Acrylamide-Induced Changes in the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Immunoreactivity in Small Intestinal Intramural Neurons in Pigs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
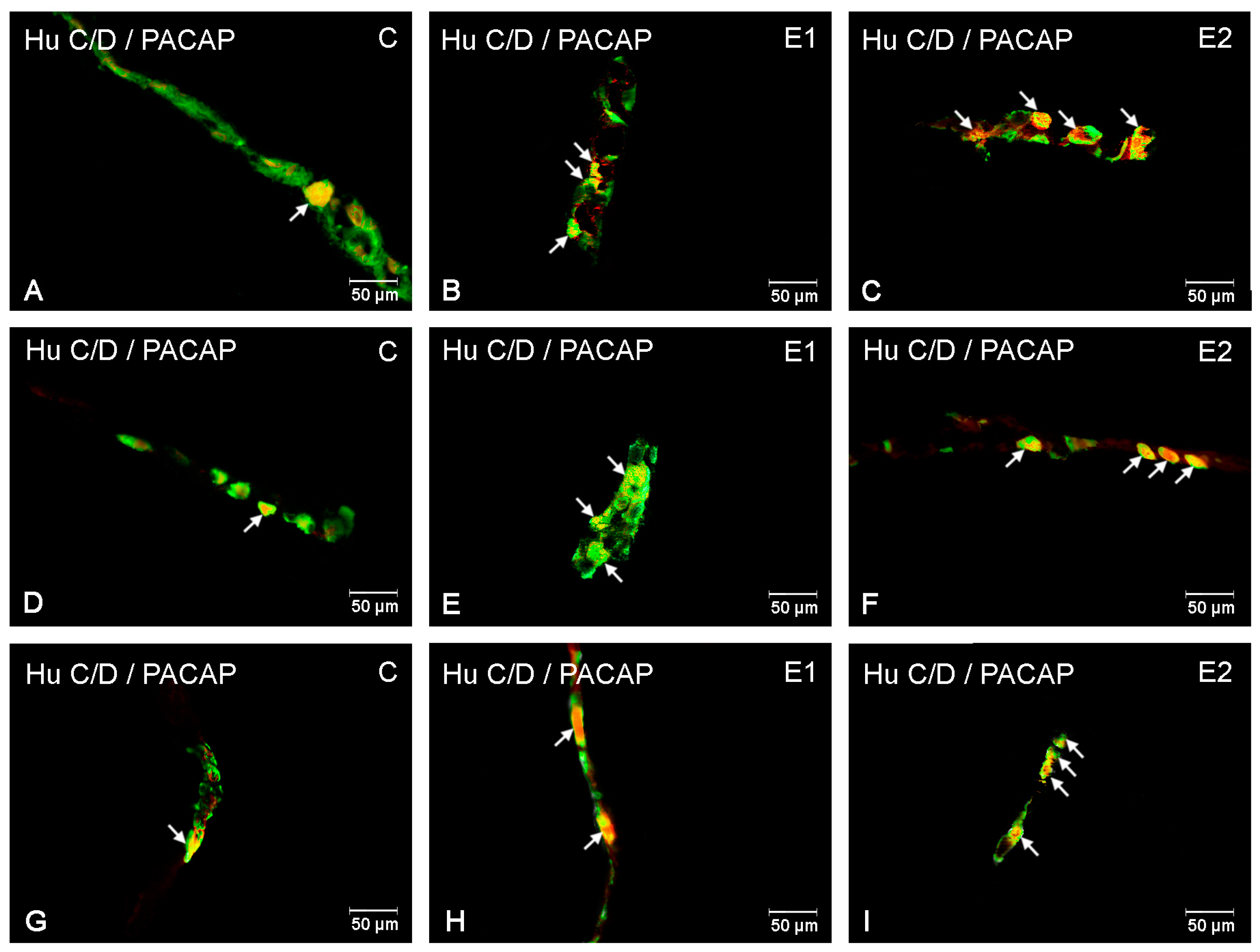
3.1. The Duodenum
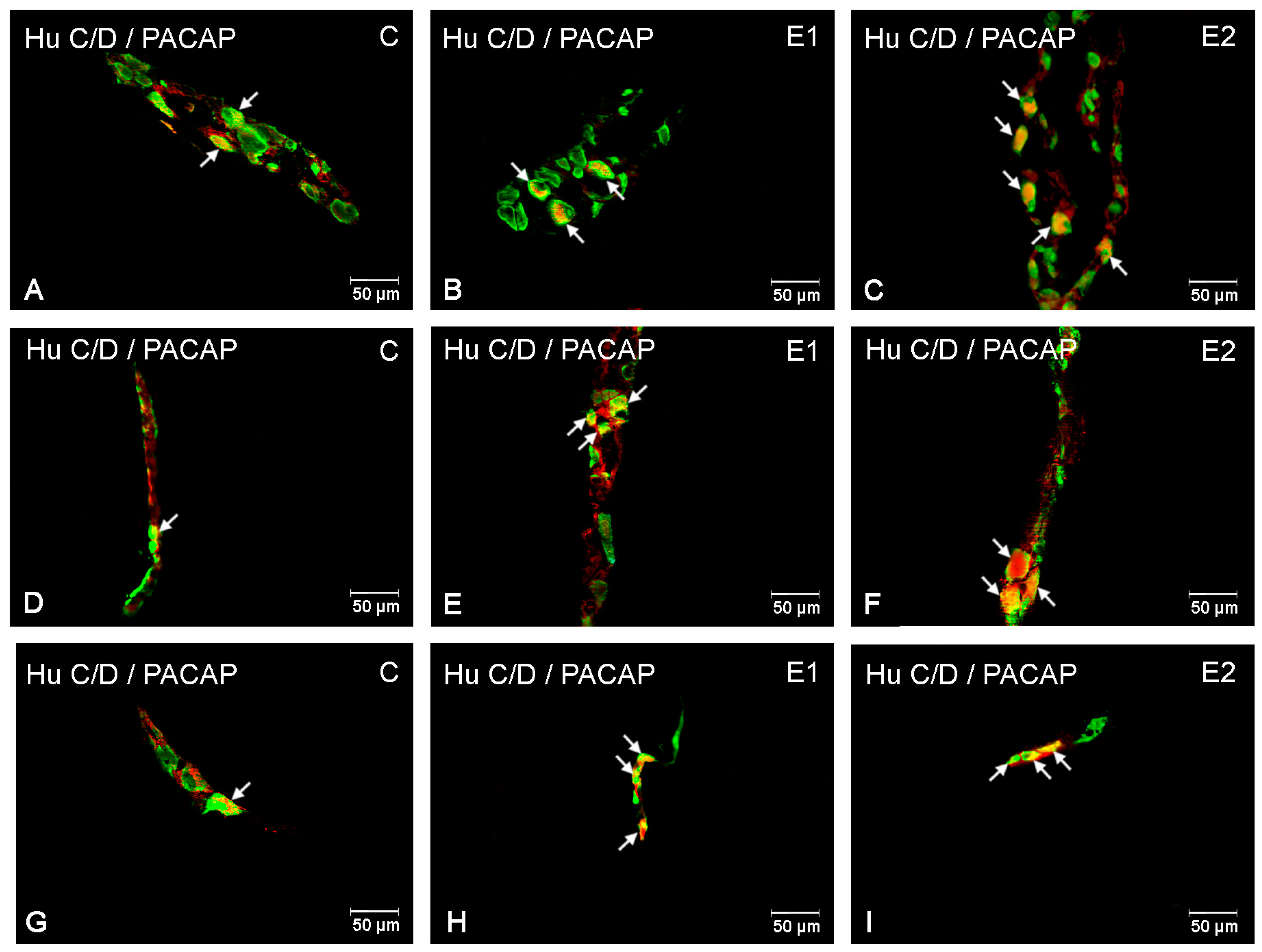
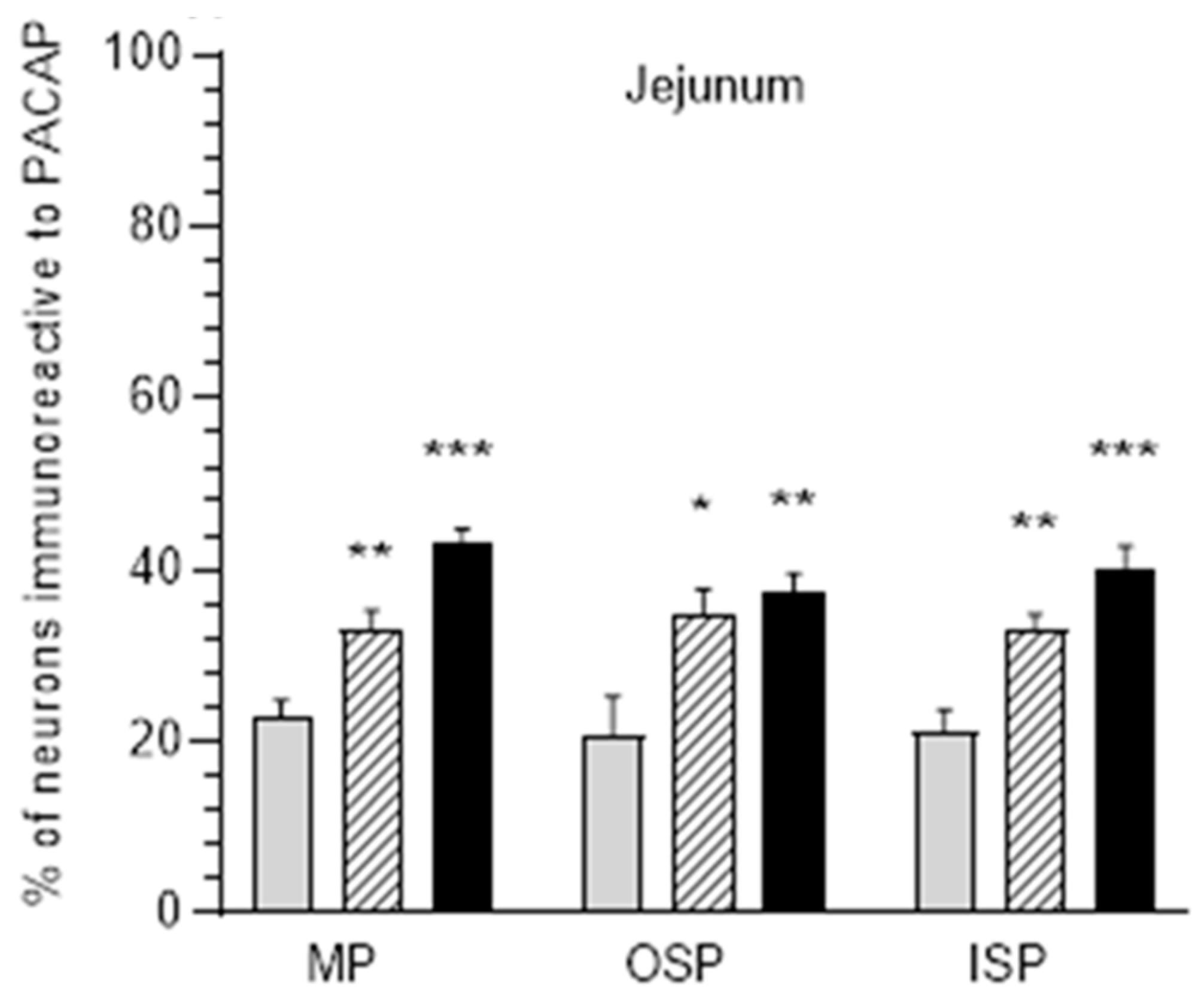
3.2. The Jejunum
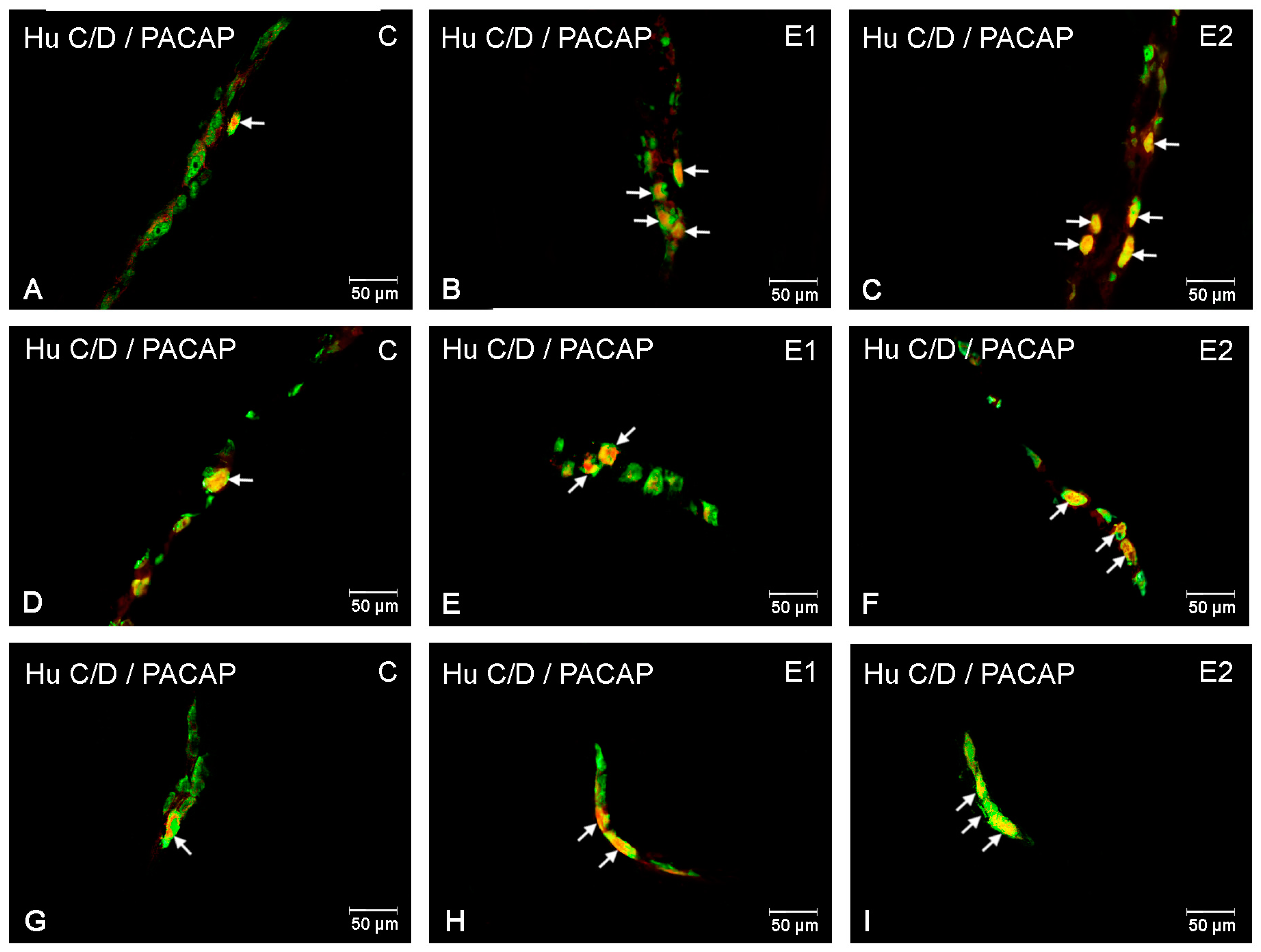
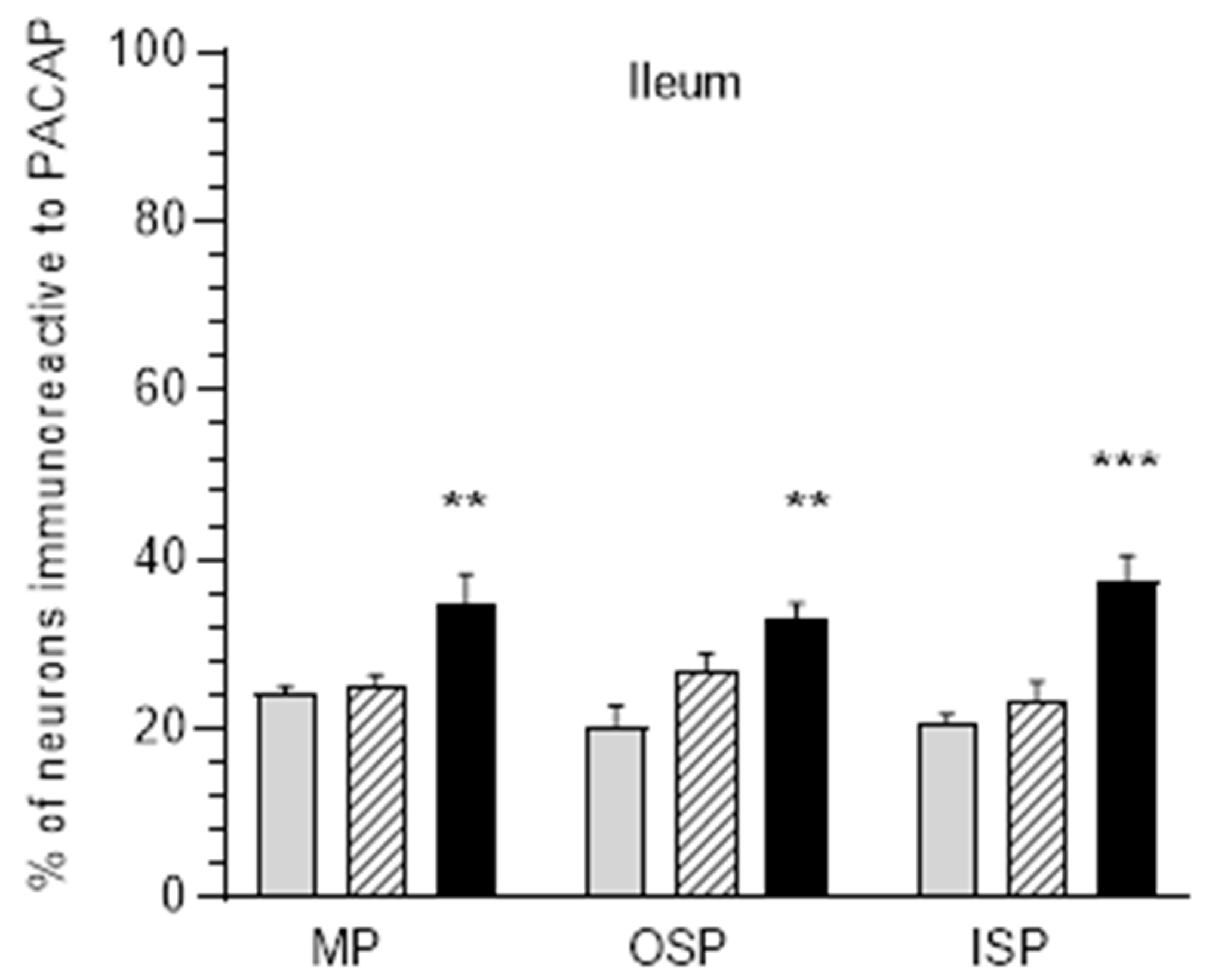
3.3. The Ileum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chico Galdo, V.; Massart, C.; Jin, L.; Vanvooren, V.; Caillet-Fauquet, P.; Andry, G.; Dequanter, D.; Friedman, M.; van Sande, J. Acrylamide an in vitro thyroid carcinogenic agent, induces DNA damage in rat thyroid cell lines and primary cultures. Mol. Cell. Endocrinol. 2006, 257–258, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Kopanska, M.; Muchacka, R.; Czech, J.; Batoryna, M.; Formicki, G. Acrylamide toxicity and cholinergic nervous system. J. Physiol. Pharmacol. 2018, 69, 847–858. [Google Scholar]
- Yousef, M.I.; El-Demerdash, F.M. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology 2006, 219, 133–141. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M. The changing view of acrylamide neurotoxicity. Neurotoxicology 2004, 25, 617–630. [Google Scholar] [CrossRef]
- WHO. Health Implications of Acrylamide in Food; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2002; Available online: http://apps.who.int/iris/handle/10665/42563 (accessed on 15 November 2016).
- Furness, J.B. The enteric nervous system and neurogastroenterology. Review. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Timmermans, J.P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Illes, A.; Opper, B.; Schafer, E.; Tamas, A.; Horvath, G. Presence and Effects of Pituitary Adenylate Cyclase Activating Polypeptide Under Physiological and Pathological Conditions in the Stomach. Front. Endocrinol. 2018, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Läuff, J.M.; Modlin, I.M.; Tang, L.H. Biological relevance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the gastrointestinal tract. Regul. Pept. 1999, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Całka, J. Changes in pituitary adenylate cyclase-activating Peptide 27-like immunoreactive nervous structures in the porcine descending colon during selected pathological processes. J. Mol. Neurosci. 2012, 48, 777–787. [Google Scholar] [CrossRef]
- Karpiesiuk, A.; Palus, K. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Physiological and Pathological Processes within the Gastrointestinal Tract: A Review. Int. J. Mol. Sci. 2021, 22, 8682. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Bulc, M.; Całka, J.; Zielonka, Ł.; Nowicki, M. Diabetes Affects the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Like Immunoreactive Enteric Neurons in the Porcine Digestive Tract. Int. J. Mol. Sci. 2021, 22, 5727. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Obremski, K.; Całka, J. The Influence of Low Doses of Zearalenone on Distribution of Selected Active Substances in Nerve Fibers Within the Circular Muscle Layer of Porcine Ileum. J. Mol. Neurosci. 2015, 56, 878–886. [Google Scholar] [CrossRef]
- Thoene, M.; Rytel, L.; Dzika, E.; Wojtkiewicz, J. Increased PACAP- and DβH-Positive Hepatic Nerve Fibers after Bisphenol A Exposure. Toxics 2021, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Łakomy, I.M. Changes in vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide and neuropeptide Y-ergic structures of the enteric nervous system in the carcinoma of the human large intestine. Folia Histochem. Cytobiol. 2010, 48, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Szanto, Z.; Sarszegi, Z.; Reglodi, D.; Nemeth, J.; Szabadfi, K.; Kiss, P.; Varga, A.; Banki, E.; Csanaky, K.; Gaszner, B.; et al. PACAP immunoreactivity in human malignant tumor samples and cardiac diseases. J. Mol. Neurosci. 2012, 48, 667–673. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Liu, J.; Ren, X.; Song, G.; Xia, X.; Qin, N. Dietary Acrylamide Intake Alters Gut Microbiota in Mice and Increases Its Susceptibility to Salmonella Typhimurium Infection. Foods 2021, 10, 2990. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Prost, L.; Kurlak, P.; Sawczuk, P.; Badzian, B.; Hulas-Stasiak, M.; Kostro, K. Acrylamide-induced prenatal programming of intestine structure in guinea pig. J. Physiol. Pharmacol. 2014, 65, 107–115. [Google Scholar]
- Su, D.; Lei, A.; Nie, C.; Chen, Y. The protective effect of Ganoderma atrum polysaccharide on intestinal barrier function damage induced by acrylamide in mice through TLR4/MyD88/NF-κB based on the iTRAQ analysis. Food Chem. Toxicol. 2023, 171, 113548. [Google Scholar] [CrossRef]
- Palus, K.; Obremski, K.; Bulc, M.; Całka, J. The impact of low and high doses of acrylamide on the intramural neurons of the porcine ileum. Food Chem. Toxicol. 2019, 132, 110673. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, H.; Liu, Q.; Shen, Y.; Jiang, Y.; Li, Y.; Liu, T.; Liu, T.; Xu, H.; Shao, M. Protective effect of seabuckthorn berry juice against acrylamide-induced oxidative damage in rats. J. Food Sci. 2020, 85, 2245–2254. [Google Scholar] [CrossRef]
- Swindle, M.M.; Smith, A.C. Comparative anatomy and physiology of the pig. Scand. J. Lab. Anim. Sci. 1998, 25, 11–21. [Google Scholar]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Porcine models of digestive disease: The future of large animal translational research. Transl. Res. 2015, 166, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palus, K.; Bulc, M.; Całka, J. Changes in Somatostatin-Like Immunoreactivity in the Sympathetic Neurons Projecting to the Prepyloric Area of the Porcine Stomach Induced by Selected Pathological Conditions. Biomed. Res. Int. 2017, 2017, 9037476. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Ekblad, E.; Mulder, H.; Sundler, F.; Fahrenkrug, J. Pituitary adenylate cyclase activating polypeptide (PACAP) in the gastrointestinal tract of the rat: Distribution and effects of capsaicin or denervation. Cell Tissue Res. 1998, 291, 65–79. [Google Scholar] [CrossRef]
- Nagahama, M.; Tsuzuki, M.; Mochizuki, T.; Iguchi, K.; Kuwahara, A. Light and electron microscopic studies of pituitary adenylate cyclase-activating peptide (PACAP)—Immunoreactive neurons in the enteric nervous system of rat small and large intestine. Anat. Embryol. 1998, 198, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sundler, F.; Ekblad, E.; Absood, A.; Håkanson, R.; Köves, K.; Arimura, A. Pituitary adenylate cyclase activating peptide: A novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience 1992, 46, 439–454. [Google Scholar] [CrossRef]
- Portbury, A.L.; McConalogue, K.; Furness, J.B.; Young, H.M. Distribution of pituitary adenylyl cyclase-activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the Palus^0 and colon. Cell. Tissue Res. 1995, 279, 385–392. [Google Scholar] [CrossRef]
- Suzuki, M.; Nokihara, K.; Ando, E.; Naruse, S. Immunohistochemical comparison of localization of pituitary adenylate cyclase activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) in the enteric nerve plexus of the guinea pig jejunum. Biomed. Pept. Proteins Nucleic Acids 1996, 2, 19–22. [Google Scholar]
- Köves, K.; Arimura, A.; Vigh, S.; Somogyvári-Vigh, A.; Miller, J. Immunohistochemical localization of PACAP in the ovine digestive system. Peptides 1993, 14, 449–455. [Google Scholar] [CrossRef]
- Cox, H.M. Pituitary adenylate cyclase activating polypeptides, PACAP-27 and PACAP-38: Stimulators of electrogenic ion secretion in the rat small intestine. Br. J. Pharmacol. 1992, 106, 498–502. [Google Scholar] [CrossRef]
- Fuchs, M.; Adermann, K.; Raab, H.R.; Forssmann, W.G.; Kuhn, M. Pituitary adenylate cyclase-activating polypeptide: A potent activator of human intestinal ion transport. Ann. N. Y. Acad. Sci. 1996, 805, 640–647. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takehara, K.; Kato, S.; Yagi, K. PACAPs stimulate duodenal bicarbonate secretion at PACAP receptors in the rat. Am. J. Physiol. 1997, 272, G646–G653. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chey, W.Y.; Braggins, L.; Coy, D.H.; Chang, T.M. Pituitary adenylate cyclase-activating polypeptide stimulates cholecystokinin secretion in STC-1 cells. Am. J. Physiol. 1996, 271, G516–G523. [Google Scholar] [CrossRef] [PubMed]
- Racké, K.; Reimann, A.; Schwörer, H.; Kilbinger, H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1996, 73, 83–87. [Google Scholar] [CrossRef]
- Chang, C.H.; Chey, W.Y.; Erway, B.; Coy, D.H.; Chang, T.M. Modulation of secretin release by neuropeptides in secretin-producing cells. Am. J. Physiol. 1998, 275, G192–G202. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, E.; Bauer, A.J. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol. Motil. 2004, 16, 123–128. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Gonkowski, S. Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 2013, 39, 542–551. [Google Scholar] [CrossRef]
- Czajkowska, M.; Całka, J. Neurochemistry of Enteric Neurons Following Prolonged Indomethacin Administration in the Porcine Duodenum. Front. Pharmacol. 2020, 11, 564457. [Google Scholar] [CrossRef]
- Czajkowska, M.; Rychlik, A.; Całka, J. Long-term treatment with naproxen changes the chemical coding of the porcine intramural duodenum neurons. Ann. Anat. 2020, 227, 151425. [Google Scholar] [CrossRef]
- Brzozowska, M.; Jana, B.; Całka, J. Effect of NSAIDs Supplementation on the PACAP, SP- and GAL-Immunoreactive Neurons in the Porcine Jejunum. Int. J. Mol. Sci. 2021, 22, 11689. [Google Scholar] [CrossRef]
- Całka, J. Increased expression of CART, Nnos, VIP, PACAP, SP and GAL in enteric neurons of the porcine stomach prepyloric region following hydrochloric acid infusion. Folia Histochem. Cytobiol. 2019, 57, 179–187. [Google Scholar] [CrossRef]
- Rytel, L.; Wojtkiewicz, J.; Snarska, A.; Mikołajczyk, A. Changes in the Neurochemical Characterization of Enteric Neurons in the Porcine Duodenum after Administration of Low-Dose Salmonella Enteritidis Lipopolysaccharides. J. Mol. Neurosci. 2020, 71, 1556–1566. [Google Scholar] [CrossRef]
- Ohtaki, H.; Satoh, A.; Nakamachi, T.; Yofu, S.; Dohi, K.; Mori, H.; Ohara, K.; Miyamoto, K.; Hashimoto, H.; Shintani, N.; et al. Regulation of oxidative stress by pituitary adenylate cyclase-activating polypeptide (PACAP) mediated by PACAP receptor. J. Mol. Neurosci. 2010, 42, 397–403. [Google Scholar] [CrossRef]
- Kasica, N.; Podlasz, P.; Sundvik, M.; Tamas, A.; Reglodi, D.; Kaleczyc, J. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Against Oxidative Stress in Zebrafish Hair Cells. Neurotox. Res. 2016, 30, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Illes, A.; Opper, B.; Reglodi, D.; Kerenyi, M.; Czetany, P.; Boronkai, A.; Schafer, E.; Toth, G.; Fabian, E.; Horvath, G. Effects of pituitary adenylate cyclase activating polypeptide on small intestinal INT 407 cells. Neuropeptides 2017, 65, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Ibrahem, M.D. Acrylamide-induced hematotoxicity, oxidative stress, and DNA damage in liver, kidney, and brain of catfish (Clarias gariepinus). Environ. Toxicol. 2020, 35, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Zapolska-Downar, D.; Kośmider, A.; Nowicka, G.; Kozłowska-Wojciechowska, M.; Vikström, A.S.; Törnqvist, M. Chronic intake of potato chips in humans increases the production of reactive oxygen radicals by leukocytes and increases plasma C-reactive protein: A pilot study. Am. J. Clin. Nutr. 2009, 89, 773–777. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Dunay, I.R.; Schulze, S.; Fischer, A.; Grundmann, U.; Alutis, M.; Kühl, A.A.; Tamas, A.; Toth, G.; Dunay, M.P.; et al. Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS ONE 2014, 9, e108389. [Google Scholar] [CrossRef]
- Bereswill, S.; Escher, U.; Grunau, A.; Kühl, A.A.; Dunay, I.R.; Tamas, A.; Reglodi, D.; Heimesaat, M.M. Pituitary Adenylate Cyclase-Activating Polypeptide-A Neuropeptide as Novel Treatment Option for Subacute Ileitis in Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Reifenberger, G.; Vicena, V.; Illes, A.; Horvath, G.; Tamas, A.; Fulop, B.D.; Bereswill, S.; Reglodi, D. Intestinal Microbiota Changes in Mice Lacking Pituitary Adenylate Cyclase Activating Polypeptide (PACAP)—Bifidobacteria Make the Difference. Eur. J. Microbiol. Immunol. 2017, 7, 187–199. [Google Scholar] [CrossRef]
- Yagi, K.; Takehara, K.; Kitamura, M.; Takeuchi, K. Effects of pituitary adenylate cyclase activating polypeptide-27 on alkaline secretory and mucosal ulcerogenic responses in rat duodenum. Life Sci. 1998, 63, 317–325. [Google Scholar] [CrossRef]
- Santhanasabapathy, R.; Vasudevan, S.; Anupriya, K.; Pabitha, R.; Sudhandiran, G. Farnesol quells oxidative stress, reactive gliosis and inflammation during acrylamide-induced neurotoxicity: Behavioral and biochemical evidence. Neuroscience 2015, 308, 212–227. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiesiuk, A.; Całka, J.; Palus, K. Acrylamide-Induced Changes in the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Immunoreactivity in Small Intestinal Intramural Neurons in Pigs. Int. J. Environ. Res. Public Health 2023, 20, 3272. https://doi.org/10.3390/ijerph20043272
Karpiesiuk A, Całka J, Palus K. Acrylamide-Induced Changes in the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Immunoreactivity in Small Intestinal Intramural Neurons in Pigs. International Journal of Environmental Research and Public Health. 2023; 20(4):3272. https://doi.org/10.3390/ijerph20043272
Chicago/Turabian StyleKarpiesiuk, Aleksandra, Jarosław Całka, and Katarzyna Palus. 2023. "Acrylamide-Induced Changes in the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Immunoreactivity in Small Intestinal Intramural Neurons in Pigs" International Journal of Environmental Research and Public Health 20, no. 4: 3272. https://doi.org/10.3390/ijerph20043272




