A Comprehensive Review on Wastewater Nitrogen Removal and Its Recovery Processes
Abstract
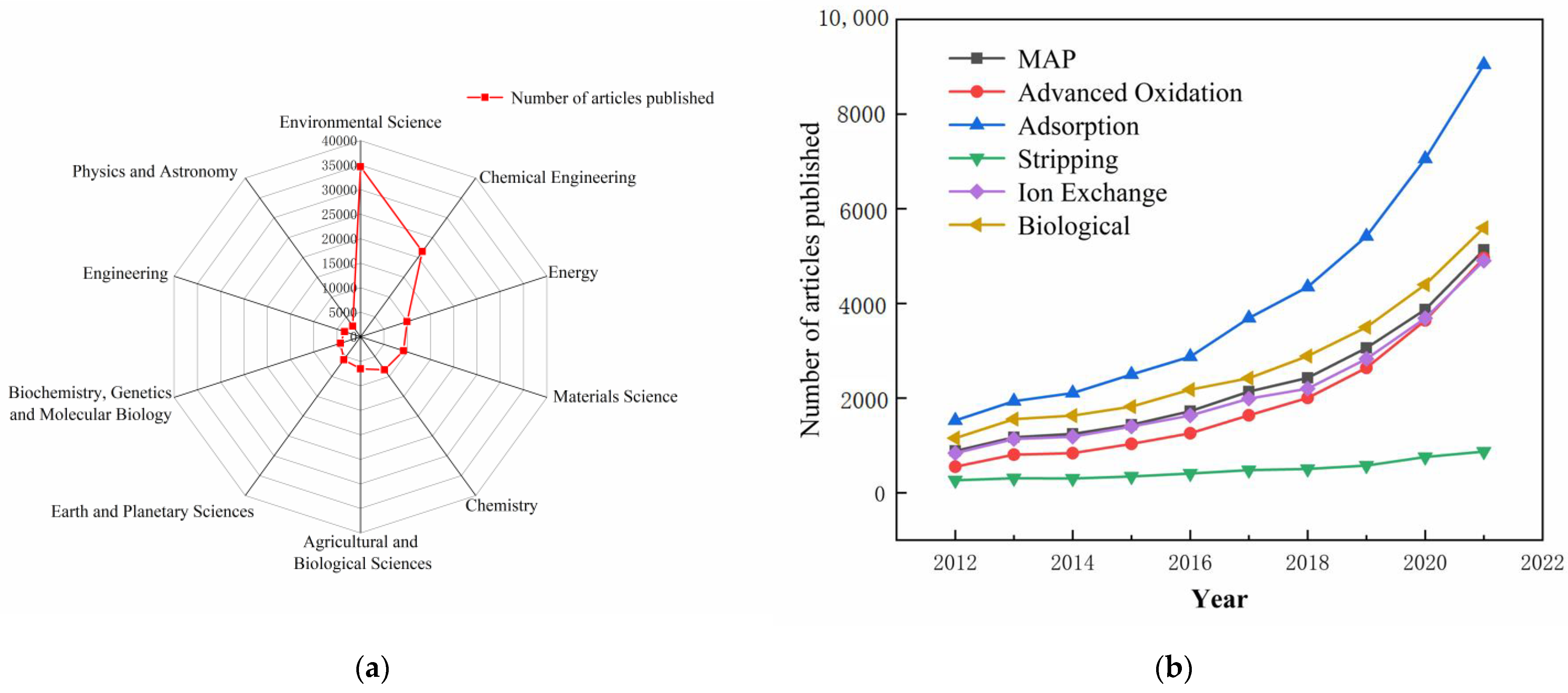
:1. Introduction
2. Nitrogen Removal Process
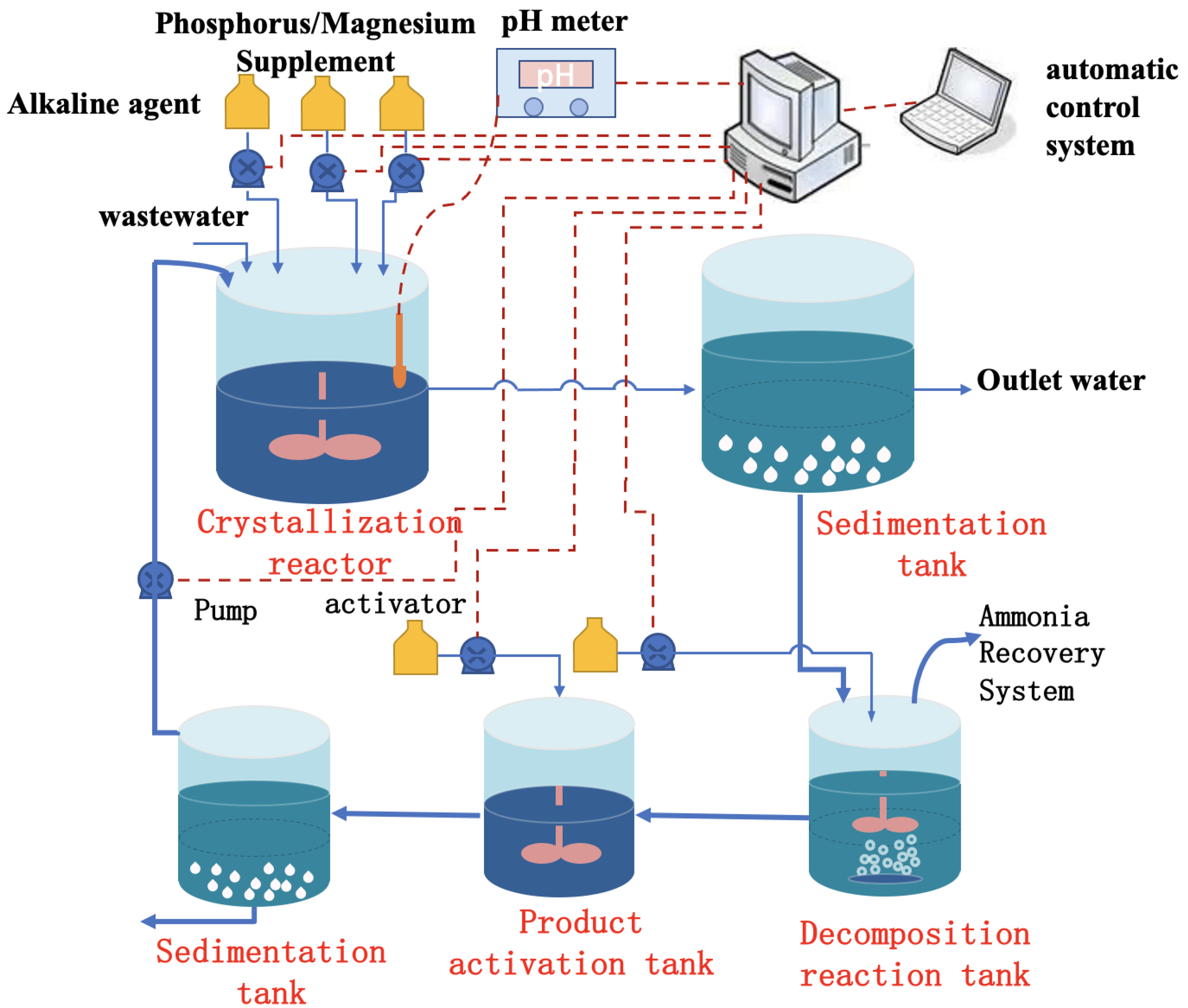
2.1. Chemical Precipitation
2.1.1. Factors Influencing Chemical Precipitation
2.1.2. Disadvantages of Chemical Precipitation
2.2. Adsorption Method
2.2.1. Factors Influencing Adsorption
2.2.2. Disadvantages of Adsorption
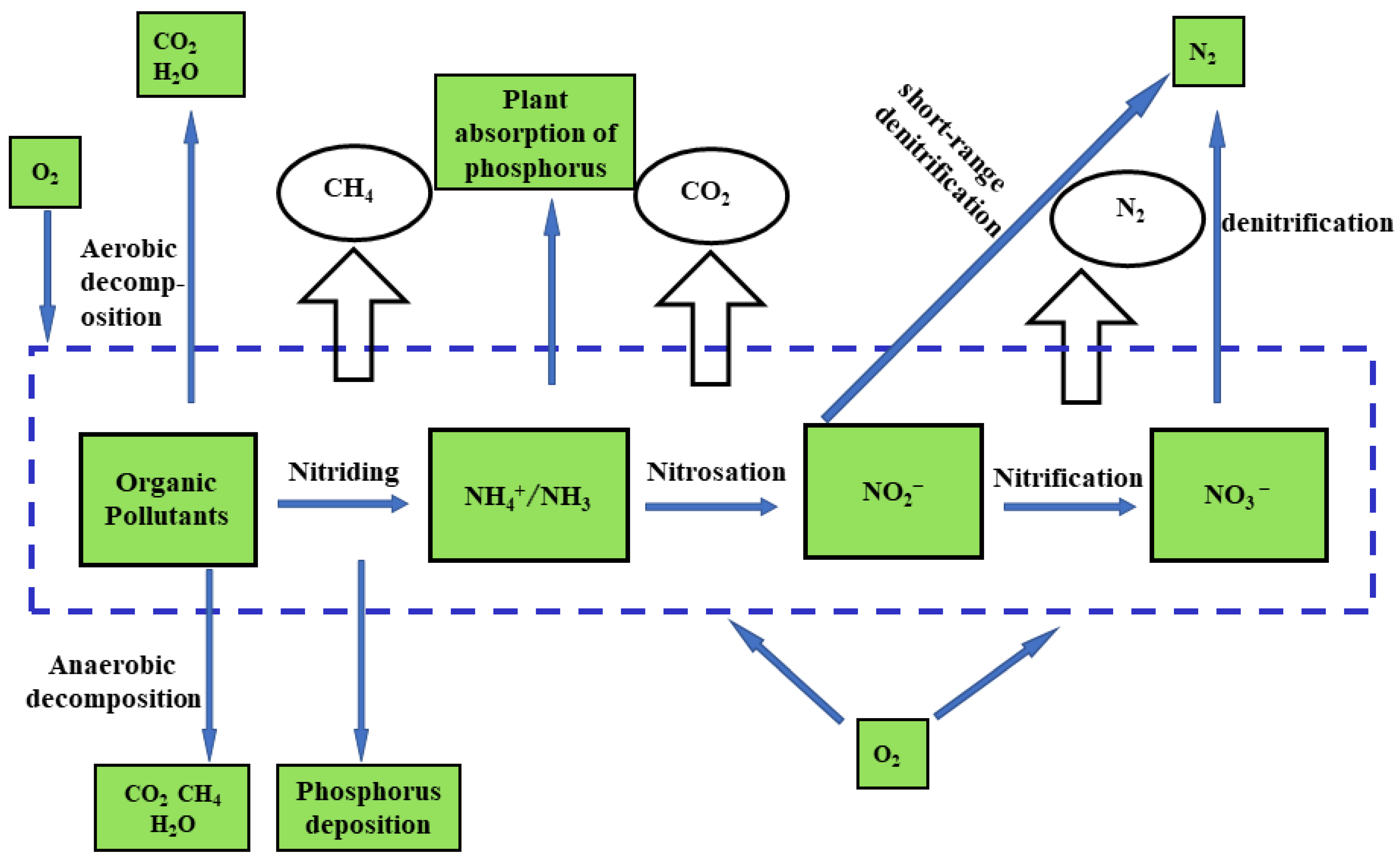
2.3. Biological Nutrient Removal (BNR)
2.3.1. Factors Influencing the Biological Method
2.3.2. Disadvantages of the Biological Method
3. Nitrogen Recovery Process
3.1. Membrane Distillation
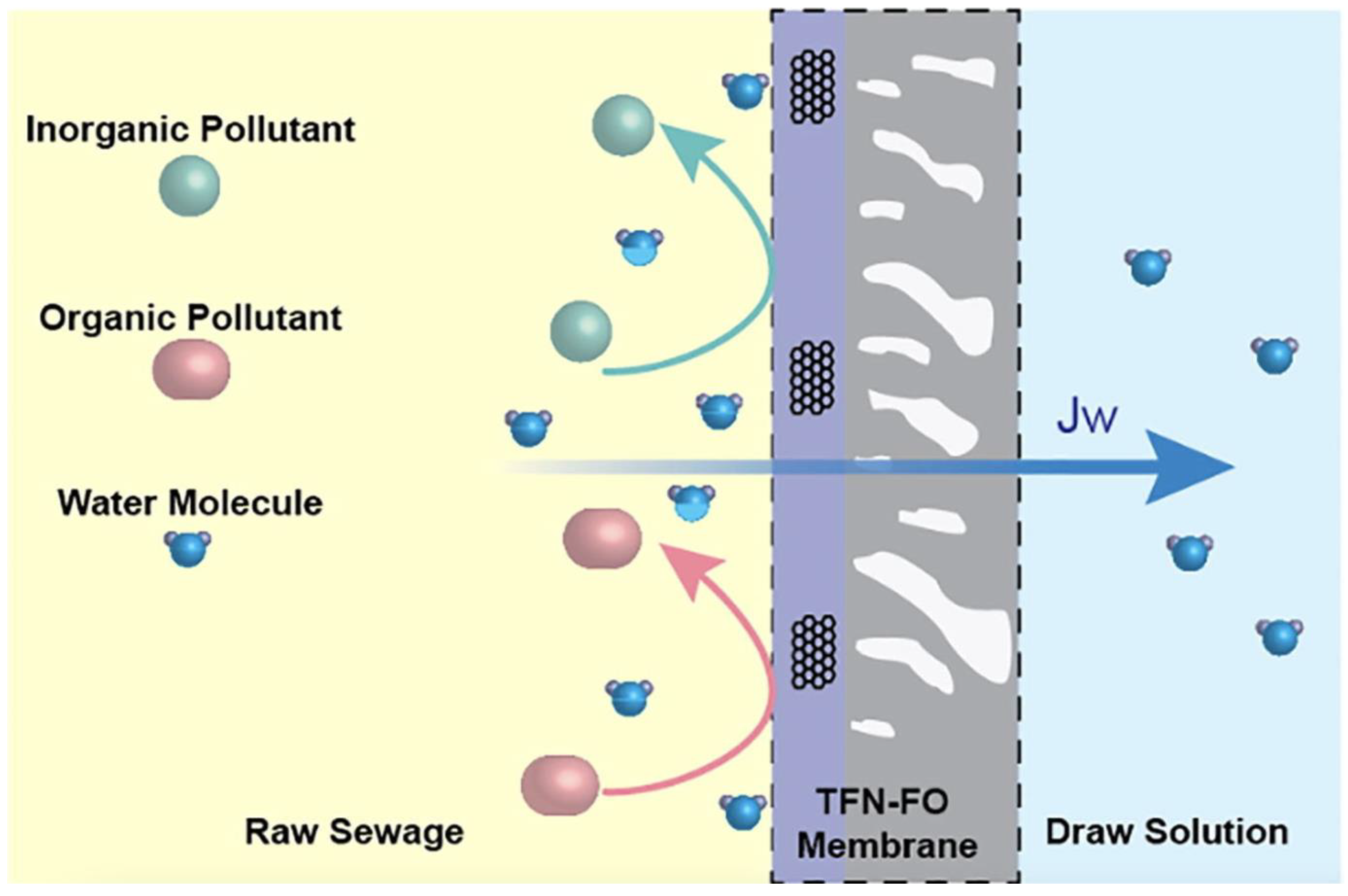
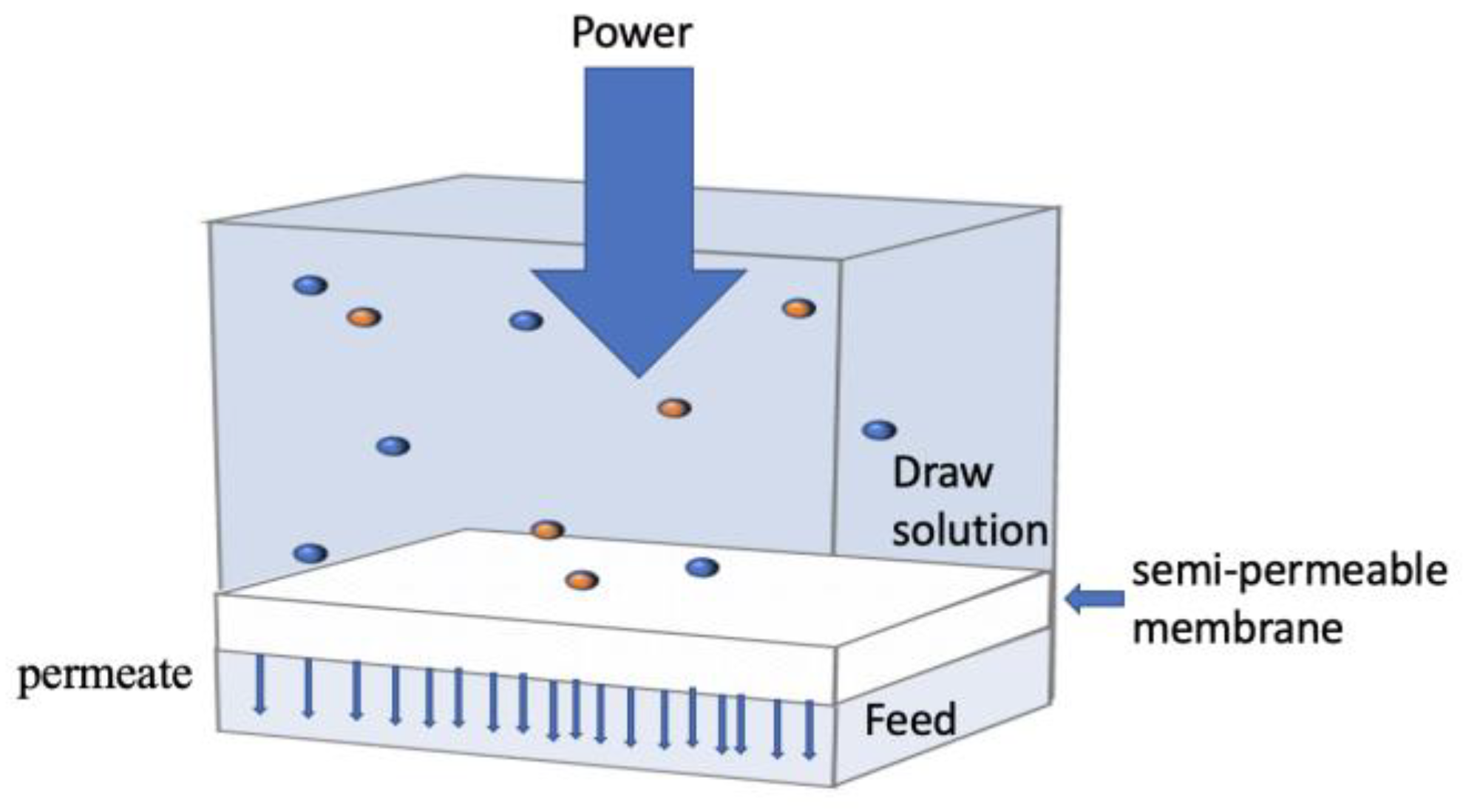
3.2. Forward Osmosis (FO)
3.3. Reverse Osmosis and Nanofiltration (RO and NF)
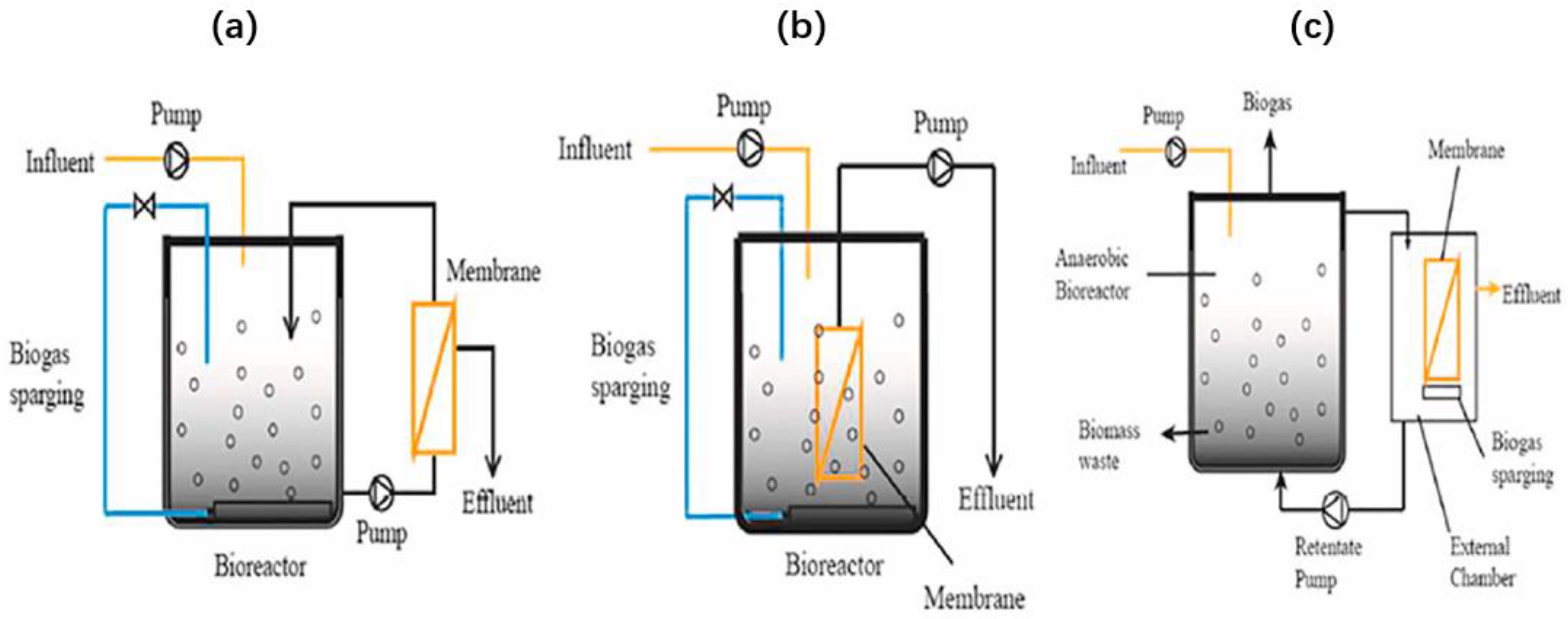
3.4. Anaerobic Membrane Bioreactor (AnMBR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, C.; Stüeken, E.E.; Rosen, C.J.; Mateos, K.; Anderson, R.E. Radiation of Nitrogen-Metabolizing Enzymes across the Tree of Life Tracks Environmental Transitions in Earth History. Geobiology 2021, 19, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.; Fergany, M.; Elhabbasha, E.F.; El-temsah, M. Productivity Improvement of Canola Genotypes Under Salinity Stress Conditions by Integration between Mineral and Nano-Scale Forms of Nitrogen Fertilizer. Arab. Univ. J. Agric. Sci. 2022, 30, 1–16. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Kabir, M.; Zuhara, F.T.; Mehjabin, A.; Tasannum, N.; Hoang, A.T.; Kabir, Z.; Mofijur, M. Threats, Challenges and Sustainable Conservation Strategies for Freshwater Biodiversity. Environ. Res. 2022, 214, 113808. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [Green Version]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of Heterotrophic Aerobic Denitrifying Bacteria in Nitrate Removal from Wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Singh, V.; Cheng, L.; Hussain, A.; Ormeci, B. Nitrogen Removal from Wastewater: A Comprehensive Review of Biological Nitrogen Removal Processes, Critical Operation Parameters and Bioreactor Design. J. Environ. Chem. Eng. 2022, 10, 107387. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of Ammonia Inhibition in Anaerobic Digestion of Nitrogen-Rich Substrates for Biogas Production by Ammonia Stripping: A Review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical Review of Abatement of Ammonia from Wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- Winkler, M.K.; Straka, L. New Directions in Biological Nitrogen Removal and Recovery from Wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, H.; Zhuo, X.; Bai, X.; Shen, J.; Zhang, H. Efficient Treatment of Aged Landfill Leachate Containing High Ammonia Nitrogen Concentration Using Dynamic Wave Stripping: Insights into Influencing Factors and Kinetic Mechanism. Waste Manag. 2022, 150, 48–56. [Google Scholar] [CrossRef]
- Scandelai, A.P.J.; Zotesso, J.P.; Jegatheesan, V.; Cardozo-Filho, L.; Tavares, C.R.G. Intensification of Supercritical Water Oxidation (ScWO) Process for Landfill Leachate Treatment through Ion Exchange with Zeolite. Waste Manag. 2020, 101, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A Comprehensive Review on Technological Advances of Adsorption for Removing Nitrate and Phosphate from Waste Water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical Treatment Technologies for Waste-Water Recycling—An Overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, M.; Meng, X.; Zhou, J.L.; Zhang, H.; Shen, X. The Role of Biochar on Alleviating Ammonia Toxicity in Anaerobic Digestion of Nitrogen-Rich Wastes: A Review. Bioresour. Technol. 2022, 351, 126924. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the Simultaneous Removal of Fluoride, Ammonia Nitrogen and Phosphate from Semiconductor Wastewater Using Chemical Precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Aghdam, E.; Xiang, Y.; Ling, L.; Shang, C. New Insights into Micropollutant Abatement in Ammonia-Containing Water by the UV/Breakpoint Chlorination Process. ACS EST Water 2021, 1, 1025–1034. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Physico-Chemical Technologies for Nitrogen Removal from Wastewaters: A Review. Rev. Ambient. Água 2015, 10, 481–498. [Google Scholar]
- Yi, H.; Li, M.; Huo, X.; Zeng, G.; Lai, C.; Huang, D.; An, Z.; Qin, L.; Liu, X.; Li, B.; et al. Recent Development of Advanced Biotechnology for Wastewater Treatment. Crit. Rev. Biotechnol. 2020, 40, 99–118. [Google Scholar] [CrossRef]
- Cruz, H.; Law, Y.Y.; Guest, J.S.; Rabaey, K.; Batstone, D.; Laycock, B.; Verstraete, W.; Pikaar, I. Mainstream Ammonium Recovery to Advance Sustainable Urban Wastewater Management. Environ. Sci. Technol. 2019, 53, 11066–11079. [Google Scholar] [CrossRef]
- Dai, H.; Han, T.; Sun, T.; Zhu, H.; Wang, X.; Lu, X. Nitrous Oxide Emission during Denitrifying Phosphorus Removal Process: A Review on the Mechanisms and Influencing Factors. J. Environ. Manag. 2021, 278, 111561. [Google Scholar] [CrossRef]
- McCarty, P.L. What Is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable Electrochemical Sensing Methodologies for On-Site Detection of Pesticide Residues in Fruits and Vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.; Huh, Y.S. Advances in Optical-Sensing Strategies for the on-Site Detection of Pesticides in Agricultural Foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Mohana Rani, G.; Lee, E.-S.; Jeon, T.-J.; Kang, S.-M.; Oh, M.-H.; Huh, Y.S. Colorimetric Based On-Site Sensing Strategies for the Rapid Detection of Pesticides in Agricultural Foods: New Horizons, Perspectives, and Challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Venkateswara Raju, C.; Hwan Cho, C.; Mohana Rani, G.; Manju, V.; Umapathi, R.; Suk Huh, Y.; Pil Park, J. Emerging Insights into the Use of Carbon-Based Nanomaterials for the Electrochemical Detection of Heavy Metal Ions. Coord. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen Availability Influences Phosphorus Removal in Microalgae-Based Wastewater Treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef]
- Conant, R.T.; Berdanier, A.B.; Grace, P.R. Patterns and Trends in Nitrogen Use and Nitrogen Recovery Efficiency in World Agriculture. Glob. Biogeochem. Cycles 2013, 27, 558–566. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, K.; Xia, Q.; Yu, S.; Zhang, M.; An, Y.; Zhao, X.; Zhou, Z. Up-Concentration of Nitrogen from Domestic Wastewater: A Sustainable Strategy from Removal to Recovery. Chem. Eng. J. 2023, 451, 138789. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for Biological Removal and Recovery of Nitrogen from Wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Li, J.; Qiu, S.; Cao, D.; Xu, Y.; Liu, Z.; Yu, X.; Sun, Y. Two Benzoyl Coumarin Amide Fluorescence Chemosensors for Cyanide Anions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 1–6. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Ding, L. Recovery of Phosphate and Ammonia Nitrogen from the Anaerobic Digestion Supernatant of Activated Sludge by Chemical Precipitation. J. Clean. Prod. 2015, 102, 437–446. [Google Scholar] [CrossRef]
- Dube, P.J.; Vanotti, M.B.; Szogi, A.A.; García-González, M.C. Enhancing Recovery of Ammonia from Swine Manure Anaerobic Digester Effluent Using Gas-Permeable Membrane Technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New Progress of Ammonia Recovery during Ammonia Nitrogen Removal from Various Wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 144. [Google Scholar] [CrossRef] [PubMed]
- Vanotti, M.B.; Dube, P.J.; Szogi, A.A.; García-González, M.C. Recovery of Ammonia and Phosphate Minerals from Swine Wastewater Using Gas-Permeable Membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Al-Juboori, R.A.; Al-Shaeli, M.; Aani, S.A.; Johnson, D.; Hilal, N. Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis. Membranes 2023, 13, 15. [Google Scholar] [CrossRef]
- Han, F.; Zhou, W. Nitrogen Recovery from Wastewater by Microbial Assimilation—A Review. Bioresour. Technol. 2022, 363, 127933. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Liu, Q.; Bui, X.T.; Hoang, N.B. Bio-Membrane Integrated Systems for Nitrogen Recovery from Wastewater in Circular Bioeconomy. Chemosphere 2022, 289, 133175. [Google Scholar] [CrossRef]
- Arora, A.S.; Nawaz, A.; Qyyum, M.A.; Ismail, S.; Aslam, M.; Tawfik, A.; Yun, C.M.; Lee, M. Energy Saving Anammox Technology-Based Nitrogen Removal and Bioenergy Recovery from Wastewater: Inhibition Mechanisms, State-of-the-Art Control Strategies, and Prospects. Renew. Sustain. Energy Rev. 2021, 135, 110126. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Resource Recovery from Municipal Wastewater: A Critical Paradigm Shift in the Post Era of Activated Sludge. Bioresour. Technol. 2022, 363, 127932. [Google Scholar] [CrossRef]
- Chan-Pacheco, C.R.; Valenzuela, E.I.; Cervantes, F.J.; Quijano, G. Novel Biotechnologies for Nitrogen Removal and Their Coupling with Gas Emissions Abatement in Wastewater Treatment Facilities. Sci. Total Environ. 2021, 797, 149228. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Nie, J.; Luo, X.; Wang, D. Chemical Precipitation and Biosorption Treating Landfill Leachate to Remove Ammonium-Nitrogen. Clean Techn. Environ. Policy 2013, 15, 395–399. [Google Scholar] [CrossRef]
- Bi, W.; Li, Y.; Hu, Y. Recovery of Phosphorus and Nitrogen from Alkaline Hydrolysis Supernatant of Excess Sludge by Magnesium Ammonium Phosphate. Bioresour. Technol. 2014, 166, 1–8. [Google Scholar] [CrossRef]
- Jeguirim, M.; Belhachemi, M.; Limousy, L.; Bennici, S. Adsorption/Reduction of Nitrogen Dioxide on Activated Carbons: Textural Properties versus Surface Chemistry—A Review. Chem. Eng. J. 2018, 347, 493–504. [Google Scholar] [CrossRef]
- Ren, Z.; Jia, B.; Zhang, G.; Fu, X.; Wang, Z.; Wang, P.; Lv, L. Study on Adsorption of Ammonia Nitrogen by Iron-Loaded Activated Carbon from Low Temperature Wastewater. Chemosphere 2021, 262, 127895. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent Advances in Nitrogen Removal from Landfill Leachate Using Biological Treatments—A Review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef]
- Khan, S.U.; Pratap, V.; Uddin, M.K.; Farooqi, I.H. Chapter 2—An Overview of Conventional and Advanced Water Defluoridation Techniques. In Green Technologies for the Defluoridation of Water; Hadi Dehghani, M., Karri, R., Lima, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 17–40. ISBN 978-0-323-85768-0. [Google Scholar]
- Izadi, P.; Izadi, P.; Eldyasti, A. Enhancement of Simultaneous Nitrogen and Phosphorus Removal Using Intermittent Aeration Mechanism. J. Environ. Sci. 2021, 109, 1–14. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Li, Z.; Ren, X.; Zuo, J.; Liu, Y.; Duan, E.; Yang, J.; Chen, P.; Wang, Y. Struvite Precipitation for Ammonia Nitrogen Removal in 7-Aminocephalosporanic Acid Wastewater. Molecules 2012, 17, 2126–2139. [Google Scholar] [CrossRef]
- Sanghavi, R.J.; Dobariya, R.; Bhatti, S.; Kumar, A. Preparation of High-Purity Magnesium-Ammonium-Phosphate Fertilizer Using Sea Bittern and Industrial Waste Streams. Environ. Sci. Pollut. Res. 2020, 27, 7720–7728. [Google Scholar] [CrossRef]
- Xu, G.; Ren, J.; Cui, K.; Guo, K. Effect of Operating Conditions on Phosphorus Recovery from Acidified Plant Oil Wastewater by Struvite Crystallization. IOP Conf. Ser. Earth Environ. Sci. 2023, 1135, 012014. [Google Scholar] [CrossRef]
- Korchef, A.; Naffouti, S.; Souid, I. Recovery of High Concentrations of Phosphorus and Ammonium through Struvite Crystallization by CO2 Repelling. Cryst. Res. Technol. 2022, 57, 2200123. [Google Scholar] [CrossRef]
- Shen, Q.; Yuan, J.; Luo, X.; Qin, Y.; Hu, S.; Liu, J.; Hu, H.; Xu, D. Simultaneous Recovery of Nitrogen and Phosphorus from Sewage by Magnesium Ammonium Phosphate Method with Magnesium-Loaded Bentonite. Langmuir 2023, 39, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Warmadewanthi, I.D.A.A.; Zulkarnain, M.A.; Ikhlas, N.; Kurniawan, S.B.; Abdullah, S.R.S. Struvite Precipitation as Pretreatment Method of Mature Landfill Leachate. Bioresour. Technol. Rep. 2021, 15, 100792. [Google Scholar] [CrossRef]
- Astals, S.; Martínez-Martorell, M.; Huete-Hernández, S.; Aguilar-Pozo, V.B.; Dosta, J.; Chimenos, J.M. Nitrogen Recovery from Pig Slurry by Struvite Precipitation Using a Low-Cost Magnesium Oxide. Sci. Total Environ. 2021, 768, 144284. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, Y.V.; Vol’khin, V.V.; Permyakova, I.A. Recovery of Nitrogen and Phosphorus in Processing of Aqueous Production Wastes by Precipitation of Struvite Using an Active Intermediate as a Reagent. Russ. J. Appl. Chem. 2022, 95, 588–601. [Google Scholar] [CrossRef]
- Ramagundam, R. A study on ammonium nitrogen removal in urea fertilizer plant sewagE. J. Inf. Comput. Sci. 2020, 13, 190–198. [Google Scholar]
- Song, L.; Li, Z.; Wang, G.; Tian, Y.; Yang, C. Supersaturation Control of Struvite Growth by Operating PH. J. Mol. Liq. 2021, 336, 116293. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of Slow Release Crystal Fertilizer from Wastewaters through Struvite Crystallization—A Review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous Recovery of Ammonium, Potassium and Magnesium from Produced Water by Struvite Precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, L.; Ren, H.; Xiong, X. Ammonium Nitrogen Removal from Coking Wastewater by Chemical Precipitation Recycle Technology. Water Res. 2009, 43, 5209–5215. [Google Scholar] [CrossRef]
- Moulessehoul, A.; Gallart-Mateu, D.; Harrache, D.; Djaroud, S.; de la Guardia, M.; Kameche, M. Conductimetric Study of Struvite Crystallization in Water as a Function of PH. J. Cryst. Growth 2017, 471, 42–52. [Google Scholar] [CrossRef]
- Jia, G. Nutrient Removal and Recovery by the Precipitation of Magnesium Ammonium Phosphate. Master’s Thesis, University of Adelaide, Adelaide, SA, Australia, 2014. [Google Scholar]
- Zhou, S.; Dong, M.; Ding, X.; Xue, X.; Yang, H. Application of RSM to Optimize the Recovery of Ammonia Nitrogen from High Chromium Effluent Produced in Vanadium Industry Using Struvite Precipitation. J. Environ. Chem. Eng. 2021, 9, 106318. [Google Scholar] [CrossRef]
- Moghaddam, S.Z.; Thormann, E. The Hofmeister Series: Specific Ion Effects in Aqueous Polymer Solutions. J. Colloid Interface Sci. 2019, 555, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Di Iaconi, C.; Pagano, M.; Ramadori, R.; Lopez, A. Nitrogen Recovery from a Stabilized Municipal Landfill Leachate. Bioresour. Technol. 2010, 101, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Türker, M.; Çelen, I. Removal of Ammonia as Struvite from Anaerobic Digester Effluents and Recycling of Magnesium and Phosphate. Bioresour. Technol. 2007, 98, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in Struvite Precipitation Technologies for Nutrients Removal and Recovery from Aqueous Waste and Wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Guan, Q.; Zeng, G.; Gong, B.; Li, Y.; Ji, H.; Zhang, J.; Song, J.; Liu, C.; Wang, Z.; Deng, C. Phosphorus Recovery and Iron, Copper Precipitation from Swine Wastewater via Struvite Crystallization Using Various Magnesium Compounds. J. Clean. Prod. 2021, 328, 129588. [Google Scholar] [CrossRef]
- Tan, J.; Wu, Z.; Sun, Y.; Zhang, J.; Yin, Z.; Chen, Q. Mechanism Analysis of Ions Interaction in MgCl2/LiCl Solution and Effect of Lithium on Ammonia Precipitation Products. Trans. Nonferrous Met. Soc. China 2015, 25, 319–328. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Zhang, Z.; Liu, J.; Xiao, J.; Gao, F. Simultaneous Removal of Ammonia Nitrogen and Recovery of Phosphate from Swine Wastewater by Struvite Electrochemical Precipitation and Recycling Technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Shu, J.; Wu, H.; Chen, M.; Wei, L.; Wang, B.; Li, B.; Liu, R.; Liu, Z. Simultaneous Optimizing Removal of Manganese and Ammonia Nitrogen from Electrolytic Metal Manganese Residue Leachate Using Chemical Equilibrium Model. Ecotoxicol. Environ. Saf. 2019, 172, 273–280. [Google Scholar] [CrossRef]
- Liang, P.; Yu, H.; Huang, J.; Zhang, Y.; Cao, H. The Review on Adsorption and Removing Ammonia Nitrogen with Biochar on Its Mechanism. MATEC Web Conf. 2016, 67, 07006. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Yuan, R.; Salam, M.; Zhang, L.; Wei, Y.; Tang, B.; Yuan, X.; Liu, B.; Yu, X.; Li, H.; et al. Achieving Simultaneous Removal of Nitrogen and Phosphorus in Sediment via Combined Adsorption and Oxygen Supplement. Chem. Eng. J. 2022, 441, 136056. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, P.; Wang, X.; Yang, J.; Zhou, Z.; Du, X.; Lu, X. Efficient Adsorption Removal of Organic Nitrogen Pesticides: Insight into a New Hollow NiO/Co@C Magnetic Nanocomposites Derived from Metal-Organic Framework. Sep. Purif. Technol. 2022, 287, 120608. [Google Scholar] [CrossRef]
- Cheng, G.; Tan, B.; Zhang, Z.; Fu, S.; Haiyan, W.; Wang, F. Characteristics of Coal-Oxygen Chemisorption at the Low-Temperature Oxidation Stage: DFT and Experimental Study. Fuel 2022, 315, 123120. [Google Scholar] [CrossRef]
- Napolitano, S. Irreversible Adsorption of Polymer Melts and Nanoconfinement Effects. Soft Matter 2020, 16, 5348–5365. [Google Scholar] [CrossRef] [PubMed]
- Yunnen, C.; Xiaoyan, L.; Changshi, X.; Liming, L. The Mechanism of Ion Exchange and Adsorption Coexist on Medium–Low Concentration Ammonium–Nitrogen Removal by Ion-Exchange Resin. Environ. Technol. 2015, 36, 2349–2356. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, H.; Xiong, Z.; Zhao, R.; Liu, Y.; Zhao, C.; Zheng, C. Novel Anionic Polyacrylamide-Modify-Chitosan Magnetic Composite Nanoparticles with Excellent Adsorption Capacity for Cationic Dyes and PH-Independent Adsorption Capability for Metal Ions. Chem. Eng. J. 2020, 392, 123706. [Google Scholar] [CrossRef]
- Dey, S.; Charan, S.S.; Pallavi, U.; Sreenivasulu, A.; Haripavan, N. The Removal of Ammonia from Contaminated Water by Using Various Solid Waste Biosorbents. Energy Nexus 2022, 7, 100119. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujimoto, M.; Minami, K.; Takahashi, A.; Parajuli, D.; Hiwatari, T.; Kawakami, M.; Kawamoto, T. Ammonium Removal and Recovery from Sewage Water Using Column-System Packed Highly Selective Ammonium Adsorbent. Environ. Pollut. 2021, 284, 117495. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, Y.; Meng, X.; Yang, X.; Hu, R.; Liu, Y.; Wu, J. Nitrogen-Functionalized Bone Chars with Developed Surface Area for Efficient Adsorption of Multiple Aquatic Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129061. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhao, J.; Zhang, Z.; Wang, H.; Zhou, S.; Wu, L. Synthesis of Zeolite P1 from Fly Ash under Solvent-Free Conditions for Ammonium Removal from Water. J. Clean. Prod. 2018, 202, 11–22. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, H.; Xu, Y.; Chen, S.; Liao, Y.; Deng, F.; Li, J. Study on the Adsorption of Nitrogen and Phosphorus from Biogas Slurry by NaCl-Modified Zeolite. PLoS ONE 2017, 12, e0176109. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of Slow Pyrolyzed Wood and Rice Husks Biochar for Adsorption of Ammonium Nitrogen from Piggery Manure Anaerobic Digestate Slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Wu, D.; Bai, X.; Lin, Y.; Wu, T.; Zhang, C.; Chen, D.; Li, H. Improving the Supercapacitor Performance of Activated Carbon Materials Derived from Pretreated Rice Husk. J. Energy Storage 2021, 44, 103432. [Google Scholar] [CrossRef]
- Hussain, Z.; Chang, N.; Sun, J.; Xiang, S.; Ayaz, T.; Zhang, H.; Wang, H. Modification of Coal Fly Ash and Its Use as Low-Cost Adsorbent for the Removal of Directive, Acid and Reactive Dyes. J. Hazard. Mater. 2022, 422, 126778. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, O.I.; Fathy, N.A.; El-Nabarawy, T.A. Sorption of Ammonium Ions onto Natural and Modified Egyptian Kaolinites: Kinetic and Equilibrium Studies. Adv. Phys. Chem. 2014, 2014, e935854. [Google Scholar] [CrossRef] [Green Version]
- Elkhalifah, A.E.I.; Maitra, S.; Bustam, M.A.; Murugesan, T. Effects of Exchanged Ammonium Cations on Structure Characteristics and CO2 Adsorption Capacities of Bentonite Clay. Appl. Clay Sci. 2013, 83–84, 391–398. [Google Scholar] [CrossRef]
- Mazloomi, F.; Jalali, M. Ammonium Removal from Aqueous Solutions by Natural Iranian Zeolite in the Presence of Organic Acids, Cations and Anions. J. Environ. Chem. Eng. 2016, 4, 1664–1673. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, H.; Zhu, B.; Li, H. Combination of Powdered Activated Carbon and Powdered Zeolite for Enhancing Ammonium Removal in Micro-Polluted Raw Water. Chemosphere 2015, 134, 127–132. [Google Scholar] [CrossRef]
- Li, A.; Ge, W.; Liu, L.; Qiu, G. Preparation, Adsorption Performance and Mechanism of MgO-Loaded Biochar in Wastewater Treatment: A Review. Environ. Res. 2022, 212, 113341. [Google Scholar] [CrossRef]
- Pellenz, L.; da Silva, L.J.S.; Mazur, L.P.; de Figueiredo, G.M.; Borba, F.H.; de Souza, A.A.U.; de Souza, S.M.A.G.U.; da Silva, A. Functionalization of Graphene with Nitrogen-Based Groups for Water Purification via Adsorption: A Review. J. Water Process Eng. 2022, 48, 102873. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R. Role of Anaerobic Sludge Digestion in Handling Antibiotic Resistant Bacteria and Antibiotic Resistance Genes—A Review. Bioresour. Technol. 2021, 330, 124970. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Shahid, M.K.; Dash, R.R.; Bhunia, P.; Liu, D.; Varjani, S.; Zhang, T.C.; Surampalli, R.Y. Nutrient Removal from Domestic Wastewater: A Comprehensive Review on Conventional and Advanced Technologies. J. Environ. Manag. 2021, 296, 113246. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial Denitrification Providing Nitrite: Opportunities of Extending Application for Anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Chai, H.; Xiang, Y.; Chen, R.; Shao, Z.; Gu, L.; Li, L.; He, Q. Enhanced Simultaneous Nitrification and Denitrification in Treating Low Carbon-to-Nitrogen Ratio Wastewater: Treatment Performance and Nitrogen Removal Pathway. Bioresour. Technol. 2019, 280, 51–58. [Google Scholar] [CrossRef]
- Wang, Q.; He, J. Complete Nitrogen Removal via Simultaneous Nitrification and Denitrification by a Novel Phosphate Accumulating Thauera sp. Strain SND5. Water Res. 2020, 185, 116300. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, M.; Chen, Y.; Hu, Y.; Luo, J. Insight into Short-Cut of Simultaneous Nitrification and Denitrification Process in Moving Bed Biofilm Reactor: Effects of Carbon to Nitrogen Ratio. Chem. Eng. J. 2020, 400, 125905. [Google Scholar] [CrossRef]
- Singh, V.; Ormeci, B.; Mishra, S.; Hussain, A. Simultaneous Partial Nitrification, ANAMMOX and Denitrification (SNAD)—A Review of Critical Operating Parameters and Reactor Configurations. Chem. Eng. J. 2022, 433, 133677. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological Nitrogen Removal from Sewage via Anammox: Recent Advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef]
- Guo, L.-J.; Zhao, B.; An, Q.; Tian, M. Characteristics of a Novel Aerobic Denitrifying Bacterium, Enterobacter Cloacae Strain HNR. Appl. Biochem. Biotechnol. 2016, 178, 947–959. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Huang, G.; Zhang, L.; Liu, Y. Effect of Dissolved Oxygen Concentration on Nitrogen Removal and Electricity Generation in Self PH-Buffer Microbial Fuel Cell. Int. J. Hydrog. Energy 2020, 45, 34099–34109. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Hassan, G.K.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Mąkinia, J. Integrating Conventional Nitrogen Removal with Anammox in Wastewater Treatment Systems: Microbial Metabolism, Sustainability and Challenges. Environ. Res. 2022, 215, 114432. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, L.; Wang, J.; Yang, S.; Hou, Z.; Wang, X.C.; Chen, R. Partial-Nitritation of Low-Strength Anaerobic Effluent: A Moderate-High Dissolved Oxygen Concentration Facilitates Ammonia-Oxidizing Bacteria Disinhibition and Nitrite-Oxidizing Bacteria Suppression. Sci. Total Environ. 2021, 770, 145337. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Hung, N.-T.; Dutta, K.; Lin, J.-G. Ambient Temperature SNAD Process Treating Anaerobic Digester Liquor of Swine Wastewater. Bioresour. Technol. 2013, 141, 191–198. [Google Scholar] [CrossRef]
- Ravishankar, H.; Nemeth, A.; Massons, G.; Puig, D.; Zardoya, D.; Carpi, N.; Lens, P.N.L.; Heffernan, B. Factors Impacting Simultaneous Nitrification and Denitrification in a Membrane Aerated Biofilm Reactor (MABR) System Treating Municipal Wastewater. J. Environ. Chem. Eng. 2022, 10, 108120. [Google Scholar] [CrossRef]
- Machat, H.; Boudokhane, C.; Roche, N.; Dhaouadi, H. Effects of C/N Ratio and DO Concentration on Carbon and Nitrogen Removals in a Hybrid Biological Reactor. Biochem. Eng. J. 2019, 151, 107313. [Google Scholar] [CrossRef]
- Chen, W.H.; Chiang, Y.A.; Huang, Y.T.; Chen, S.Y.; Sung, S.; Lin, J.G. Tertiary Nitrogen Removal Using Simultaneous Partial Nitrification, Anammox and Denitrification (SNAD) Process in Packed Bed Reactor. Int. Biodeterior. Biodegrad. 2017, 120, 36–42. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent Advances in the Use of Different Substrates in Microbial Fuel Cells toward Wastewater Treatment and Simultaneous Energy Recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Recent Advances in Nutrient Removal and Recovery in Biological and Bioelectrochemical Systems. Bioresour. Technol. 2016, 215, 173–185. [Google Scholar] [CrossRef]
- Pandey, B.; Chen, L. Technologies to Recover Nitrogen from Livestock Manure—A Review. Sci. Total Environ. 2021, 784, 147098. [Google Scholar] [CrossRef]
- Folino, A.; Zema, D.A.; Calabrò, P.S. Environmental and Economic Sustainability of Swine Wastewater Treatments Using Ammonia Stripping and Anaerobic Digestion: A Short Review. Sustainability 2020, 12, 4971. [Google Scholar] [CrossRef]
- Gonzalez-Salgado, I.; Guigui, C.; Sperandio, M. Transmembrane Chemical Absorption Technology for Ammonia Recovery from Wastewater: A Critical Review. Chem. Eng. J. 2022, 444, 136491. [Google Scholar] [CrossRef]
- Alkhatib, A.; Ayari, M.A.; Hawari, A.H. Fouling Mitigation Strategies for Different Foulants in Membrane Distillation. Chem. Eng. Process.—Process Intensif. 2021, 167, 108517. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and Wetting in the Membrane Distillation Driven Wastewater Reclamation Process—A Review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef]
- Kalla, S. Use of Membrane Distillation for Oily Wastewater Treatment—A Review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, M.; Chen, G.; Huang, M.; Tan, W. Nitrogen Recovery from a Palladium Leachate via Membrane Distillation: System Performance and Ammonium Chloride Crystallization. Resour. Conserv. Recycl. 2022, 183, 106368. [Google Scholar] [CrossRef]
- Tun, L.L.; Jeong, D.; Jeong, S.; Cho, K.; Lee, S.; Bae, H. Dewatering of Source-Separated Human Urine for Nitrogen Recovery by Membrane Distillation. J. Membr. Sci. 2016, 512, 13–20. [Google Scholar] [CrossRef]
- Gao, L.; Li, J.-D.; Yang, G.; Zhang, J.; Xie, Z. De-Ammonification Using Direct Contact Membrane Distillation—An Experimental and Simulation Study. Sep. Purif. Technol. 2020, 250, 117158. [Google Scholar] [CrossRef]
- Yang, X.; Pang, H.; Zhang, J.; Liubinas, A.; Duke, M. Sustainable Waste Water Deammonification by Vacuum Membrane Distillation without PH Adjustment: Role of Water Chemistry. Chem. Eng. J. 2017, 328, 884–893. [Google Scholar] [CrossRef]
- Qiu, B.; Fan, S.; Tang, X.; Qi, B.; Deng, L.; Wang, W.; Liu, J.; Wang, Y.; Xiao, Z. Simultaneous Recovery of Phosphorus and Nitrogen from Liquid Digestate by Vacuum Membrane Distillation with Permeate Fractional Condensation. Chin. J. Chem. Eng. 2020, 28, 1558–1565. [Google Scholar] [CrossRef]
- Davey, C.J.; Liu, P.; Kamranvand, F.; Williams, L.; Jiang, Y.; Parker, A.; Tyrrel, S.; McAdam, E.J. Membrane Distillation for Concentrated Blackwater: Influence of Configuration (Air Gap, Direct Contact, Vacuum) on Selectivity and Water Productivity. Sep. Purif. Technol. 2021, 263, 118390. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, P.; Guan, K.; Gonzales, R.R.; Ishigami, T.; Xue, M.; Yoshioka, T.; Matsuyama, H. An Experimental Study on Recovering and Concentrating Ammonia by Sweep Gas Membrane Distillation. Process Saf. Environ. Prot. 2023, 171, 555–560. [Google Scholar] [CrossRef]
- Yu, C.; Yin, W.; Yu, Z.; Chen, J.; Huang, R.; Zhou, X. Membrane Technologies in Toilet Urine Treatment for Toilet Urine Resource Utilization: A Review. RSC Adv. 2021, 11, 35525–35535. [Google Scholar] [CrossRef]
- Jacob, P.; Phungsai, P.; Fukushi, K.; Visvanathan, C. Direct Contact Membrane Distillation for Anaerobic Effluent Treatment. J. Membr. Sci. 2015, 475, 330–339. [Google Scholar] [CrossRef]
- Zico, M.M.; Ricci, B.C.; Reis, B.G.; Magalhães, N.C.; Amaral, M.C.S. Sustainable Ammonia Resource Recovery from Landfill Leachate by Solar-Driven Modified Direct Contact Membrane Distillation. Sep. Purif. Technol. 2021, 264, 118356. [Google Scholar] [CrossRef]
- He, Q.; Tu, T.; Yan, S.; Yang, X.; Duke, M.; Zhang, Y.; Zhao, S. Relating Water Vapor Transfer to Ammonia Recovery from Biogas Slurry by Vacuum Membrane Distillation. Sep. Purif. Technol. 2018, 191, 182–191. [Google Scholar] [CrossRef]
- Qu, D.; Sun, D.; Wang, H.; Yun, Y. Experimental Study of Ammonia Removal from Water by Modified Direct Contact Membrane Distillation. Desalination 2013, 326, 135–140. [Google Scholar] [CrossRef]
- EL-Bourawi, M.S.; Khayet, M.; Ma, R.; Ding, Z.; Li, Z.; Zhang, X. Application of Vacuum Membrane Distillation for Ammonia Removal. J. Membr. Sci. 2007, 301, 200–209. [Google Scholar] [CrossRef]
- Shi, M.; Xiao, M.; Feng, L.; Tu, T.; He, Q.; Yan, S. Water and Green Ammonia Recovery from Anaerobic Digestion Effluent by Two-Stage Membrane Distillation. J. Water Process Eng. 2022, 49, 102949. [Google Scholar] [CrossRef]
- Wu, X.; Tanner, J.; Ng, D.; Acharya, D.; Xie, Z. Sewage Concentration via a Graphene Oxide Modified Thin-Film Nanocomposite Forward Osmosis Membrane: Enhanced Performance and Mitigated Fouling. Chem. Eng. J. 2021, 420, 127718. [Google Scholar] [CrossRef]
- Ji, C.; Zhai, Z.; Jiang, C.; Hu, P.; Zhao, S.; Xue, S.; Yang, Z.; He, T.; Niu, Q.J. Recent Advances in High-Performance TFC Membranes: A Review of the Functional Interlayers. Desalination 2021, 500, 114869. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S. A Short Review of Membrane Fouling in Forward Osmosis Processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpin, F.; Heo, H.; Hasan Johir, M.A.; Cho, J.; Phuntsho, S.; Shon, H.K. Techno-Economic Feasibility of Recovering Phosphorus, Nitrogen and Water from Dilute Human Urine via Forward Osmosis. Water Res. 2019, 150, 47–55. [Google Scholar] [CrossRef]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining Nutrients (N, K, P) from Urban Source-Separated Urine by Forward Osmosis Dewatering. Environ. Sci. Technol. 2014, 48, 3386–3394. [Google Scholar] [CrossRef]
- Engelhardt, S.; Vogel, J.; Duirk, S.E.; Moore, F.B.; Barton, H.A. Assessment of Urea Hydrolysis as a Pretreatment Strategy to Improve Total Nitrogen Rejection from Urine Using Aquaporin-Based Membranes in Forward Osmosis. J. Water Process Eng. 2020, 34, 101135. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent Developments in Forward Osmosis: Opportunities and Challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Sasaki, Y.; Istirokhatun, T.; Li, J.; Matsuyama, H. Ammonium Enrichment and Recovery from Synthetic and Real Industrial Wastewater by Amine-Modified Thin Film Composite Forward Osmosis Membranes. Sep. Purif. Technol. 2022, 297, 121534. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T.-S. Draw Solutions for Forward Osmosis Processes: Developments, Challenges, and Prospects for the Future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Gulied, M.; Al Momani, F.; Khraisheh, M.; Bhosale, R.; AlNouss, A. Influence of Draw Solution Type and Properties on the Performance of Forward Osmosis Process: Energy Consumption and Sustainable Water Reuse. Chemosphere 2019, 233, 234–244. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y.-Q. Enhancing Ammonium Rejection in Forward Osmosis for Wastewater Treatment by Minimizing Cation Exchange. J. Membr. Sci. 2022, 648, 120365. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Q.; Ge, Q. Recent Advances in Forward Osmosis (FO) Membrane: Chemical Modifications on Membranes for FO Processes. Desalination 2017, 419, 101–116. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and Advanced Membrane Technology for Wastewater Treatment: A Review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Bardhan, A.; Akhtar, A.; Subbiah, S. Chapter 1—Microfiltration and Ultrafiltration Membrane Technologies. In Advancement in Polymer-Based Membranes for Water Remediation; Nayak, S.K., Dutta, K., Gohil, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–42. ISBN 978-0-323-88514-0. [Google Scholar]
- Liu, C.; An, Y.-P.; Yang, J.; Guo, B.-B.; Yu, H.-H.; Xu, Z.-K. Osmotic Pressure as Driving Force for Recovering Ionic Liquids from Aqueous Solutions. J. Membr. Sci. 2020, 599, 117835. [Google Scholar] [CrossRef]
- Lumami Kapepula, V.; García Alvarez, M.; Sang Sefidi, V.; Buleng Njoyim Tamungang, E.; Ndikumana, T.; Musibono, D.-D.; Van Der Bruggen, B.; Luis, P. Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technol. 2022, 4, 1300–1316. [Google Scholar] [CrossRef]
- Courtney, C.; Randall, D.G. A Hybrid Nanofiltration and Reverse Osmosis Process for Urine Treatment: Effect on Urea Recovery and Purity. Water Res. 2022, 222, 118851. [Google Scholar] [CrossRef]
- Arola, K.; Van der Bruggen, B.; Mänttäri, M.; Kallioinen, M. Treatment Options for Nanofiltration and Reverse Osmosis Concentrates from Municipal Wastewater Treatment: A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2049–2116. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse Osmosis Desalination: A State-of-the-Art Review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Malaeb, L.; Ayoub, G.M. Reverse Osmosis Technology for Water Treatment: State of the Art Review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Papac Zjačić, J.; Morović, S.; Košutić, K.; Ašperger, D. Combined Application of Membrane and Advanced Oxidation Processes for Removal of Pharmaceuticals from Water. Kem. Ind. 2022, 71, 719–727. [Google Scholar] [CrossRef]
- Häyrynen, K.; Pongrácz, E.; Väisänen, V.; Pap, N.; Mänttäri, M.; Langwaldt, J.; Keiski, R.L. Concentration of Ammonium and Nitrate from Mine Water by Reverse Osmosis and Nanofiltration. Desalination 2009, 240, 280–289. [Google Scholar] [CrossRef]
- Moresi, M.; Ceccantoni, B.; Lo Presti, S. Modelling of Ammonium Fumarate Recovery from Model Solutions by Nanofiltration and Reverse Osmosis. J. Membr. Sci. 2002, 209, 405–420. [Google Scholar] [CrossRef]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of Olive Mill Wastewaters by Nanofiltration and Reverse Osmosis Membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]
- Al-Mutaz, I.; Ghunaimi, M. Performance of Reverse Osmosis Units at High Temperatures; IDA: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kaya, Y.; Dayanir, S. Application of Nanofiltration and Reverse Osmosis for Treatment and Reuse of Laundry Wastewater. J. Environ. Health Sci. Engineer. 2020, 18, 699–709. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Rejection of Nitrogen Species in Real Fresh and Hydrolyzed Human Urine by Reverse Osmosis and Nanofiltration. J. Environ. Chem. Eng. 2020, 8, 103993. [Google Scholar] [CrossRef]
- Cancino-Madariaga, B.; Hurtado, C.F.; Ruby, R. Effect of Pressure and PH in Ammonium Retention for Nanofiltration and Reverse Osmosis Membranes to Be Used in Recirculation Aquaculture Systems (RAS). Aquac. Eng. 2011, 45, 103–108. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Song, X.; Zhang, B.; Li, G.; Huda, N.; Luo, W. Membrane Processes for Resource Recovery from Anaerobically Digested Livestock Manure Effluent: Opportunities and Challenges. Curr. Pollution Rep. 2020, 6, 123–136. [Google Scholar] [CrossRef]
- Sudeep, B.; Kumar, K.S.; Kamalakannan, V.P.; Ratnam, M.V.; Bharathiraja, B. Membrane Process Technologies for Industrial Saline Wastewater Treatment and Its Applications. In Removal of Pollutants from Saline Water; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-318543-7. [Google Scholar]
- Wang, Y.; Shen, C.; Tang, Z.; Yao, Y.; Wang, X.; Park, B. Interaction between Particulate Fouling and Precipitation Fouling: Sticking Probability and Deposit Bond Strength. Int. J. Heat Mass Transf. 2019, 144, 118700. [Google Scholar] [CrossRef]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral Scaling in Membrane Desalination: Mechanisms, Mitigation Strategies, and Feasibility of Scaling-Resistant Membranes. J. Membr. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhammad, M.H.; Ismail, N.I. A Review of Biological Drinking Water Treatment Technologies for Contaminants Removal from Polluted Water Resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Dardouri, M.; Bettencourt, A.; Martin, V.; Carvalho, F.; Santos, C.; Monge, N.; Santos, N.; Fernandes, M.; Gomes, P.; Ribeiro, I. Using Plasma-Mediated Covalent Functionalization of Rhamnolipids on Polydimethylsiloxane towards the Antimicrobial Improvement of Catheter Surfaces. Mater. Sci. Eng. C 2021, 134, 112563. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, R.; Nir, O.; Lahav, O.; Green, M. Selective Nitrate Removal from Groundwater Using a Hybrid Nanofiltration–Reverse Osmosis Filtration Scheme. Chem. Eng. J. 2015, 279, 372–378. [Google Scholar] [CrossRef]
- Skouteris, G.; Hermosilla, D.; López, P.; Negro, C.; Blanco, Á. Anaerobic Membrane Bioreactors for Wastewater Treatment: A Review. Chem. Eng. J. 2012, 198–199, 138–148. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane Bioreactor for Wastewater Treatment: A Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic Membrane Bioreactors (AnMBRs) for Municipal Wastewater Treatment- Potential Benefits, Constraints, and Future Perspectives: An Updated Review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef]
- Grossman, A.D.; Belete, Y.Z.; Boussiba, S.; Yogev, U.; Posten, C.; Ortiz Tena, F.; Thomsen, L.; Wang, S.; Gross, A.; Leu, S.; et al. Advanced Near-Zero Waste Treatment of Food Processing Wastewater with Water, Carbon, and Nutrient Recovery. Sci. Total Environ. 2021, 779, 146373. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, J.; Rong, C.; Wang, T.; Li, L.; Luo, Z.; Ji, J.; Hanaoka, T.; Sakemi, S.; Ito, M.; et al. Large Pilot-Scale Submerged Anaerobic Membrane Bioreactor for the Treatment of Municipal Wastewater and Biogas Production at 25 °C. Bioresour. Technol. 2021, 319, 124123. [Google Scholar] [CrossRef]
- Gao, D.; Sui, L.; Liang, H. How Microbial Community and Membrane Biofouling Respond to Temperature Changes in an Anaerobic Membrane Bioreactor. Environ. Technol. Innov. 2022, 28, 102675. [Google Scholar] [CrossRef]
- Yu, G.; Peng, H.; Fu, Y.; Yan, X.; Du, C.; Chen, H. Enhanced Nitrogen Removal of Low C/N Wastewater in Constructed Wetlands with Co-Immobilizing Solid Carbon Source and Denitrifying Bacteria. Bioresour. Technol. 2019, 280, 337–344. [Google Scholar] [CrossRef]
- Hao, L.; Liss, S.N.; Liao, B.Q. Influence of COD:N Ratio on Sludge Properties and Their Role in Membrane Fouling of a Submerged Membrane Bioreactor. Water Res. 2016, 89, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, W.S.; Ngo, H.H.; Chang, S.W.; Nguyen, D.D.; Zhang, J.; Liang, S.; Guo, J.B.; Zhang, X.B. Effects of C/N Ratio on the Performance of a Hybrid Sponge-Assisted Aerobic Moving Bed-Anaerobic Granular Membrane Bioreactor for Municipal Wastewater Treatment. Bioresour. Technol. 2018, 247, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Pollice, A.; Tandoi, V.; Lestingi, C. Influence of Aeration and Sludge Retention Time on Ammonium Oxidation to Nitrite and Nitrate. Water Res. 2002, 36, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged Anaerobic Membrane Bioreactor for Low-Strength Wastewater Treatment: Effect of HRT and SRT on Treatment Performance and Membrane Fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A Review on Anaerobic Membrane Bioreactors: Applications, Membrane Fouling and Future Perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Ji, J.; Kakade, A.; Yu, Z.; Khan, A.; Liu, P.; Li, X. Anaerobic Membrane Bioreactors for Treatment of Emerging Contaminants: A Review. J. Environ. Manag. 2020, 270, 110913. [Google Scholar] [CrossRef]
- Chen, F.; Bi, X.; Ng, H.Y. Effects of Bio-Carriers on Membrane Fouling Mitigation in Moving Bed Membrane Bioreactor. J. Membr. Sci. 2016, 499, 134–142. [Google Scholar] [CrossRef]
- Wang, K.M.; Jiang, S.F.; Zhang, Z.H.; Ye, Q.Q.; Zhang, Y.C.; Zhou, J.H.; Hong, Q.K.; Yu, J.M.; Wang, H.Y. Impact of Static Biocarriers on the Microbial Community, Nitrogen Removal and Membrane Fouling in Submerged Membrane Bioreactor at Different COD:N Ratios. Bioresour. Technol. 2020, 301, 122798. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Yu, M.; Wang, Y.; Yang, F. Performance of Anaerobic Forward Osmosis Membrane Bioreactor (AnOMBR) for Dissolved Gases Transfer and Energy Recovery. J. Clean. Prod. 2019, 235, 943–952. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Lee, D.-J.; Tung, K.-L.; Jin, P.; Wang, J.; Wu, Y. Challenges in Biogas Production from Anaerobic Membrane Bioreactors. Renew. Energy 2016, 98, 120–134. [Google Scholar] [CrossRef]

| Method | Working Environment | Advantage | Shortcoming | Outlet Concentration (mg/L NH4–N) | Removal Efficiency |
|---|---|---|---|---|---|
| Chemical precipitation [41,42] | Requires a specific pH and temperature | Produces valuable fertilizers at a moderate cost | Requires additional magnesium source; incurs phosphate cost; introduces new contaminants | 29–100 | 20–98% |
| Adsorption method [43,44] | Broad temperature and pH range | Simple and effective removal of NH4+; able to work at low NH4+ concentrations | Adsorbents have different removal efficiencies | 1 | 43–97% |
| Biological method [29,45] | Heterotrophic, photosynthetic algal, or bacterial growth is temperature sensitive | No need for chemical reagents and complicated configurations; high denitrification efficiency | High cost; requires external carbon source; only operates at low input/output concentrations; long start-up time | <5 | 70–99.9% |
| Operational Technology | Total Nitrogen Removal Efficiency | Cost | Effect | Main Parameters |
|---|---|---|---|---|
| Partial nitrification of nitrite [96] | The nitrite reduction rate is increased by 1.5–2 times in the subsequent denitrification stage | 40% reduction in COD 1 | 25% reduction in oxygen demand and 20% reduction in CO2 emissions during denitrification | pH, temperature, DO 2, real-time aeration control, SRT 3, and substrate concentration |
| Simultaneous nitrification and denitrification [97,98] | Nitrogen removal rate 99% | Requires external carbon sources | Almost complete removal of organic matter and NH4+-N, no accumulation of by-products | DO, carbon source, reactor design, oxygen availability for nitrification, and efficient carbon source utilization for denitrification |
| Short-path nitrification and denitrification [99] | The nitrite denitrification rate is 1.5–2 times higher than the nitrate denitrification rate | 40% reduction in electron donor requirement during the anaerobic phase | In the aerobic stage, the oxygen demand is reduced by 25%, and the energy saving is 60%. It is suitable for wastewater with high ammonia concentration or low C/N ratio | DO, HRT 4, pH, C/N 5 ratio, substrate concentration, and aeration mode |
| Simultaneous partial nitrification, anammox, and denitrification [100] | 99% denitrification | Low concentration of organic matter | Simultaneous removal of inorganic nitrogen and organic carbon, suitable for wastewater with complex composition, high ammonia concentration, and low C/N ratio | Intermittent aeration, pH, DO, C/N ratio, and free ammonia concentration |
| Anammox [101] | The denitrification rate is over 90% | 100% Reduction in organic carbon source requirements | Oxygen demand is reduced by 60%, and N2O production is reduced. In addition, the anammox process produces 90% less sludge, which reduces sludge disposal costs | Reactor configuration, initial biomass concentration, usually for high NH4+ ion wastewater |
| Method | Principle | Nitrogen Removal Efficiency | By-Product | Shortcoming |
|---|---|---|---|---|
| Ammonia stripping [113] | Through the difference in gas partial pressure, free ammoniacal nitrogen escapes from wastewater in a gaseous state | 50–98% | Ammonium sulfate | Large air consumption, high energy consumption, secondary pollution. Easy scaling, |
| MAP precipitation [41] | By adding chemical reagents to form precipitation to achieve solid-liquid separation, to separate ammoniacal nitrogen | 65–98% | MAP precipitation | Requires additional phosphorus and magnesium sources, and causes secondary pollution |
| Membrane technology [114] | Separation of nitrogen gas by selective ion permeation through membranes | 64–99.8% | Ammonium salt fertilizer | Membrane fouling and wastewater contaminants settle on the membrane surface, reducing the efficiency |
| Configure | Advantage | Shortcoming | Nitrogen Recovery Efficiency | Reference |
|---|---|---|---|---|
| DCMD | 1. High permeation flux 2. Simple design and operation 3. The flux is more stable, and the output ratio is high | 1. Low thermal efficiency 2. Temperature and concentration polarization have great influence 3. The water quality is seriously polluted. | >90% | [119,120,121] |
| VMD | 1. High permeation flux 2. Less conduction heat loss 3. The water quality of the product is good | 1. The wettability of membrane pores are strong, and the membrane fouling rate is higher. 2. Heat recovery is difficult, requiring a vacuum pump and an external condenser. | >95% | [122,123] |
| AGMD | 1. Seawater can be used as the cooling water flow on the permeate side. 2. High thermal efficiency 3. Relatively high throughput | 1. Further resistance to water vapor results in a lower permeate flux. 2. The air gap provides additional resistance to steam; the module is difficult to design | 88% | [124] |
| SGMD | 1. High mass transfer rate 2. Low heat loss by conduction | 1. Heat recovery is difficult. 2. Handling sweep gas is difficult. 3. Larger condenser is required, and the cost is higher | 85% | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhu, Y.; Zhu, J.; Li, C.; Chen, G. A Comprehensive Review on Wastewater Nitrogen Removal and Its Recovery Processes. Int. J. Environ. Res. Public Health 2023, 20, 3429. https://doi.org/10.3390/ijerph20043429
Zhou Y, Zhu Y, Zhu J, Li C, Chen G. A Comprehensive Review on Wastewater Nitrogen Removal and Its Recovery Processes. International Journal of Environmental Research and Public Health. 2023; 20(4):3429. https://doi.org/10.3390/ijerph20043429
Chicago/Turabian StyleZhou, Yifan, Yingying Zhu, Jinyuan Zhu, Chaoran Li, and Geng Chen. 2023. "A Comprehensive Review on Wastewater Nitrogen Removal and Its Recovery Processes" International Journal of Environmental Research and Public Health 20, no. 4: 3429. https://doi.org/10.3390/ijerph20043429
APA StyleZhou, Y., Zhu, Y., Zhu, J., Li, C., & Chen, G. (2023). A Comprehensive Review on Wastewater Nitrogen Removal and Its Recovery Processes. International Journal of Environmental Research and Public Health, 20(4), 3429. https://doi.org/10.3390/ijerph20043429







