Oleate Impacts on Acetoclastic and Hydrogenotrophic Methanogenesis under Mesophilic and Thermophilic Conditions
Abstract
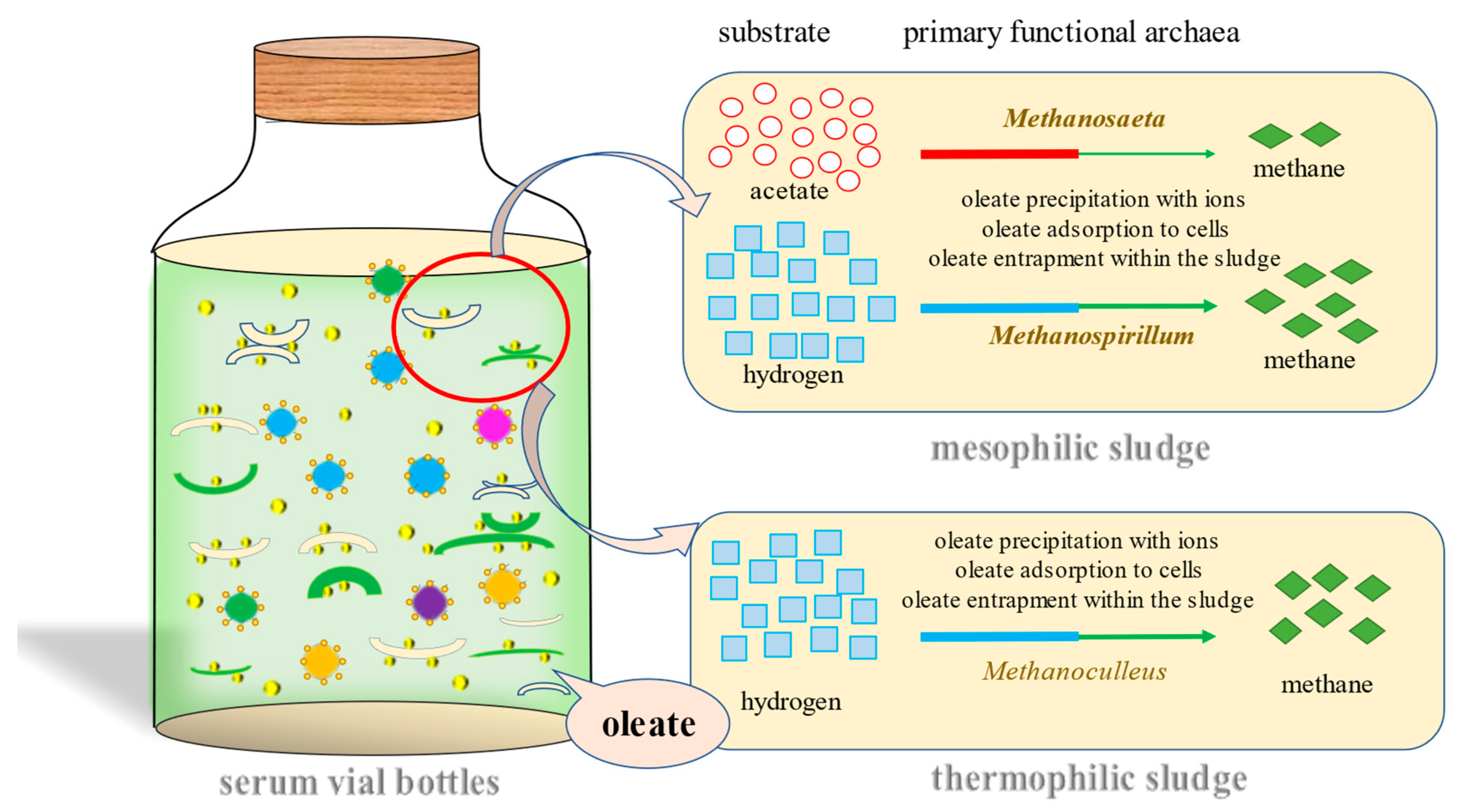
1. Introduction
2. Materials and Methods
2.1. 16S rRNA Sequencing Analysis
2.2. Seed Sludge and Substrate
2.3. Experimental Design
2.4. Analytical Methods
3. Results and Discussion
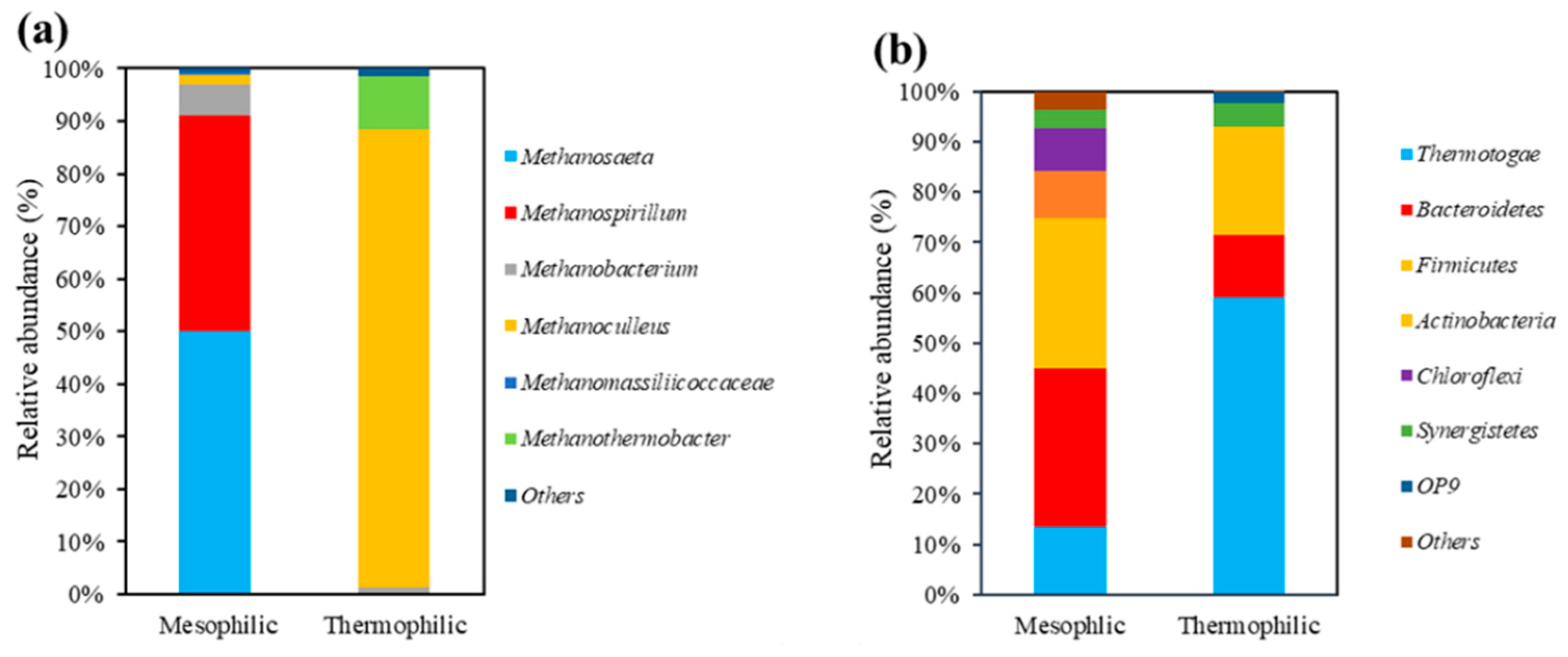
3.1. Microorganism Composition in Initial Mesophilic and Thermophilic Sludge
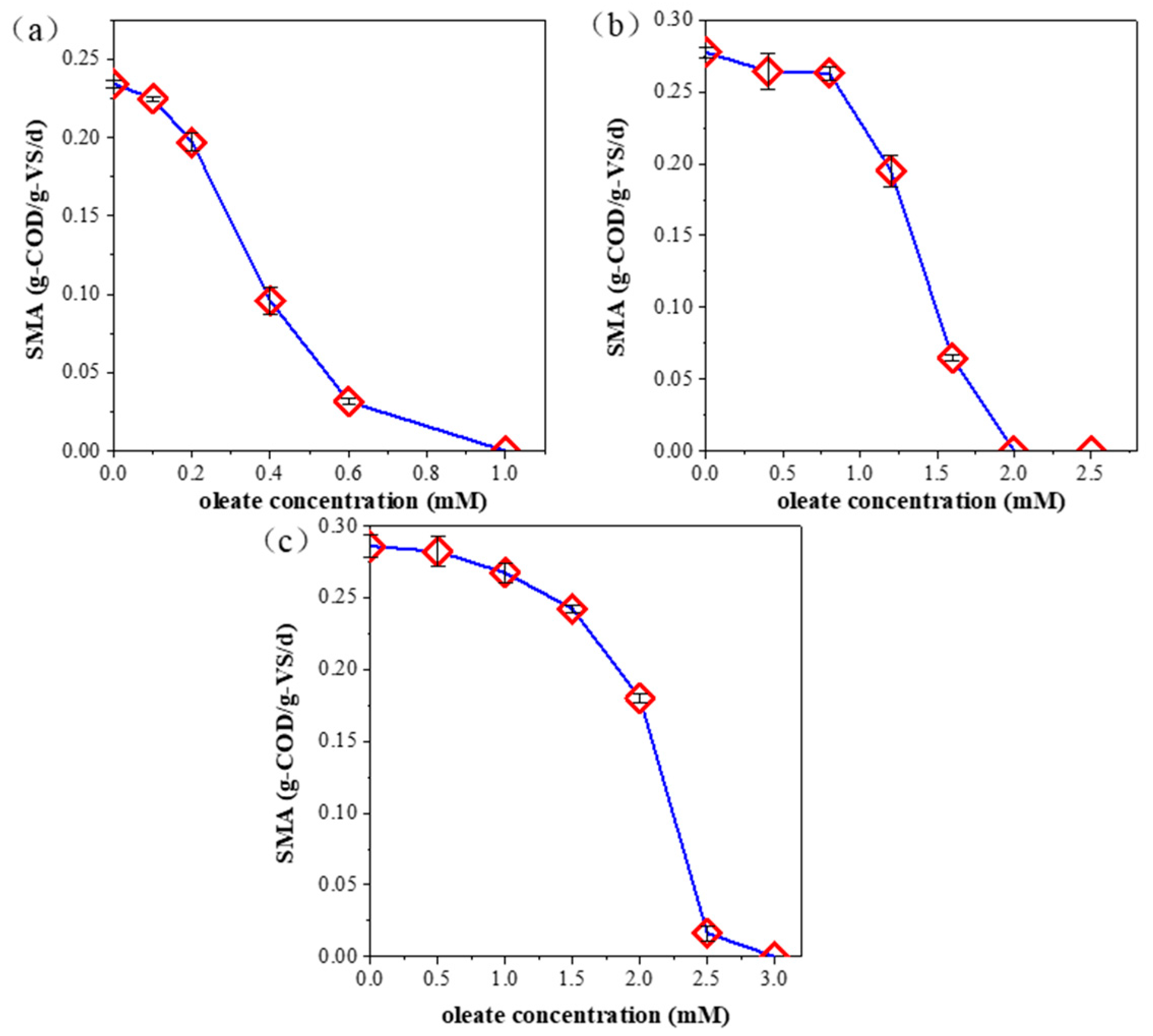
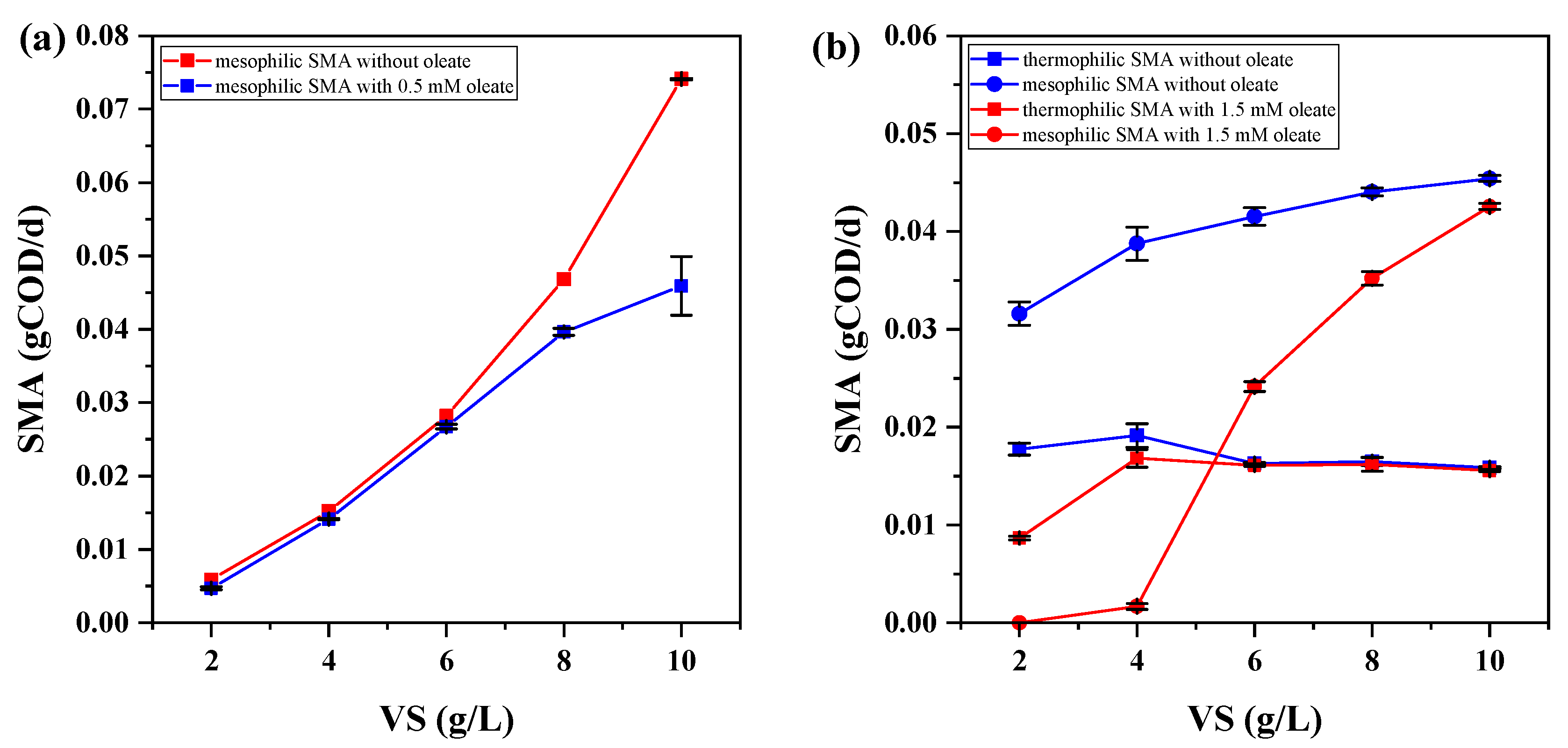
3.2. Effect of Oleate Concentration on Specific Methanogenic Activity
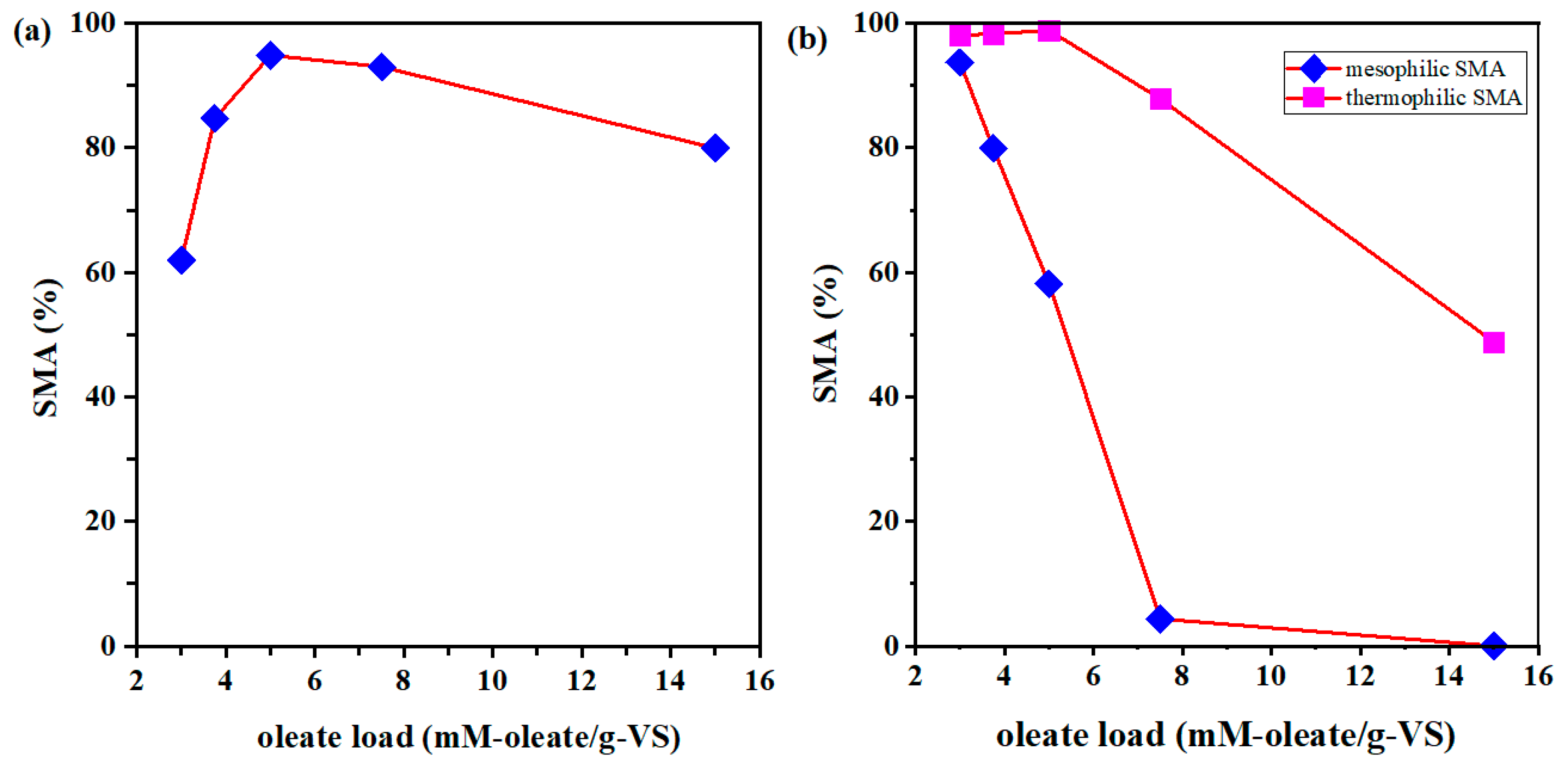
3.3. Effect of Oleate Loads on Specific Methanogenic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchetti, R.; Vasmara, C.; Bertin, L.; Fiume, F. Conversion of waste cooking oil into biogas: Perspectives and limits. Appl. Microbiol. Biotechnol. 2020, 104, 2833–2856. [Google Scholar]
- Kibler, K.M.; Reinhart, D.; Hawkins, C.; Motlagh, A.M.; Wright, J. Food waste and the food-energy-water nexus: A review of food waste management alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef]
- Talan, A.; Tiwari, B.; Yadav, B.; Tyagi, R.; Wong, J.; Drogui, P. Food waste valorization: Energy production using novel integrated systems. Bioresour. Technol. 2021, 322, 124538. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Zhang, H.; Xu, S.; Lichtfouse, E.; Yun, Y. Anaerobic digestion and recycling of kitchen waste: A review. Environ. Chem. Lett. 2022, 20, 1745–1762. [Google Scholar] [CrossRef]
- Salama, E.S.; Saha, S.; Kurade, M.B.; Dev, S.; Chang, S.W.; Jeon, B.H. Recent trends in anaerobic co-digestion: Fat, oil, and grease (FOG) for enhanced biomethanation. Prog. Energy Combust. Sci. 2019, 70, 22–42. [Google Scholar]
- He, X.; Yan, T. Impact of microbial activities and hydraulic retention time on the production and profile of long chain fatty acids in grease interceptors: A laboratory study. Environ. Sci. Water Res. Technol. 2016, 2, 474–482. [Google Scholar] [CrossRef]
- Kurade, M.B.; Saha, S.; Salama, E.-S.; Patil, S.M.; Govindwar, S.P.; Jeon, B.-H. Acetoclastic methanogenesis led by Methanosarcina in anaerobic co-digestion of fats, oil and grease for enhanced production of methane. Bioresour. Technol. 2019, 272, 351–359. [Google Scholar] [CrossRef]
- Elsamadony, M.; Mostafa, A.; Fujii, M.; Tawfik, A.; Pant, D. Advances towards understanding long chain fatty acids-induced inhibition and overcoming strategies for efficient anaerobic digestion process. Water Res. 2021, 190, 116732. [Google Scholar]
- Rodríguez-Méndez, R.; Le Bihan, Y.; Béline, F.; Lessard, P. Long chain fatty acids (LCFA) evolution for inhibition forecasting during anaerobic treatment of lipid-rich wastes: Case of milk-fed veal slaughterhouse waste. Waste Manag. 2017, 67, 51–58. [Google Scholar] [CrossRef]
- Palatsi, J.; Laureni, M.; Andrés, M.; Flotats, X.; Nielsen, H.; Angelidaki, I. Strategies for recovering inhibition caused by long chain fatty acids on anaerobic thermophilic biogas reactors. Bioresour. Technol. 2009, 100, 4588–4596. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Pereira, M.A.; Guedes, A.P.; Stams, A.J.M.; Alves, M.M.; Sousa, D.Z. Conversion of Cn-Unsaturated into Cn-2-Saturated LCFA Can Occur Uncoupled from Methanogenesis in Anaerobic Bioreactors. Environ. Sci. Technol. 2016, 50, 3082–3090. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.Z.; Salvador, A.F.; Ramos, J.; Guedes, A.; Barbosa, S.L.; Stams, A.; Alves, M.; Pereira, M.A. Activity and viability of methanogens in anaerobic digestion of unsaturated and saturated long-chain fatty acids. Appl. Environ. Microbiol. 2013, 79, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.A.; Salvador, A.F.; Cavaleiro, A.J.; Pereira, M.A.; Stams, A.J.M.; Alves, M.M.; Sousa, D.Z. Toxicity of long chain fatty acids towards acetate conversion by Methanosaeta concilii and Methanosarcina mazei. Microb. Biotechnol. 2016, 9, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Hwu, C.-S.; Lettinga, G. Acute toxicity of oleate to acetate-utilizing methanogens in mesophilic and thermophilic anaerobic sludges. Enzym. Microb. Technol. 1997, 21, 297–301. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.; Cui, M.; Zhang, F.; Liu, Y.; Liu, Y.; Long, J.; Guo, Y. Anaerobic digestion of kitchen waste: The effects of source, concentration, and temperature. Biochem. Eng. J. 2018, 135, 91–97. [Google Scholar] [CrossRef]
- Mahmoud, N.; Zeeman, G.; Gijzen, H.; Lettinga, G. Anaerobic stabilisation and conversion of biopolymers in primary sludge—Effect of temperature and sludge retention time. Water Res. 2004, 38, 983–991. [Google Scholar] [CrossRef]
- Alves, M.M.; Pereira, M.A.; Sousa, D.Z.; Cavaleiro, A.; Picavet, M.; Smidt, H.; Stams, A. Waste lipids to energy: How to optimize methane production from long-chain fatty acids (LCFA). Microb. Biotechnol. 2009, 2, 538–550. [Google Scholar] [CrossRef]
- Jing, Z.; Hu, Y.; Niu, Q.; Liu, Y.; Li, Y.-Y.; Wang, X. UASB performance and electron competition between methane-producing archaea and sulfate-reducing bacteria in treating sulfate-rich wastewater containing ethanol and acetate. Bioresour. Technol. 2013, 137, 349–357. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, H.; Wu, J.; Kobayashi, T.; Xu, K.-Q. Performance Comparison of CSTR and CSFBR in Anaerobic Co-Digestion of Food Waste with Grease Trap Waste. Energies 2022, 15, 8929. [Google Scholar] [CrossRef]
- Penning, H.; Claus, P.; Casper, P.; Conrad, R. Carbon isotope fractionation during acetoclastic methanogenesis by Methanosaeta concilii in culture and a lake sediment. Appl. Environ. Microbiol. 2006, 72, 5648–5652. [Google Scholar] [CrossRef]
- de Lucena, R.M.; Gavazza, S.; Florencio, L.; Kato, M.T.; de Morais, M.A. Study of the microbial diversity in a full-scale UASB reactor treating domestic wastewater. World J. Microbiol. Biotechnol. 2011, 27, 2893–2902. [Google Scholar] [CrossRef]
- Dolfing, J.; Tiedje, J.M. kinetics of 2 complementary hydrogen sink reactions in a defined 3-chlorobenzoate degrading methanogenic coculture. Fems. Microbiol. Ecol. 1991, 86, 25–32. [Google Scholar] [CrossRef]
- Barret, M.; Gagnon, N.; Kalmokoff, M.L.; Topp, E.; Verastegui, Y.; Brooks, S.P.J.; Matias, F.; Neufeld, J.D.; Talbot, G. Identification of Methanoculleus spp. as Active Methanogens during Anoxic Incubations of Swine Manure Storage Tank Samples. Appl. Environ. Microbiol. 2013, 79, 424–433. [Google Scholar] [CrossRef]
- Maus, I.; Wibberg, D.; Stantscheff, R.; Eikmeyer, F.G.; Seffner, A.; Boelter, J.; Szczepanowski, R.; Blom, J.; Jaenicke, S.; König, H.; et al. Complete Genome Sequence of the Hydrogenotrophic, Methanogenic Archaeon Methanoculleus bourgensis Strain MS2(T), Isolated from a Sewage Sludge Digester. J. Bacteriol. 2012, 194, 5487–5488. [Google Scholar] [CrossRef] [PubMed]
- Kouzuma, A.; Tsutsumi, M.; Ishii, S.; Ueno, Y.; Abe, T.; Watanabe, K. Non-autotrophic methanogens dominate in anaerobic digesters. Sci. Rep. 2017, 7, 1510. [Google Scholar] [CrossRef]
- Lanzilli, M.; Esercizio, N.; Vastano, M.; Xu, Z.; Nuzzo, G.; Gallo, C.; Manzo, E.; Fontana, A.; D’Ippolito, G. Effect of Cultivation Parameters on Fermentation and Hydrogen Production in the Phylum Thermotogae. Int. J. Mol. Sci. 2021, 22, 341. [Google Scholar] [CrossRef]
- Baserba, M.G.; Angelidaki, I.; Karakashev, D. Effect of continuous oleate addition on microbial communities involved in anaerobic digestion process. Bioresour. Technol. 2012, 106, 74–81. [Google Scholar] [CrossRef]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.-J.; Duan, M.-L. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure. Sci. Rep. 2016, 6, 30237. [Google Scholar] [CrossRef]
- Petriglieri, F.; Nierychlo, M.; Nielsen, P.H.; McIlroy, S.J. In situ visualisation of the abundant Chloroflexi populations in full-scale anaerobic digesters and the fate of immigrating species. PLoS ONE 2018, 13, e0206255. [Google Scholar] [CrossRef]
- Ao, T.; Xie, Z.; Zhou, P.; Liu, X.; Wan, L.; Li, D. Comparison of microbial community structures between mesophilic and thermophilic anaerobic digestion of vegetable waste. Bioprocess Biosyst. Eng. 2021, 44, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Ike, M.; Inoue, D.; Miyano, T.; Liu, T.T.; Sei, K.; Soda, S.; Kadoshin, S. Microbial population dynamics during startup of a full-scale anaerobic digester treating industrial food waste in Kyoto eco-energy project. Bioresour. Technol. 2010, 101, 3952–3957. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Ziels, R.M.; Beck, D.A.; Stensel, H.D. Long-chain fatty acid feeding frequency in anaerobic codigestion impacts syntrophic community structure and biokinetics. Water Res. 2017, 117, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Pires, O.; Mota, M.; Alves, M. Anaerobic biodegradation of oleic and palmitic acids: Evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Overview of some theoretical approaches for derivation of the Monod equation. Appl. Microbiol. Biotechnol. 2007, 73, 1241–1250. [Google Scholar] [CrossRef]
- Lokshina, L.Y.; Vavilin, V.A.; Kettunen, R.H.; Rintala, J.A.; Holliger, C.; Nozhevnikova, A.N. Evaluation of kinetic coefficients using integrated Monod and Haldane models for low-temperature acetoclastic methanogenesis. Water Res. 2001, 35, 2913–2922. [Google Scholar] [CrossRef]
- Wu, L.-J.; Li, X.-X.; Yang, F.; Zhou, Q.; Ren, R.-P.; Lyu, Y.-K. The distinctive responses of hyperthermophilic, thermophilic and mesophilic anaerobic digesters to restaurant-discharged oily waste. Process Biochem. 2021, 106, 149–157. [Google Scholar] [CrossRef]
- Theander, K.; Pugh, R.J. The influence of pH and temperature on the equilibrium and dynamic surface tension of aqueous solutions of sodium oleate. J. Colloid. Interface Sci. 2001, 239, 209–216. [Google Scholar] [CrossRef]
- Levén, L.; Eriksson, A.R.B.; Schnürer, A. Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste. Fems. Microbiol. Ecol. 2007, 59, 683–693. [Google Scholar] [CrossRef]
- Hatamoto, M.; Imachi, H.; Yashiro, Y.; Ohashi, A.; Harada, H. Diversity of anaerobic microorganisms involved in long-chain fatty acid degradation in methanogenic sludges as revealed by RNA-based stable isotope probing. Appl. Environ. Microbiol. 2007, 73, 4119–4127. [Google Scholar] [CrossRef]
- KKim, S.-H.; Han, S.-K.; Shin, H.-S. Kinetics of LCFA inhibition on acetoclastic methanogenesis, propionate degradation and beta-oxidation. J. Env. Sci. Health A Tox Hazard Subst. Env. Eng. 2004, 39, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; De la Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Astals, S.; Chávez-Fuentes, J.J.; Capson-Tojo, G.; Hutňan, M.; Jensen, P.D. The interaction between lipids and ammoniacal nitrogen mitigates inhibition in mesophilic anaerobic digestion. Waste Manag. 2021, 136, 244–252. [Google Scholar] [CrossRef] [PubMed]
| Number | VS (g/L) | Oleate Concentration (mM) | Number | VS (g/L) | Oleate Concentration (mM) |
|---|---|---|---|---|---|
| MA1 | 4 | 0 | MA2 | 4 | 0.1 |
| MA3 | 4 | 0.2 | MA4 | 4 | 0.4 |
| MA5 | 4 | 0.6 | MA6 | 4 | 1.0 |
| MH1 | 4 | 0 | TH1 | 4 | 0 |
| MH2 | 4 | 0.4 | TH2 | 4 | 0.5 |
| MH3 | 4 | 0.8 | TH3 | 4 | 1.0 |
| MH4 | 4 | 1.2 | TH4 | 4 | 1.5 |
| MH5 | 4 | 1.6 | TH5 | 4 | 2.0 |
| MH6 | 4 | 2.0 | TH6 | 4 | 2.5 |
| MH7 | 4 | 2.5 | TH7 | 4 | 3.0 |
| MA7 | 2 | 0 | MA8 | 2 | 0.5 |
| MA9 | 4 | 0 | MA10 | 4 | 0.5 |
| MA11 | 6 | 0 | MA12 | 6 | 0.5 |
| MA13 | 8 | 0 | MA14 | 8 | 0.5 |
| MA15 | 10 | 0 | MA16 | 10 | 0.5 |
| MH8 | 2 | 0 | TH8 | 2 | 0 |
| MH9 | 2 | 1.5 | TH9 | 2 | 1.5 |
| MH10 | 4 | 0 | TH10 | 4 | 0 |
| MH11 | 4 | 1.5 | TH11 | 4 | 1.5 |
| MH12 | 6 | 0 | TH12 | 6 | 0 |
| MH13 | 6 | 1.5 | TH13 | 6 | 1.5 |
| MH14 | 8 | 0 | TH14 | 8 | 0 |
| MH15 | 8 | 1.5 | TH15 | 8 | 1.5 |
| MH16 | 10 | 0 | TH16 | 10 | 0 |
| MH17 | 10 | 1.5 | TH17 | 10 | 1.5 |
| Temperature | Phylum | Genus | Number of OTU | Percentage (%) | Similarity (%) |
|---|---|---|---|---|---|
| Mesophilic | Euryarchaeota | Methanosaeta | 1928 | 50.00 | 99.70 |
| Methanospirillum | 1583 | 41.05 | 99.96 | ||
| Methanobacterium | 226 | 5.86 | |||
| Methanoculleus | 72 | 1.87 | |||
| Methanomassiliicoccaceae | 26 | 0.67 | |||
| Others | 21 | 0.54 | |||
| Total | 3856 | 100.00 | |||
| Thermophilic | Euryarchaeota | Methanoculleus | 1529 | 86.92 | 99.92 |
| Methanothermobacter | 179 | 10.18 | 99.99 | ||
| Methanobacterium | 25 | 1.42 | |||
| Others | 26 | 1.48 | |||
| Total | 1759 | 100.00 |
| Temperature | Substrate | Dominant Archaea | IC50 (mM) | Ref. |
|---|---|---|---|---|
| mesophilic | acetate | Methanosaeta | 0.42 | this study |
| mesophilic | hydrogen | Methanospirillum | 1.57 | this study |
| thermophilic | hydrogen | Methanoculleus | 1.66 | this study |
| mesophilic | acetate | Methanosaeta and Methanosarcina | <0.50 | [13] |
| mesophilic | hydrogen | Methanobacterium | 1.00 | [12] |
| mesophilic | hydrogen | Methanospirillum | 0.30 | [12] |
| mesophilic | acetate | 0.35 | [14] | |
| thermophilic | acetate | 0.53 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, Y.; Lu, C.-S.; Kobayashi, T.; Kong, Z.; Hu, Y. Oleate Impacts on Acetoclastic and Hydrogenotrophic Methanogenesis under Mesophilic and Thermophilic Conditions. Int. J. Environ. Res. Public Health 2023, 20, 3423. https://doi.org/10.3390/ijerph20043423
Li X, Yang Y, Lu C-S, Kobayashi T, Kong Z, Hu Y. Oleate Impacts on Acetoclastic and Hydrogenotrophic Methanogenesis under Mesophilic and Thermophilic Conditions. International Journal of Environmental Research and Public Health. 2023; 20(4):3423. https://doi.org/10.3390/ijerph20043423
Chicago/Turabian StyleLi, Xiang, Yang Yang, Chen-Shun Lu, Takuro Kobayashi, Zhe Kong, and Yong Hu. 2023. "Oleate Impacts on Acetoclastic and Hydrogenotrophic Methanogenesis under Mesophilic and Thermophilic Conditions" International Journal of Environmental Research and Public Health 20, no. 4: 3423. https://doi.org/10.3390/ijerph20043423
APA StyleLi, X., Yang, Y., Lu, C.-S., Kobayashi, T., Kong, Z., & Hu, Y. (2023). Oleate Impacts on Acetoclastic and Hydrogenotrophic Methanogenesis under Mesophilic and Thermophilic Conditions. International Journal of Environmental Research and Public Health, 20(4), 3423. https://doi.org/10.3390/ijerph20043423







