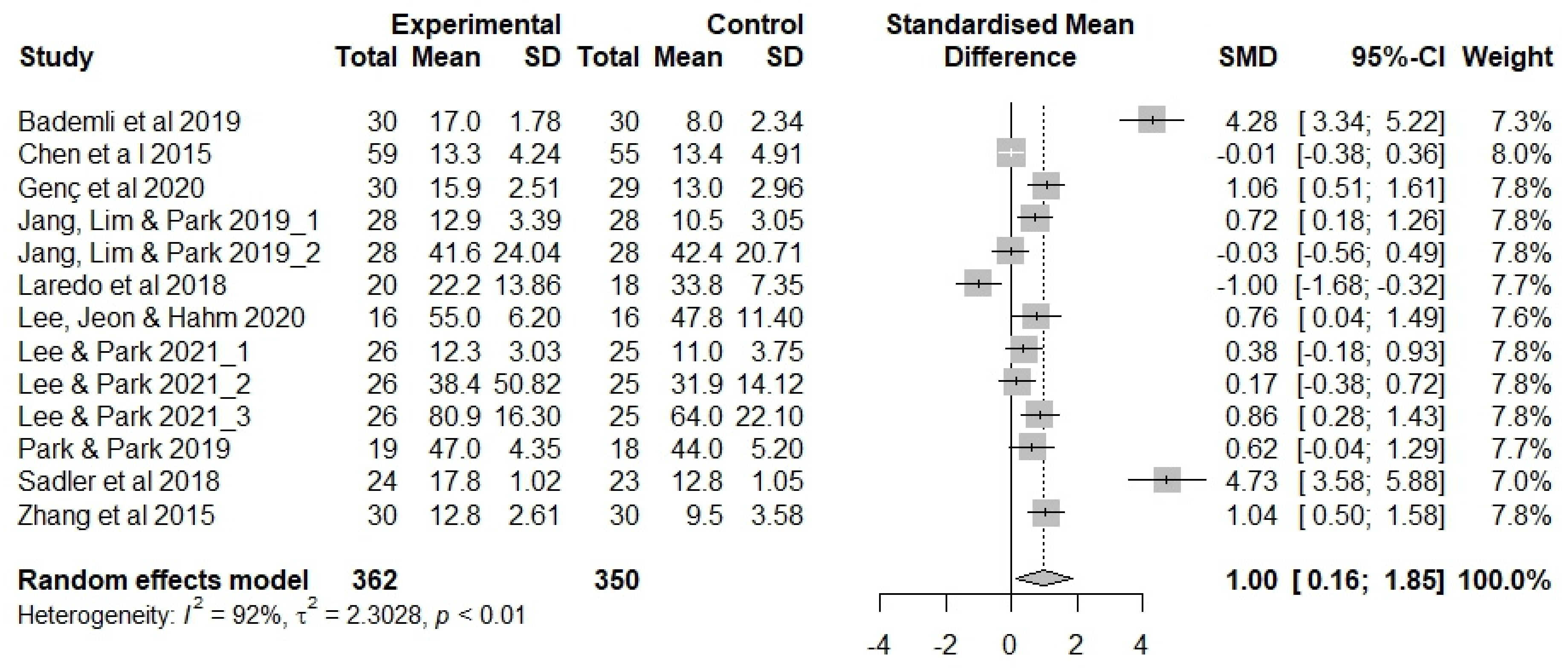
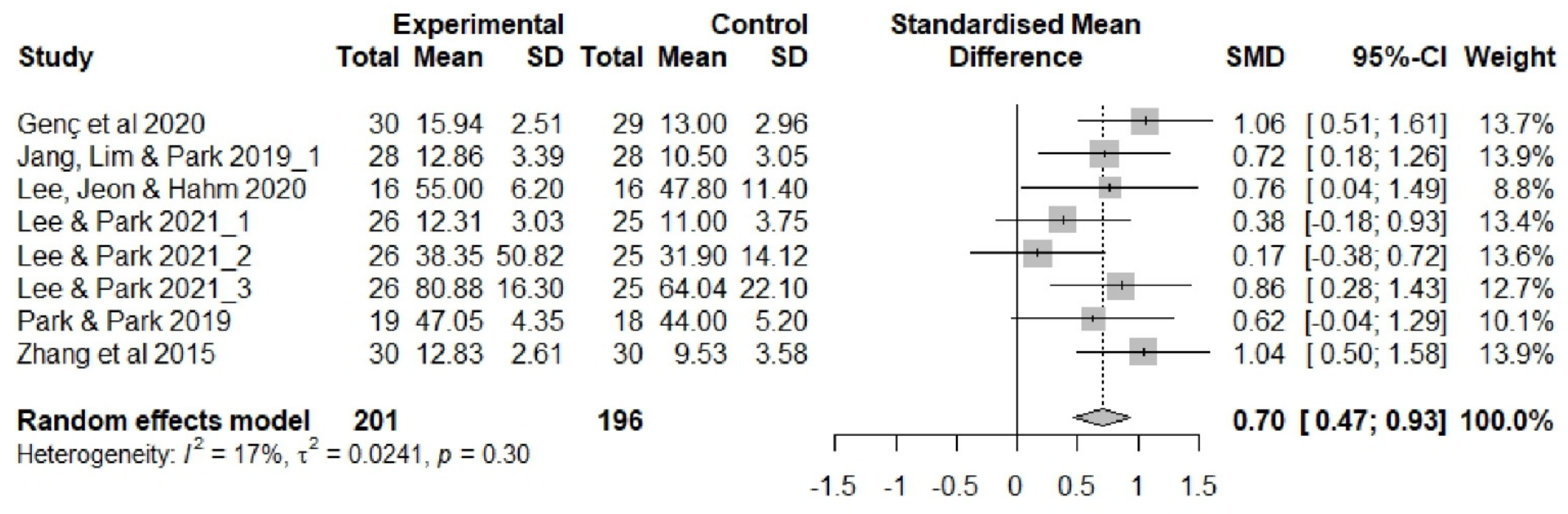
3.4. Effectiveness of Sleep Interventions
3.5. Analysis of the Moderating Effect
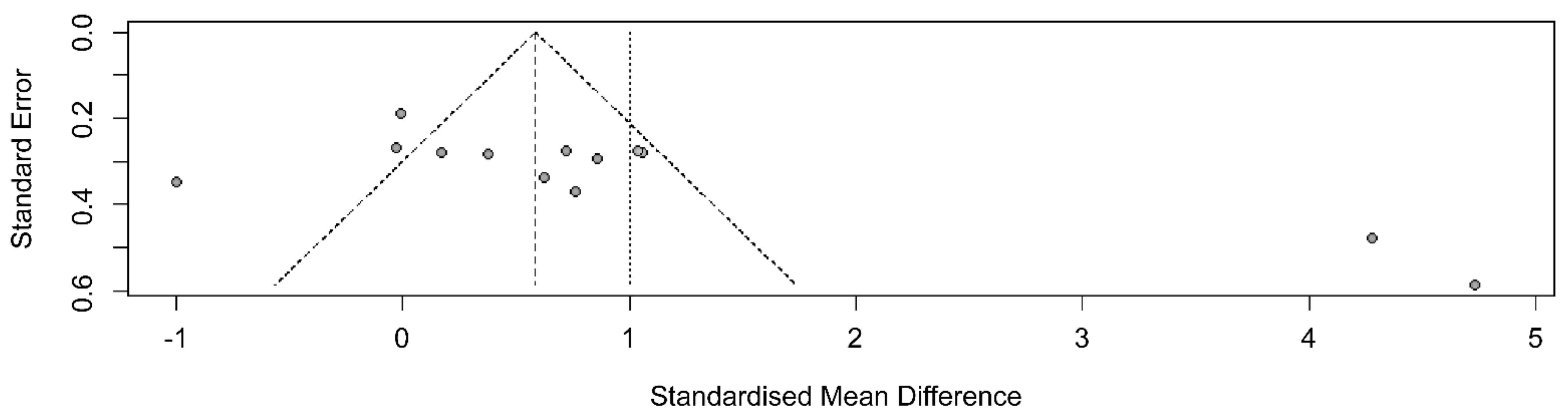
3.6. Publication Bias
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, G.B.; Lim, F.D. The Impact of Nonpharmacological Interventions on Sleep Quality among Older Adult Patients in the Intensive Care Unit. Crit. Care Nurs. Q. 2021, 44, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Voiss, P.; Höxtermann, M.D.; Dobos, G.; Cramer, H. Cancer, sleep problems, and mind-body medicine use: Results of the 2017 National Health Interview Survey. Cancer 2019, 125, 4490–4497. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.; Zhuang, J.-H.; Xu, J.; Li, P.; Peng, H. The effects of different meditation exercises on sleep quality in older people: A network meta-analysis. Eur. Geriatr. Med. 2019, 10, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Valle, V.I.-D.; Silva, J.; Castelló-Domenech, A.-B.; Martinez-Martinez, M.; Verdejo, Y.; Sanantonio-Camps, L.; Cauli, O. Subjective and objective sleep quality in elderly individuals: The role of psychogeriatric evaluation. Arch. Gerontol. Geriatr. 2018, 76, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, T.A.; Kitiş, Y. The Effect of Aromatherapy Application on Cognitive Functions and Daytime Sleepiness in Older Adults Living in a Nursing Home. Holist. Nurs. Pract. 2020, 34, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Manousakis, J.E.; Nicholas, C.; Scovelle, A.J.; Naismith, S.L.; Anderson, C. Associations between sleep and verbal memory in subjective cognitive decline: A role for semantic clustering. Neurobiol. Learn. Mem. 2019, 166, 107086. [Google Scholar] [CrossRef] [PubMed]
- Romanella, S.M.; Roe, D.; Tatti, E.; Cappon, D.; Paciorek, R.; Testani, E.; Rossi, A.; Rossi, S.; Santarnecchi, E. The Sleep Side of Aging and Alzheimer’s Disease. Sleep Med. 2021, 77, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Lunsford-Avery, J.R.; Engelhard, M.M.; Navar, A.M.; Kollins, S.H. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Sci. Rep. 2018, 8, 14158. [Google Scholar] [CrossRef] [PubMed]
- Blackman, J.; Swirski, M.; Clynes, J.; Harding, S.; Leng, Y.; Coulthard, E. Pharmacological and non-pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer’s disease: A systematic review. J. Sleep Res. 2021, 30, e13229. [Google Scholar] [CrossRef]
- Snow, A.L.; Loup, J.; Morgan, R.O.; Richards, K.; Parmelee, P.A.; Baier, R.R.; McCreedy, E.; Frank, B.; Brady, C.; Fry, L.; et al. Enhancing sleep quality for nursing home residents with dementia: A pragmatic randomized controlled trial of an evidence-based frontline huddling program. BMC Geriatr. 2021, 21, 281. [Google Scholar] [CrossRef] [PubMed]
- Ball, E.L.; Owen-Booth, B.; Gray, A.; Shenkin, S.D.; Hewitt, J.; McCleery, J. Aromatherapy for dementia. Cochrane Database Syst. Rev. 2020, 8, CD003150. [Google Scholar] [CrossRef] [PubMed]
- Aibar-Almazán, A.; Hita-Contreras, F.; Cruz-Díaz, D.; de la Torre-Cruz, M.; Jiménez-García, J.D.; Martínez-Amat, A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: A randomized controlled trial. Maturitas 2019, 124, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Taraghi, Z.; Khalili, Z.; Ilali, E.; Mousavinasab, N. Comparison of the effect of aromatherapy with essential of Damask Rose and Citrus aurantium on the sleep quality of the elderly people. J. Nurs. Midwifery Sci. 2021, 8, 9–14. [Google Scholar] [CrossRef]
- Jang, M.J.; Lim, Y.-M.; Park, H.J. Effects of Auricular Acupressure on Joint Pain, Range of Motion, and Sleep in the Elderly with Knee Osteoarthritis. J. Korean Acad. Community Health Nurs. 2019, 30, 79–89. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.J. The Effect of Auricular Acupressure on Sleep in Older Adults with Sleep Disorders. J. Korean Gerontol. Nurs. 2021, 23, 117–128. [Google Scholar] [CrossRef]
- Lovato, N.; Micic, G.; Lack, L. Sleep misestimation among older adults suffering from insomnia with short and normal objective sleep duration and the effects of cognitive behavior therapy. Sleep 2021, 44, zsaa250. [Google Scholar] [CrossRef]
- Vaughan, C.P.; Markland, A.D.; Huang, A.J.; Alessi, C.A.; Guzman, A.; Martin, J.L.; Bliwise, D.L.; Johnson, T.M.; Burgio, K.L.; Fung, C.H. Considerations for integrated cognitive behavioural treatment for older adults with coexisting nocturia and insomnia. Age Ageing 2022, 51, afac024. [Google Scholar] [CrossRef]
- Huberty, J.; Puzia, M.E.; Larkey, L.; Vranceanu, A.-M.; Irwin, M.R. Can a meditation app help my sleep? A cross-sectional survey of Calm users. PLoS ONE 2021, 16, e0257518. [Google Scholar] [CrossRef]
- Wahbeh, H.; Nelson, M. iRest Meditation for Older Adults with Depression Symptoms: A Pilot Study. Int. J. Yoga Ther. 2019, 29, 9–17. [Google Scholar] [CrossRef]
- Ferster, M.L.; Da Poian, G.; Menachery, K.; Schreiner, S.J.; Lustenberger, C.; Maric, A.; Huber, R.; Baumann, C.R.; Karlen, W. Benchmarking Real-Time Algorithms for In-Phase Auditory Stimulation of Low Amplitude Slow Waves with Wearable EEG Devices during Sleep. IEEE Trans. Biomed. Eng. 2022, 69, 2916–2925. [Google Scholar] [CrossRef]
- Voiß, P.; Höxtermann, M.D.; Dobos, G.; Cramer, H. The use of mind-body medicine among US individuals with sleep problems: Analysis of the 2017 National Health Interview Survey data. Sleep Med. 2019, 56, 151–156. [Google Scholar] [CrossRef]
- Statistics Korea. Dependency Ratio and Aging Index. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_2KAA202&vw_cd=MT_RTITLE&list_id=101_003_A&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=K1&path (accessed on 5 July 2022).
- Faydalı, S.; Çetinkaya, F. The Effect of Aromatherapy on Sleep Quality of Elderly People Residing in a Nursing Home. Holist. Nurs. Pract. 2018, 32, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Yun, H.J. Effects of Non-Pharmacological Interventions to Improve the Sleep of Korean Elderly: A Systematic Review. J. Korean Acad. Fundam. Nurs. 2022, 29, 67–83. [Google Scholar] [CrossRef]
- Her, J.; Cho, M.-K. Effect of aromatherapy on sleep quality of adults and elderly people: A systematic literature review and meta-analysis. Complement. Ther. Med. 2021, 60, 102739. [Google Scholar] [CrossRef]
- Roozbeh, N.; Ghazanfarpour, M.; Khadivzadeh, T.; Kargarfard, L.; Dizavandi, F.R.; Shariati, K. Effect of Lavender on Sleep, Sexual Desire, Vasomotor, Psychological and Physical Symptom among Menopausal and Elderly Women: A Systematic Review. J. Menopausal Med. 2019, 25, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, J.; Boen, F.; Van Uffelen, J.G.Z. Effects of physical activity programs on sleep outcomes in older adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; He, W.-B.; Gao, Y.-Y.; Han, X.-M. Effects of traditional Chinese exercises and general aerobic exercises on older adults with sleep disorders: A systematic review and meta-analysis. J. Integr. Med. 2021, 19, 493–502. [Google Scholar] [CrossRef]
- Weber, M.; Schnorr, T.; Morat, M.; Morat, T.; Donath, L. Effects of Mind–Body Interventions Involving Meditative Movements on Quality of Life, Depressive Symptoms, Fear of Falling and Sleep Quality in Older Adults: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6556. [Google Scholar] [CrossRef]
- Jang, M.J.; Park, H.J. The effects of auricular acupressure on sleep disorder in the elderly: A systematic review and meta-analysis. J. Korea Acad. Ind. Coop. Soc. 2020, 21, 116–126. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chung, B.-Y.; Park, H.-S. Effects of non-pharmacological interventions for adults with insomnia in Korea: A meta-analysis. J. Korea Acad. Ind. Coop. Soc. 2017, 18, 95–106. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G. A systematic review of workplace interventions to prevent low back pain. Aust. J. Physiother. 2000, 46, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. Theory and Practice of Meta-Analysis. J. Rhinol. 2020, 27, 83–89. [Google Scholar] [CrossRef]
- Bademli, K.; Lok, N.; Canbaz, M.; Lok, S. Effects of Physical Activity Program on cognitive function and sleep quality in elderly with mild cognitive impairment: A randomized controlled trial. Perspect. Psychiatr. Care 2019, 55, 401–408. [Google Scholar] [CrossRef]
- Cassidy-Eagle, E.; Siebern, A.; Unti, L.; Glassman, J.; O’Hara, R. Neuropsychological Functioning in Older Adults with Mild Cognitive Impairment and Insomnia Randomized to CBT-I or Control Group. Clin. Gerontol. 2018, 41, 136–144. [Google Scholar] [CrossRef]
- Chen, K.-M.; Huang, H.-T.; Cheng, Y.-Y.; Li, C.-H.; Chang, Y.-H. Sleep quality and depression of nursing home older adults in wheelchairs after exercises. Nurs. Outlook 2015, 63, 357–365. [Google Scholar] [CrossRef]
- Genç, F.P.; Karadağ, S.P.; Akça, N.P.K.; Tan, M.P.; Cerit, D. The Effect of Aromatherapy on Sleep Quality and Fatigue Level of the Elderly: A randomized controlled study. Holist. Nurs. Pract. 2020, 34, 155–162. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Chen, K.-M.; Tsai, H.-Y.; Huang, H.-T.; Cheng, Y.-Y.; Tsai, A.Y. Self-Perceived Health and Sleep Quality of Community Older Adults after Acupunch Exercises. Am. J. Geriatr. Psychiatry 2018, 26, 511–520. [Google Scholar] [CrossRef]
- Kouzuki, M.; Kitao, S.; Kaju, T.; Urakami, K. Evaluation of the effect of aroma oil as a bath salt on cognitive function. Psychogeriatrics 2020, 20, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Laredo-Aguilera, J.A.; Carmona-Torres, J.M.; García-Pinillos, F.; Latorre-Román, P.Á. Effects of a 10-week functional training programme on pain, mood state, depression, and sleep in healthy older adults. Psychogeriatrics 2018, 18, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Jeon, J.J.; Hahm, S.C. A comparison of the effects of barefoot walking and sneakers walking on a sandy beach on pain, disability, motor function, sleep satisfaction, and quality of life in the elderly with low back pain. J. Korean. Soc. Integr. Med. 2020, 8, 29–38. [Google Scholar] [CrossRef]
- Park, M.J.; Park, H.O. Is Hand Massage with the Preferred Aroma Oil better than Lavender on Stress and Sleep for Long-term Care Facility Residents? Korean J. Adult Nurs. 2019, 31, 156–164. [Google Scholar] [CrossRef]
- Sadler, P.; McLaren, S.; Klein, B.; Harvey, J.; Jenkins, M. Cognitive behavior therapy for older adults with insomnia and depression: A randomized controlled trial in community mental health services. Sleep 2018, 41, zsy104. [Google Scholar] [CrossRef]
- Takamoto, K.; Saitoh, T.; Taguchi, T.; Nishimaru, H.; Urakawa, S.; Sakai, S.; Ono, T.; Nishijo, H. Lip closure training improves eating behaviors and prefrontal cortical hemodynamic activity and decreases daytime sleep in elderly persons. J. Bodyw. Mov. Ther. 2018, 22, 810–816. [Google Scholar] [CrossRef]
- Yagli, N.V.; Ulger, O. The effects of yoga on the quality of life and depression in elderly breast cancer patients. Complement. Ther. Clin. Pract. 2015, 21, 7–10. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Liu, X.-H.; Xie, X.-H.; Zhao, D.; Shan, M.-S.; Zhang, X.-L.; Kong, X.-M.; Cui, H. Mindfulness-Based Stress Reduction for Chronic Insomnia in Adults Older than 75 Years: A Randomized, Controlled, Single-Blind Clinical Trial. Explore 2015, 11, 180–185. [Google Scholar] [CrossRef]
- Statistics Korea. Statistics on the Elderly; Statistics Korea: Daejeon, Republic of Korea, 2021; pp. 1–81. [Google Scholar]
- OECD. Elderly Population (Indicator). 2023. Available online: https://doi.org/10.1787/8d805ea1-en (accessed on 27 January 2023). [CrossRef]
- Meikis, L.; Wicker, P.; Donath, L. Effects of Pilates Training on Physiological and Psychological Health Parameters in Healthy Older Adults and in Older Adults with Clinical Conditions Over 55 Years: A Meta-Analytical Review. Front. Neurol. 2021, 12, 724218. [Google Scholar] [CrossRef]
- Hwang, D.H.; Lee, M.N. Basic study on forest walk program for reduction of insomnia and depression in the elderly. Asia Pac. J. Multimed. Serv. Converg. Art Humanit. Sociol. 2020, 10, 117–126. [Google Scholar]
- Leung, L.Y.L.; Tam, H.L.; Ho, J.K.M. Effectiveness of Tai Chi on older adults: A systematic review of systematic reviews with re-meta-analysis. Arch. Gerontol. Geriatr. 2022, 103, 104796. [Google Scholar] [CrossRef] [PubMed]
- Chun, N.; Kim, M.; Noh, G.O. Effects of a Sleep Improvement Program Combined with Aroma-Necklace on Sleep, Depression, Anxiety and Blood Pressure in Elderly Women. J. Korean Acad. Nurs. 2017, 47, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chao, Y.-H.; Lu, S.-F.; Shiung, T.-F.; Chao, Y.-F. The effectiveness of valerian acupressure on the sleep of ICU patients: A randomized clinical trial. Int. J. Nurs. Stud. 2012, 49, 913–920. [Google Scholar] [CrossRef]
- Huberty, J.; Vranceanu, A.-M.; Carney, C.; Breus, M.; Gordon, M.; Puzia, M.E. Characteristics and Usage Patterns among 12,151 Paid Subscribers of the Calm Meditation App: Cross-Sectional Survey. JMIR mHealth uHealth 2019, 7, e15648. [Google Scholar] [CrossRef]
- Gunst, M.; De Meyere, I.; Willems, H.; Schoenmakers, B. Effect of exergaming on wellbeing of residents in a nursing home: A single blinded intervention study. Aging Clin. Exp. Res. 2022, 34, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Nanthakwang, N.; Siviroj, P.; Matanasarawoot, A.; Sapbamrer, R.; Lerttrakarnnon, P.; Awiphan, R. Effectiveness of Deep Breathing and Body Scan Meditation Combined with Music to Improve Sleep Quality and Quality of Life in Older Adults. Open Public Health J. 2020, 13, 232–239. [Google Scholar] [CrossRef]
- Rodrigo-Claverol, M.; Casanova-Gonzalvo, C.; Malla-Clua, B.; Rodrigo-Claverol, E.; Jové-Naval, J.; Ortega-Bravo, M. Animal-Assisted Intervention Improves Pain Perception in Polymedicated Geriatric Patients with Chronic Joint Pain: A Clinical Trial. Int. J. Environ. Res. Public Health 2019, 16, 2843. [Google Scholar] [CrossRef]
- Robbins, R.; Weaver, M.D.; Quan, S.F.; Sullivan, J.P.; Cohen-Zion, M.; Glasner, L.; Qadri, S.; Czeisler, C.A.; Barger, L.K. A clinical trial to evaluate the dayzz smartphone app on employee sleep, health, and productivity at a large US employer. PLoS ONE 2022, 17, e0260828. [Google Scholar] [CrossRef]
- Coombes, J.S.; Keating, S.E.; Mielke, G.I.; Fassett, R.G.; Coombes, B.K.; O’Leary, K.P.; Cox, E.R.; Burton, N.W. Personal Activity Intelligence e-Health Program in People with Type 2 Diabetes: A Pilot Randomized Controlled Trial. Med. Sci. Sports Exerc. 2022, 54, 18–27. [Google Scholar] [CrossRef]

| Author(s) (Year) (No.) | Country | Participants | Intervention | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (n or % by Sex) | Mean Age or Range | Characteristics | Type | Contents | No. of Sessions/ Time of Session/Duration | Setting | Control Group | Sleep Measure | Sleep Outcome | Other Measures | ||
| Bademli et al. (2019) [37] (A1) | Turkey | Exp.: 30 (M: 12/F: 18) Con.: 30 (M: 13/F: 17) | Exp.: 72.2 Con.: 70.7 | Older adults with mild cognitive impairment | Exercise | Physical activity program | Three, four, or seven days per week/80 min/20 weeks | Nursing home | N/A | PSQI | Effective | SMMSE |
| Cassidy-Eagle et al. (2018) [38] (A2) | USA | Exp.: 14 Con.: 13 (M < F) | Exp.: 89.4 Con.: 88.7 | Older adults with mild cognitive impairment and insomnia | Cognitive behavior therapy | Sleep hygiene, relaxation, sleep scheduling, and cognitive therapy | Six sessions/60 min/- | Two local independent and assisted living facilities | N/A | ISI sleep measure (ActiGraph, wGT3x) | Effective | HVLT-R, D-KEFS, ADL, IADL, MoCA |
| Chen et al. (2015) [39] (A3) | Taiwan | Exp.: 59 Con.: 55 (M: 50.9%/F: 49.1%) | Mean: 79.2 | Older adults in wheelchairs | Exercise | Wheelchair-bound senior elastic band exercise program | Three times per week/40 min/six months | Nursing home | N/A | Chinese PSQI | Effective | TDQ |
| Genç et al. (2020) [40] (A4) | Turkey | Exp.: 30 (M: 22/F: 8) Con.: 29 (M: 25/F: 4) | Exp.: 74.5 Con.: 72.0 | Older adults | Aromatherapy | Aroma inhalation (lavender oil) | Every day/-/one month | Nursing home | N/A | PSQI | Effective | FSS |
| Hsiao et al. (2018) [41] (A5) | Taiwan | Exp.: 113 (M: 20/F: 93) Con.: 119 (M: 30/F: 89) | Exp.: 74.7 Con.: 73.9 | Older adults | Exercise | Healthy Beat Acupunch exercises | Three times per week/40 min/12 months | Community care centers | N/A | PSQI | Effective | SF-12 |
| Jang, Lim, and Park (2019) [14] (A6) | Republic of Korea | Exp.: 28 (M: 0/F: 28) Con.: 28 (M: 2/F: 26) | Exp.: 79.2 Con.: 78.4 | Older adults with knee osteoarthritis | Acupressure | Auricular acupressure (Shen Men, knee liver, heart occiput) | Eight sessions/-/eight weeks | Elderly welfare facility | Auricular acupressure (Helix five-point) | Korean PSQI, wrist sleep device (Fitbit alta HR) | Effective | VAS, PPTs Korean WOMAC, knee flexion, knee extension |
| Kouzuki et al. (2020) [42] (A7) | Japan | 0.1%: 13 (M: 9/F: 4) 0.5%: 11 (M: 4/F: 7) 1.0%: 11 (M: 4/F: 7) | 0.1%: 76.0 0.5%: 78.0 1.0%: 82.0 | Older adults with Alzheimer’s disease, mild cognitive impairment | Aromatherapy | Bath salt containing an aroma component (0.1%) | Every day/≥ 10 min/24 weeks | Hospital outpatient clinic | Bath salt containing an aroma component (0.5%, 1.0%) | Japanese PSQI | Not effective | TDAS, OSIT-J |
| Laredo et al. (2018) [43] (A8) | Spain | Exp.: 20 (M: 22.2%/F: 77.8%) Con.: 18 (M: 28.5%/F: 71.5%) | Exp.: 75.4 Con.: 76.4 | Healthy older adults | Exercise | 10-week functional training program | Three times per week/60 min/10 weeks | Community in Loja, Granada | N/A | OSQ | Effective | VAS, PMS, GDS |
| Lee, Jeon, and Hahm (2020) [44] (A9) | Republic of Korea | Exp.: 16 (M: 1/F: 15) Con.: 16 (M: 1/F: 15) | Exp.: 76.6 Con.: 76.1 | Older adults with low back pain | Exercise | Barefoot walking | Three times per week/30 min/four weeks | Sandy beach in community | Sneaker walking | VSH | Effective | VAS, ODI, Berg balance scale, timed UP and Go test, Korean QoL |
| Lee and Park (2021) [15] (A10) | Republic of Korea | Exp.: 26 (M: 13/F: 13) Con.: 25 (M: 12/F: 13) | Exp.: 74.8 Con.: 72.6 | Older adults with sleep disorders | Acupressure | Auricular acupressure (Shen Men, heart, anterior occipital lobe) | Six sessions/-/six weeks | Elderly welfare facility | Auricular acupressure (helix four-point) | Korean PSQI, wrist sleep device (Fitbit Charge HR) blood melatonin levels | Effective | N/A |
| Park and Park (2019) [45] (A11) | Republic of Korea | Exp.: 19 (M: 4/F: 15) Con.: 18 (M: 4/F: 14) | Exp.: <75, n = 9; ≥75, n = 10 Con.: <75, n = 5; ≥75 n = 13 | Older adults long-term care facility residents | Aromatherapy | Hand massage with preferred aroma oil | Three times per week/5 min/four weeks | Long-term care facility | Hand massage with lavender oil | Korean VSH | Effective | PSQ |
| Sadler et al. (2018) [46] (A12) | Australia | CBT-I: 24 (M: 9/F: 15) CBT-I+: 25 (M: 12/F: 13) Con.: 23 (M: 10/F: 12/transgender: 1) | Exp.: 74.7 Con.: 72.3 | Older adults with insomnia and depression | Cognitive behavior therapy | Combination of educational, cognitive, and behavioral interventions | CBT-I (standard): eight sessions/60~90 min/eight weeks CBT-I + (advanced): eight sessions/75~90 min/eight weeks | Case managed by an aged persons’ community mental health service | N/A | ISI, CDS, DBAS-10, and SLEEP-50 scale | Effective | GDS, GAI-SF, BHS, EQ-5D-3L |
| Takamoto et al. (2018) [47] (A13) | Japan | Exp.: 10 (M: 2/F: 8) Con.: 10 (M: 0/F: 10) | Exp.: 87.3 Con: 85.3 | Older adults | Exercise | Lip closure training | Three times per day/3 min/four weeks | Elder care facility | N/A | Upper arm sleep device (ActiSleep Monitor) | Effective | LIP DE CUM, Everio GZ-MG275, NIRS |
| Yagli and Ulger (2015) [48] (A14) | Turkey | Exp.: 10 (F: 10) Con.: 10 (F: 10) | Exp.: 68.6 Con.: 68.9 | Older adults with breast cancer patients | Exercise | Yoga | Eight sessions/60 min/eight weeks | Department of Physiotherapy and Rehabilitation | Exercise program | VAS | Effective | Turkish-NHP, Turkish-BDI, VAS |
| Zhang et al. (2015) [49] (A15) | China | Exp.: 30 (M: 16/F: 14) Con.: 30 (M: 19/F: 11) | Exp.: 78.6 Con.: 77.6 | Older adults with chronic insomnia | Meditation | Mindfulness- based stress reduction | Every day/45 min/eight weeks | Medical Psychology Division of the general hospital | N/A | PSQI | Effective | SAS, GDS |
| PEDro Criterion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author(s) (Year) | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | Total | Quality |
| Bademli et al. (2019) [37] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Chen et al. (2015) [39] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Genç et al. (2020) [40] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Jang, Lim, and Park (2019) [14] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Laredo et al. (2018) [43] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | Good |
| Lee, Jeon, and Hahm (2020) [44] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Lee and Park (2021) [15] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 | Good |
| Park and Park (2019) [45] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Sadler et al. (2018) [46] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent |
| Zhang et al. (2015) [49] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent |
| Category | Subgroup | k | Hedge’s g | 95% CI | Z (p) | I2 | Q (p) | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| Sex | Male | 3 | 0.03 | −0.06 | 0.12 | 0.63 (0.530) | 0% | 0.60 (0.740) |
| Female | 10 | 0.09 | −0.29 | 0.47 | 0.46 (0.648) | |||
| Age | <75 | 6 | 0.09 | −0.05 | 0.21 | 1.17 (0.243) | 0% | 1.37 (0.505) |
| ≥75 | 7 | −0.00 | −0.12 | 0.11 | −0.06 (0.955) | |||
| Intervention type | Acupressure therapy | 5 | 0.04 | −0.08 | 0.17 | 0.68 (0.498) | 0% | 1.27 (0.945) |
| Aromatherapy | 2 | 0.02 | −0.19 | 0.22 | 0.16 (0.873) | |||
| Cognitive behavioral therapy | 1 | 0.17 | −0.39 | 0.74 | 0.61 (0.545) | |||
| Exercise | 4 | 0.00 | −0.16 | 0.16 | 0.02 (0.982) | |||
| Meditation | 1 | 0.22 | −0.506 | 0.94 | 0.59 (0.553) | |||
| Intervention period | 4 wks | 3 | 0.03 | −0.11 | 0.17 | 0.36 (0.717) | 0% | 1.14 (0.888) |
| 6 wks | 3 | 0.05 | −0.09 | 0.20 | 0.72 (0.470) | |||
| 8 wks | 4 | 0.06 | 0.16 | 0.28 | 0.51 (0.612) | |||
| ≥10wks | 3 | −0.07 | −0.36 | 0.22 | −0.48 (0.631) | |||
| Sleep measurement | Blood sample | 1 | 0.06 | −0.24 | 0.37 | 0.39 (0.695) | 0% | 3.53 (0.741) |
| Fitbit | 2 | 0.04 | −0.11 | 0.18 | 0.48 (0.634) | |||
| ISI | 1 | 0.17 | −0.39 | 0.74 | 0.61 (0.545) | |||
| Oviedo.S.Q | 1 | −0.21 | −0.56 | 0.13 | −1.21 (0.228) | |||
| PSQI | 6 | 0.16 | −0.12 | 0.43 | 1.12 (0.264) | |||
| VSH | 2 | 0.02 | −0.12 | 0.17 | 0.28 (0.777) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.-J.; Lee, O.-S. Effects of Non-Pharmacological Sleep Interventions in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3101. https://doi.org/10.3390/ijerph20043101
Gu H-J, Lee O-S. Effects of Non-Pharmacological Sleep Interventions in Older Adults: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(4):3101. https://doi.org/10.3390/ijerph20043101
Chicago/Turabian StyleGu, Hye-Ja, and Oi-Sun Lee. 2023. "Effects of Non-Pharmacological Sleep Interventions in Older Adults: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 4: 3101. https://doi.org/10.3390/ijerph20043101





