Influence of Agaricus bisporus Mushroom on Pb Toxicokinetic in Pregnant Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Pb Levels
2.3. Statistical Analysis
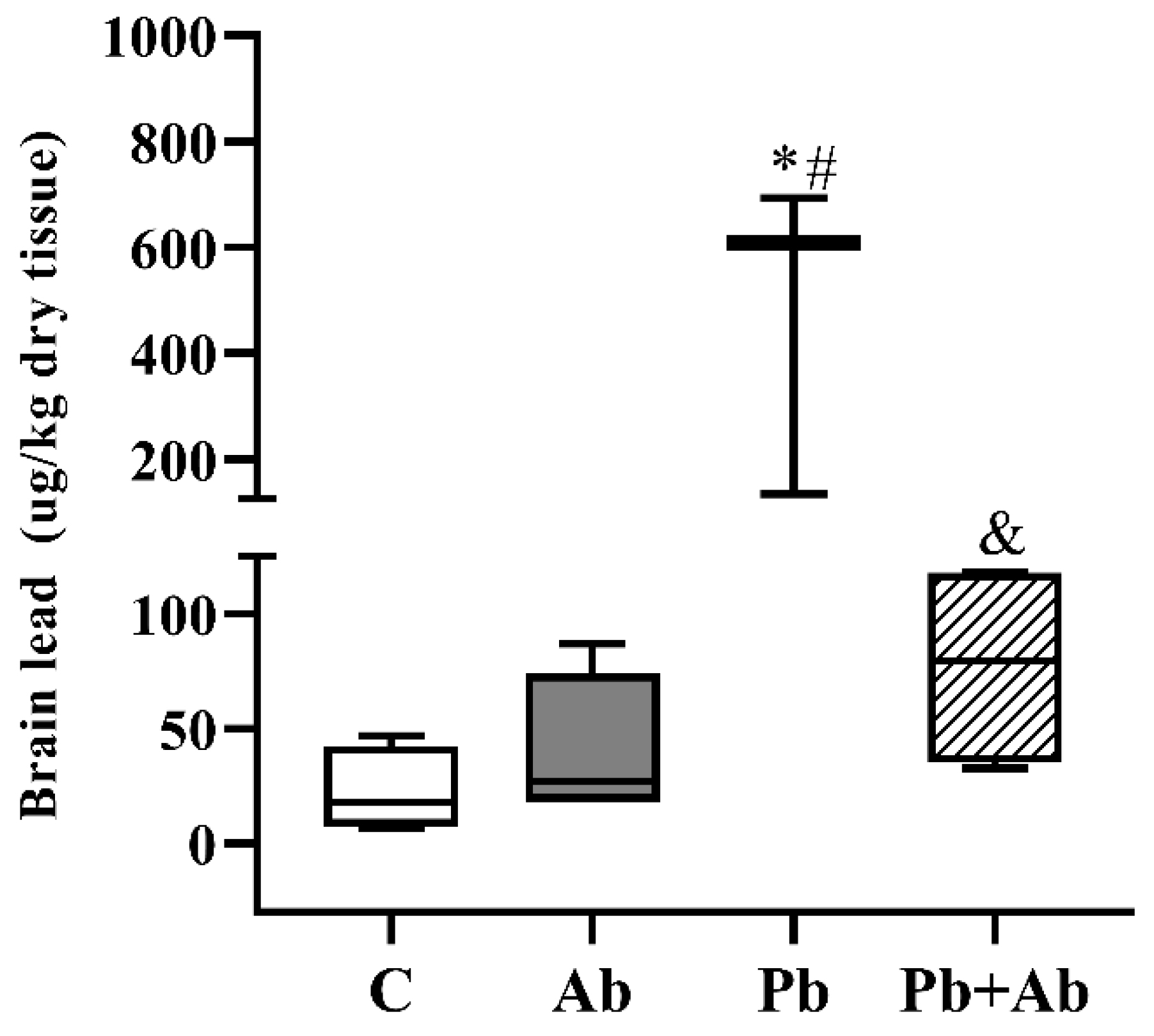
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Needleman, H. Lead poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, L.S.; Masood, S.; Anderson, D.W.; Schneider, J.S. The a ttention set-shifting test is sensitive for revealing sex-based impairments in executive functions following developmental lead exposure in rats. Behav. Brain Res. 2019, 366, 126–134. [Google Scholar] [CrossRef]
- US CDC Advisory Committee on Childhood Lead Poisoning Prevention. CDC Updates Blood Lead Reference Value to 3.5µg/dL. Atlanta: US Centres for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/nceh/lead/news/cdc-updates-blood-lead-reference-value.html (accessed on 9 January 2023).
- Al-Masri, S.A. Effect of pumpkin oil and vitamin E on lead induced testicular toxicity in male rats. J. Anim. Plant Sci. 2015, 25, 72–77. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Blood Lead Levels in Children. 2020. Available online: https://www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm (accessed on 21 December 2022).
- de Freitas, C.U.; De Capitani, E.M.; Gouveia, N.; Simonetti, M.H.; Silva, M.R.D.P.E.; Kira, C.S.; Sakuma, A.M.; Carvalho, M.D.F.H.; Duran, M.C.; Tiglea, P.; et al. Lead exposure in an urban community: Investigation of risk factors and assessment of the impact of lead abatement measures. Environ. Res. 2007, 103, 338–344. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Refaat, B.; Qasem, A.H.; Khan, A.; Ghaith, M.; Almasmoum, H.; Mahbub, A.; Almaimani, R.A. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 22736–22746. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Faulkner, K.A.; Greenspan, S.L.; Cauley, J.A. Osteoporotic Fractures in Men Research Group. Associations between bone mineral density, grip strength, and lead body burden in older men. J. Am. Geriatr. Soc. 2014, 62, 141–146. [Google Scholar] [CrossRef]
- Dongre, N.N.; Suryakar, A.N.; Patil, A.J.; Hundekari, I.A.; Devarnavadagi, B.B. Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian J. Clin. Biochem. 2013, 28, 65–70. [Google Scholar] [CrossRef]
- World Health Organization. ‘Lead Poisoning and Health’ Fact Sheet. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 9 January 2023).
- Wood, K.A.; Brown, M.J.; Cromie, R.L.; Hilton, G.M.; MacKenzie, C.; Newth, J.L.; Pain, D.J.; Perrins, C.M.; Rees, E.C. Regulation of lead fishing weights results in mute swan population recovery. Biol. Conserv. 2019, 230, 67–74. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Mazumdar, I.; Goswami, K. Congenital lead poisoning: An unusual presentation. Indian J. Clin. Biochem. 2014, 29, 257–259. [Google Scholar] [CrossRef]
- Andersen, O. Chemical and biological considerations in the treatment of metal intoxications by chelating agents. Mini-Rev. Med. Chem. 2004, 4, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef]
- Chong, P.S.; Khairuddin, S.; Tse, A.C.K.; Hiew, L.F.; Lau, C.L.; Tipoe, G.L.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Hericium erinaceus potentially rescues be-havioural motor deficits through ERK-CREB-PSD95 neuroprotective mechanisms in rat model of 3-acetylpyridine-induced cerebellar ataxia. Sci. Rep. 2020, 10, 14945. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Khan, R.; Shakir, H.A.; Tahir, H.M.; Mumtaz, S.; Andleeb, S. Therapeutic role of garlic and vitamins C and E against toxicity induced by lead on various organs. Environ. Sci. Pollut. Res. 2020, 27, 8953–8964. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract reduces blood pressure in hypertensives: A dose–response trial. Eur. J. Clin. Nutr. 2013, 67, 64–72. [Google Scholar] [CrossRef]
- Hassan, A.A.; Jassim, H.M. Effect of treating lactating rats with lead acetate and its interaction with vitamin E or C on neurobehavior, development and some biochemical parameters in their pups. Iraqi J. Vet. Sci. 2010, 24, 45–52. [Google Scholar] [CrossRef]
- Atila, F.; Owaid, M.N.; Shariati, M.A. The nutritional and medical benefits of Agaricus bisporus: A review. J. Microbiol. Biotechnol. Food Sci. 2021, 7, 281–286. [Google Scholar] [CrossRef]
- Morosanova, M.A.; Fedorova, T.V.; Polyakova, A.; Morosanova, E.I. Agaricus bisporus Crude Extract: Characterization and Analytical Application. Molecules 2020, 25, 5996. [Google Scholar] [CrossRef]
- Elhusseiny, S.; El-Mahdy, T.; Awad, M.; Elleboudy, N.; Farag, M.; Aboshanab, K.; Yassien, M. Antiviral, Cytotoxic, and Antioxidant Activities of Three Edible Agaricomycetes Mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild Mushrooms as Beta-Glucan Sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Li, L.; Luo, C.; Li, X.; Duan, H.; Wang, X. Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment. Int. J. Biol. Macromol. 2014, 66, 172–178. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 118, e3000410. [Google Scholar] [CrossRef]
- Gerenutti, M.; Del Fiol, F.S.; Groppo, F.C. Reproductive performance of pregnant rats and embryotoxic effects of ciprofloxacin. Pharmazie 2006, 61, 79–80. [Google Scholar] [PubMed]
- Gerenutti, M.; Tribuiani, N.; Oliveira, B.R.; Rosa-Castro, R.M.; Frizo, I.; Oshima-Franco, Y.; Grotto, D. Safety assessment of the royal sun mushroom, Agaricus brasiliensis (higher Basidiomycetes) intake during rat pregnancy. Int. J. Med. Mushrooms 2014, 16, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Laurino, L.F.; Viroel, F.J.M.; Caetano, E.; Spim, S.; Pickler, T.B.; Rosa-Castro, R.M.; Vasconcelos, E.A.; Jozala, A.F.; Hataka, A.; Grotto, D.; et al. Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus. Nutrients 2019, 11, 2720. [Google Scholar] [CrossRef]
- Weizsaecker, K. Lead toxicity during pregnancy. Prim. Care Update OB/GYNS 2003, 10, 304–309. [Google Scholar] [CrossRef]
- Vieira, J.S. Efeitos da Exposição Pré-Natal ao Etanol e ao Chumbo, Isoladamente e em Associação, Sobre a Pressão Arterial e a Reatividade da Aorta de Ratos Recém-Desmamados. Diploma Thesis, Universidade Estadual Paulista, Instituto de Biociência de Botucatu, Botucatu, Brazil, 2014. [Google Scholar]
- Batista, B.L.; Jairo, L.R.; Luciano, T.; Adilson, J.C.; Fernando, B.J. Reference concentrations for trace elements in urine for the Brazilian population based on q-ICP-MS with a simple dilute-and-shoot procedure. J. Braz. Chem. Soc. 2009, 10, 1406–1413. [Google Scholar] [CrossRef]
- Paniz, F.P.; Tatiana, P.; Bruna, M.F.; Daiane, P.T. Effective procedures for the determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and rare earth elements in plants and foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- Ortega, D.R.; Esquivel, D.G.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M.; Mora, P.C.; de la Cruz, V.P. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- CDC (Centers for Disease Control and Prevention) of U.S. Fourth National Report on Human Exposure to Environmental Chemicals. 2019. Available online: http://www.cdc.gov/exposurereport/ (accessed on 11 January 2023).
- Brasil Ministério da Saúde Secretaria de Atenção à Saúde Departamento de Ações Programáticas Estratégicas. Atenção à saúde dos trabalhadores expostos ao chumbo metálico; Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Ações Programáticas Estratégicas, Ministério da Saúde: Brasília, Brazil, 2006.
- Saleh, H.A.; El-Aziz, G.A.; Mustafa, H.N.; Saleh, A.H.A.; Mal, A.O.; Deifalla, A.H.S.; Aburas, M. Protective effect of garlic extract against maternal and foetal cerebellar damage induced by lead administration during pregnancy in rats. Folia Morphol. 2018, 77, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Khordad, E.; Ebrahimi, V.; Raoofi, A.; Alipour, F.; Ebrahimzadeh-Bideskan, A. Neuroprotective effects of vitamin C and garlic on glycoconjugates changes of cerebellar cortex in lead-exposed rat offspring. J. Chem. Neuroanat. 2021, 114, 101948. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Choi, S.-H.; Cho, H.-J.; Seo, J.S.; Choi, M.; Nahm, S.-S.; Chang, B.-J.; Nah, S.-Y. Ginseng Gintonin Attenuates Lead-Induced Rat Cer-ebellar Impairments during Gestation and Lactation. Biomolecules 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Barcelos, G.R.M.; Valentini, J.; Antunes, L.M.G.; Angeli, J.P.F.; Garcia, S.C.; Jr, F.B. Low levels of methylmercury induce DNA damage in rats: Protective effects of selenium. Arch. Toxicol. 2009, 83, 249–254. [Google Scholar] [CrossRef]
- Takano, T.; Okutomi, Y.; Mochizuki, M.; Ochiai, Y.; Yamada, F.; Mori, M.; Ueda, F. Biological index of environmental lead pol-lution: Accumulation of lead in liver and kidney in mice. Environ. Monit. Assess. 2015, 187, 744. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Nakayama, S.M.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Yohannes, Y.B.; Mizukawa, H.; Ishizuka, M. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. Environ. Saf. 2015, 111, 160–167. [Google Scholar] [CrossRef]
- Nakayama, S.M.M.; Ikenaka, Y.; Hamada, K.; Muzandu, K.; Choongo, K.; Yabe, J.; Umemura, T.; Ishizuka, M. Accumulation and biological effects of metals in wild rats in mining areas of Zambia. Environ. Monit. Assess. 2013, 185, 4907–4918. [Google Scholar] [CrossRef]
- Togao, M.; Nakayama, S.M.; Ikenaka, Y.; Mizukawa, H.; Makino, Y.; Kubota, A.; Matsukawa, T.; Yokoyama, K.; Hirata, T.; Ishizuka, M. Bioimaging of Pb and STIM1 in mice liver, kidney and brain using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and immuno-histochemistry. Chemosphere 2020, 238, 124581. [Google Scholar] [CrossRef]
- Riess, M.L.; Halm, J.K. Lead poisoning in an adult: Lead mobilization by pregnancy? J. Gen. Intern. Med. 2007, 22, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- CDC (Centers for Disease Control and Prevention). Morbidity and Mortality Weekly Report MMWR; CDC: Atlanta, GE, USA, 2007.
- Villeda-Hernández, J.; Méndez Armenta, M.; Barroso-Moguel, R.; Trejo-Solis, M.C.; Guevara, J.; Rios, C. Morphometric analysis of brain lesions in rat fetuses prenatally exposed to low-level lead acetate: Correlation with lipid peroxidation. Histol. Histopathol. 2006, 21, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Müller, Y.M.; Rivero, L.B.; Carvalho, M.C.; Kobus, K.; Farina, M.; Nazari, E.M. Behavioral impairments related to lead-induced developmental neurotoxicity in chicks. Arch. Toxicol. 2008, 82, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.M.; Mousavi, S.H.; Jorjani, M. Effect of chronic lead exposure on pro-apoptotic Bax and anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo. Cell. Mol. Neurobiol. 2010, 30, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Antonio-García, M.T.; Massó-Gonzalez, E.L. Toxic effects of perinatal lead exposure on the brain of rats: Involvement of oxidative stress and the beneficial role of antioxidants. Food Chem. Toxicol. 2008, 46, 2089–2095. [Google Scholar] [CrossRef]
- Pachauri, V.; Saxena, G.; Mehta, A.; Mishra, D.; Flora, S.J. Combinational chelation therapy abrogates lead-induced neuro-degeneration in rats. Toxicol. Appl. Pharmacol. 2009, 1240, 255–264. [Google Scholar] [CrossRef]
- Flora, S.J.; Pande, M.; Mehta, A. Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem. Biol. Interact. 2003, 145, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Visweswar, K.N.S.; Sunil, A.; Harsha, A.S.; Janardhana, C. Interaction studies of lead(II) ion with cyclic β-(1→3),(1→6) glucans extracted from Bradyrhizobium japonicum based on ‘chelation enhanced fluorescence’ (CHEF) effect. Luminescence 2018, 33, 1202–1208. [Google Scholar] [CrossRef] [PubMed]

| ICP-MS: Operating Conditions | |
|---|---|
| Monitored isotope | 208Pb |
| Radio frequency power | 1550 W |
| Argon flow | 15 L min−1 |
| Nebulization chamber | 0.9 L min−1 |
| Collision cell | Helium (purity > 99.999%) |
| Nebulization chamber | Scott (double pass) |
| Interface | Nickel cones |
| Sample | 0.90 mm |
| Skimmer | 0.45 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caetano, É.L.A.; Pedron, T.; Freire, B.M.; Lange, C.N.; Batista, B.L.; Grotto, D. Influence of Agaricus bisporus Mushroom on Pb Toxicokinetic in Pregnant Rats. Int. J. Environ. Res. Public Health 2023, 20, 3114. https://doi.org/10.3390/ijerph20043114
Caetano ÉLA, Pedron T, Freire BM, Lange CN, Batista BL, Grotto D. Influence of Agaricus bisporus Mushroom on Pb Toxicokinetic in Pregnant Rats. International Journal of Environmental Research and Public Health. 2023; 20(4):3114. https://doi.org/10.3390/ijerph20043114
Chicago/Turabian StyleCaetano, Érika Leão Ajala, Tatiana Pedron, Bruna Moreira Freire, Camila Neves Lange, Bruno Lemos Batista, and Denise Grotto. 2023. "Influence of Agaricus bisporus Mushroom on Pb Toxicokinetic in Pregnant Rats" International Journal of Environmental Research and Public Health 20, no. 4: 3114. https://doi.org/10.3390/ijerph20043114
APA StyleCaetano, É. L. A., Pedron, T., Freire, B. M., Lange, C. N., Batista, B. L., & Grotto, D. (2023). Influence of Agaricus bisporus Mushroom on Pb Toxicokinetic in Pregnant Rats. International Journal of Environmental Research and Public Health, 20(4), 3114. https://doi.org/10.3390/ijerph20043114









