Cost–Benefit of Real-Time Multiplex PCR Testing of SARS-CoV-2 in German Hospitals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test System
2.2. Model Approach
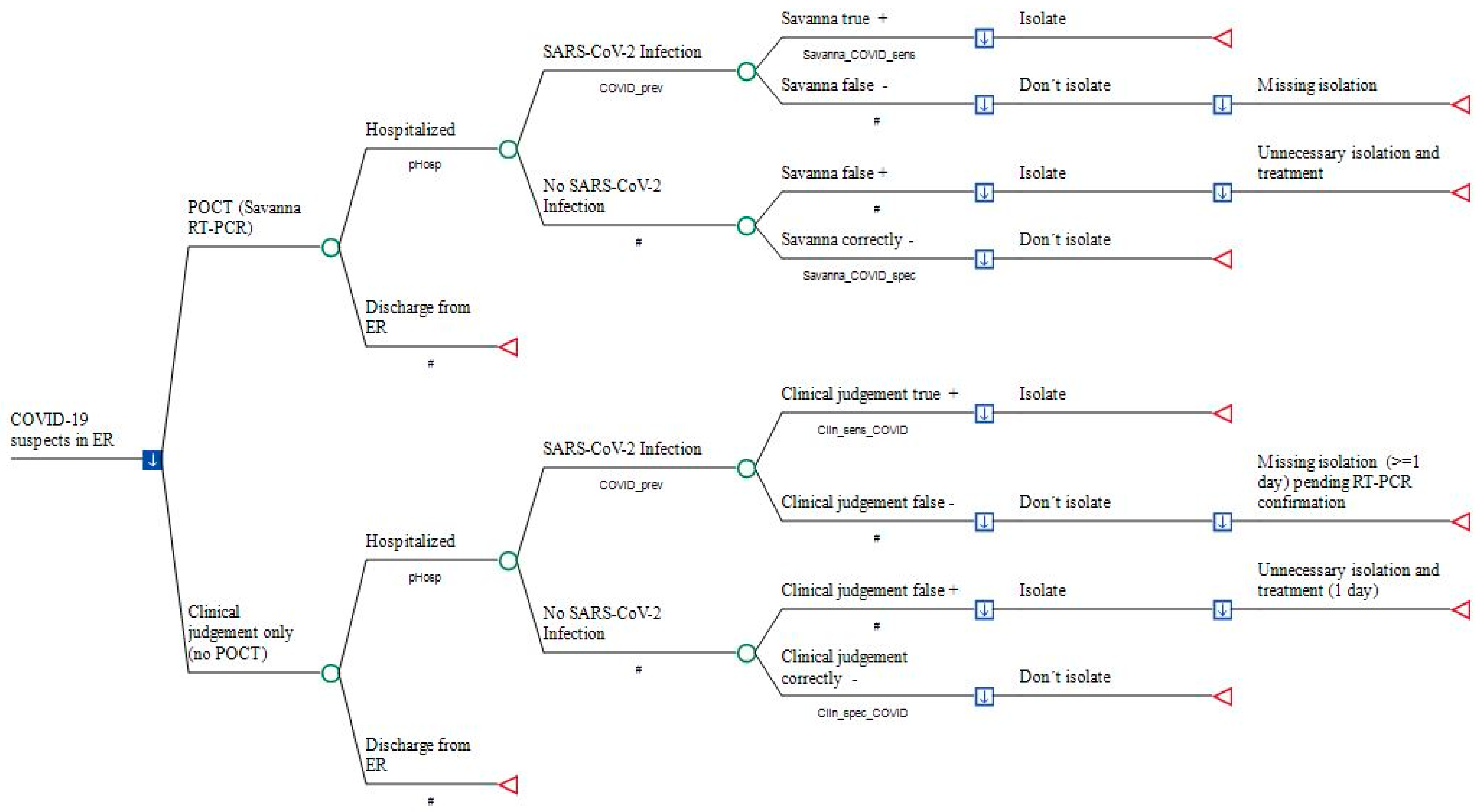
2.3. Model Structure
2.4. Model Input
| Variables Category | Variable Name | Distribution * | Value (Base Case) | Relative Change (Range) | Reference (in Supplement) |
|---|---|---|---|---|---|
| Prevalence of COVID-19 | COVID_prev | PERT | 0.156 | 0.079–0.412 | [19] |
| Additional revenue per day due to earlier discharge | cRev_day_POCT | uniform | €329.42 | ±20% (€263.54–€395.30) | Calculated using data from Institute for the Hospital Remuneration System (InEK) [16] |
| Specificity of Savanna® testing | Savanna_COVID_ spec | uniform | 1 | 95% CI (0.9542–1) | [20] |
| Opportunity costs due to blocking twin bed | cOpp_POCT | uniform | €734.53 | ±20% (€587.62–€881.44) | Calculated from InEK data [16] |
| Clinical probability of correctly excluding SARS-CoV-2 | Clin_spec_ COVID | PERT | 0.683 | 95% CI (0.60–0.758) | [21] |
| Clinical sensitivity of diagnosing SARS-CoV-2 infection | Clin_sens_ COVID | PERT | 0.806 | 95% CI (0.729–869) | [21] |
| Costs of enoxaparin per day | cAntithromb_day | uniform | €6.91 | ±20% (€5.53–€8.30) | Rote Liste [Red List] 2022 |
| Costs of dexamethasone per day | cDexa_day | uniform | €1.3572 | ±20% (€1.0857–€1.6286) | Rote Liste [Red List] 2022 |
| Costs of Savanna® PCR | cSavanna_COVID | uniform | €40 | ±20% (€32–€48) | As declared by manufacturer |
| Sensitivity of Savanna® testing | Savanna_COVID_ sens | PERT | 0.9931 | 95% CI (0.9617–0.9988) | [20] |
| Secondary cases in HCW due to one unknown COVID-19 case | sec_COVID_ HCW | PERT | 0.0248 | 95% CI (0.0085–0.0704) | [22] |
| Costs of productivity loss per day | cPL_day | uniform | €170.90 | +20% (€205.8) | Calculated from [23] |
| Number of days of health care workers out of work due to COVID-19 | sick_days | uniform | 15 | +12 (27) | [24,25] |
| Probability that hospitalization is required | pHosp | PERT | 0.6044 | 95% CI (0.5645–0.6429) [PSA: 0.043–0.6429] | [26] |
| Length of hospital stay (median) | dHosp | PERT | 10 | 5–19 (IQR) | [27] |
| Costs of RT-PCR performed in external laboratory | cRT-PCR_ext | uniform | €37.8 | +50% (€56.70) | Nationwide laboratory inquiry |
| Base-Case Analysis | Comparators | Mean Cost Per Patient (€) | Incremental Cost (€) * | Absolute Cost Savings (€) |
|---|---|---|---|---|
| COVID-19 patients prior to hospitalization | Savanna® Multiplex RT-PCR | −25.31 | 0 | −25.31 |
| Conventional approach | 123.06 | 148.37 |
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, P.; Vu, D.L.; L’Huillier, A.G.; Schibler, M.; Kaiser, L.; Jacquerioz, F. Clinical features of covid-19. BMJ 2020, 369, m1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Weekly Epidemiological Update on COVID-19. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---2-november-2022 (accessed on 2 November 2022).
- Robert Koch Institute. COVID-19: Number of Cases in Germany and Worldwide. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html/ (accessed on 7 November 2022).
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Thelwall, S. Comparative analysis of the risks of hospitalisation and death associated with SARSCoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 369, m1470. [Google Scholar]
- Liu, Y.; Rocklöv, J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J. Travel. Med. 2022, 29, taac037. [Google Scholar] [CrossRef] [PubMed]
- Federal Statistical Office of Germany. Wiesbaden–DESTATIS. Health. In Basic Data of the Hospitals; Subject Series 12, row 6.1.1. Hospitals 2020, 2.8 Special Equipment and Special Services in Hospitals; Federal Statistical Office of Germany: Wiesbaden, Germany, 2022. [Google Scholar]
- Multiple Choices. Available online: https://www.drg-research-group.de/index.php?option=com_webgrouper&view=webgrouper&Itemid=112 (accessed on 7 November 2022).
- Vogl, M. Assessing DRG cost accounting with respect to resource allocation and tariff calculation: The case of Germany. Health Econ. Rev. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert Koch Institute. COVID-19: Isolation and Quarantine Recommendations for SARS-CoV-2 Infection and Exposure. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Quarantaene/Absonderung.html?nn=2386228 (accessed on 2 May 2022).
- Robert Koch Institute. COVID-19: Isolation of Patients in the Inpatient Area and Residents in Old People’s and Nursing Homes. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Entlassmanagement.html?nn=13490888 (accessed on 2 May 2022).
- Quidel: Savanna Respiratory Viral Panel-4. Package insert, page 18. Available online: https://www.quidel.com/molecular-diagnostics/savanna-respiratory-viral-panel-4 (accessed on 7 November 2022).
- Tolksdorf, K.; Buda, S.; Schuler, E.; Wieler, L.H.; Haas, W. A higher mortality rate and long ventilation duration distinguish COVID-19 from severe respiratory infections in flu waves. EpiBull 2020, 41, 3–10. [Google Scholar]
- AWMF 113/001LG Guideline-Recommendations for Inpatient Treatment of Patients with COVID-19. Available online: https://register.awmf.org/de/leitlinien/detail/113-001LG (accessed on 12 September 2022).
- Coronavirus Test Regulation. Available online: https://www.bundesgesundheitsministerium.de/service/gesetze-und-verordnungen/detail/coronavirus-testverordnung-testv.html (accessed on 16 September 2022).
- Vereinbarung nach § 26 Absatz 2 KHG über ein Zusatzentgelt für Testungen auf das Coronavirus SARS-CoV-2 im Krankenhaus zwischen dem GKV-Spitzenverband, Berlin, sowie dem Verband der Privaten Krankenversicherung, Köln—gemeinsam—und der Deutschen Krankenhausgesellschaft, Berlin vom 27.06.2022 [Third agreement according to § 26 paragraph 2 KHG on an additional charge for testing for the coronavirus SARS-CoV-2 in hospitals dated 27.06.2022]. Available online: https://www.dkgev.de/themen/finanzierung-leistungskataloge/covid-19/vereinbarung-nach-26-abs-2-khg/ (accessed on 7 November 2022).
- Federal Ministry of Justice. Act to strengthen vaccination prevention against COVID-19 and to amend other provisions in connection with the COVID-19 pandemic dated 10.12.2021. Fed. Law Gaz. 2021, 2021, 5162. [Google Scholar]
- UK Health Security Agency. Investigation of SARS-CoV-2 Variants of Concern: Variant Risk Assessments. Available online: https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-of-concern-variant-risk-assessments (accessed on 7 November 2022).
- Slagman, A.; Behringer, W.; Greiner, F.; Klein, M.; Weismann, D.; Erdmann, B.; Pigorsch, M.; Möckel, M.; AKTIN Emergency Department Registry; German Forum of University Emergency Departments (FUN) in the Society of University Clinics of Germany E.V. Medical Emergencies during the COVID-19 Pandemic — an Analysis of Emergency Department Data in Germany. Dtsch. Arztebl. Int. 2020, 117, 545–552. [Google Scholar] [PubMed]
- Quidel. Available online: https://www.quidel.com/immunoassays/rapid-sars-tests/Savanna-sars-antigen-fia (accessed on 10 November 2022).
- Mei, X.; Lee, H.-C.; Diao, K.-Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Untersuchung und Eindämmung eines SARS-CoV-2-Alpha-Ausbruchs in einer Pflegeeinrichtung im Landkreis Dithmarschen, Juni 2021. Available online: https://edoc.rki.de/handle/176904/10372 (accessed on 10 November 2022).
- Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden) - ESTATIS. Fachserie 16, Reihe 2.3: Verdienste und Arbeitskosten –Arbeitnehmerverdienste. 4.5.1 Durchschnittliche Bruttojahresverdienste und Sonderzahlungen nach Wirtschaftszweigen Arbeitnehmer im Jahr 2021 im Krankenhaus (Q861 Krankenhäuser). Available online: https://www.destatis.de/DE/Service/Bibliothek/_publikationen-fachserienliste-16.html#605986 (accessed on 10 November 2022).
- Rommel, A.; von der Lippe, E.; Plass, D.; Ziese, T.; Diercke, M.; der Heiden, M.A.; Haller, S.; Wengler, A.; BURDEN 2020 Study Group. The COVID-19 Disease Burden in Germany in 2020—Years of Life Lost to Death and Disease Over the Course of the Pandemic. Dtsch. Arzteblatt Int. 2021, 118, 145–151. [Google Scholar]
- Jo, M.-W.; Go, D.-S.; Kim, R.; Lee, S.W.; Ock, M.; Kim, Y.-E.; Oh, I.-H.; Yoon, S.-J.; Park, H. The Burden of Disease due to COVID-19 in Korea Using Disability-Adjusted Life Years. J. Korean Med Sci. 2020, 35, e199. [Google Scholar] [CrossRef] [PubMed]
- Suárez, V.; Koehler, F.C.; Hackl, M.J.; Möckel, M.; Slagman, A.; Pudasaini, S.; Risse, J.; Schunk, D.; Blaschke, S.; Kümpers, P.; et al. Universitäre Notaufnahmen in der Coronapandemie—Ergebnisse des ReCovER-Registers. Med. Klin. Intensiv. Und Notf. 2021, 117, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Tolksdorf, K.; Buda, S.; Schuler, E.; Wieler, L.H.; Haas, W. Eine höhere Letalität und lange Beatmungsdauer unterscheiden COVID-19 von schwer verlaufenden Atemwegsinfektionen in Grippewellen. Epid. Bull. 2020, 41, 3–10. [Google Scholar]
- Xia, Y.; Chen, W.; Ren, H.; Zhao, J.; Wang, L.; Jin, R.; Zhou, J.; Wang, Q.; Yan, F.; Zhang, B.; et al. A rapid screening classifier for diagnosing COVID-19. Int. J. Biol. Sci. 2021, 17, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, U.; Malouhi, A.; Ingwersen, M.; Neumann, R.; Reljic, M.; Deinhardt-Emmer, S.; Löffler, B.; Behringer, W.; Lewejohann, J.-C.; Stallmach, A.; et al. Ruling out COVID-19 by chest CT at emergency admission when prevalence is low: The prospective, observational SCOUT study. Respir. Res. 2021, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Plecko, T.; Sethi, S.; Schilling, T.; Wienand, O.; Jürgensen, J.S.; Menzel, C.U. Klinische Performance eines neuen SARS-CoV-2-Antigen-Tests in der Notaufnahme eines Maximalversorgers. Epid. Bull. 2021, 3, 10–15. [Google Scholar]
- Robert Koch Institut. SARS-CoV-2 Steckbrief zur Coronavirus-Krankheit-2019 (COVID-19) 2020. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html (accessed on 6 October 2020).
- Auvinen, R.; Nohynek, H.; Syrjänen, R.; Ollgren, J.; Kerttula, T.; Mäntyläet, J.; Ikonen, N.; Loginov, R.; Haveri, A.; Kurkela, S.; et al. Comparison of the Clinical Characteristics and Outcomes of Hospitalized Adult COVID-19 and Influenza Patients – a Prospective Observational Study. Infect. Dis. 2021, 53, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Shorten, R.J.; Haslam, S.; Hurley, M.A.; Rowbottom, A.; Myers, M.; Wilkinson, P.; Orr, D. Seroprevalence of SARS-CoV-2 Infection in Healthcare Workers in a Large Teaching Hospital in the North West of England: A Period Prevalence Survey. BMJ Open. 2021, 11, e045384. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gameros, C.A.; Colin-Martínez, T.; Waizel-Haiat, S.; Vargas-Ortega, G.; Ferat-Osorio, E.; Guerrero-Paz, J.A.; Intriago-Alor, M.; López-Moreno, M.A.; Cuevas-García, C.F.; Mendoza-Zubieta, V.; et al. Diagnostic accuracy of symptoms as a diagnostic tool for SARS-CoV 2 infection: A cross-sectional study in a cohort of 2,173 patients. BMC Infect. Dis. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, A.; Kiefl, D.; Baacke, M.; Zöllner, R.; Mencía, E.L.; Schellein, O.; Avan, N.; Pemmerl, S. Risikostratifizierung durch Implementierung und Evaluation eines COVID-19-Scores. Med. Klin. Intensiv. Und Notfallmedizin 2020, 115, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. COVID-19-Fälle nach Meldewoche und Geschlecht sowie Anteile mit für COVID-19 relevanten Symptomen, Anteile Hospitalisierter/Verstorbener und Altersmittelwert/-median (Tabelle wird jeden Donnerstag aktualisiert). Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Klinische_Aspekte.html (accessed on 5 November 2022).
- Robert Koch Institut. KROCO – Krankenhausbasierte Online-Befragung zur COVID-19-Impfung. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Projekte_RKI/KROCO.html (accessed on 10 November 2022).
- Coronavirus-Testverordnung vom 21. September 2021. Available online: https://www.bundesanzeiger.de/pub/publication/wXbxqfkUdLM85aXaEg9/content/wXbxqfkUdLM85aXaEg9/BAnz%20AT%2024.11.2022%20V2.pdf?inline (accessed on 10 November 2022).
- Haubrock, M. Krankenhausfinanzwirtschaft. In Betriebswirtschaft und Management im Krankenhaus; Haubrock, M., Schär, W., Eds.; Huber: Bern, Switzerland, 2007; pp. 394–453. [Google Scholar]
- S3-Leitlinie—Empfehlungen zur stationären Therapie von Patienten mit COVID-19. Available online: https://register.awmf.org/assets/guidelines/113-001LGl_S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19_2022-09_1.pdf (accessed on 10 November 2022).

| Variable Name | Variable Description | Lowest Value | Basecase Value | Highest Value | Savings at Lowest Value (€) | Savings at Highest Value (€) | Spread (€) Ƭ | Risk % ¥ | Cum Risk % | Threshold Variable Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Savanna_COVID_ spec | Specificity of Savanna for correctly excluding SARS-CoV | 0.9542 | 1 | 1 | −148.37 | 27.74 | 176.11 | 0.808 | 0.808 | 0.9614 |
| Clin_spec_ COIVD | Probability of correctly excluding SARS-CoV-2 by clinical judgement | 0.6 | 0.683 | 0.758 | −180.28 | −119.53 | 60.75 | 0.096 | 0.904 | - |
| cOpp_POCT (€) | Opportunity costs due to blocking twin bed | 587.62 | 745.53 | 881.44 | −122.83 | −170.35 | 47.51 | 0.059 | 0.963 | - |
| pHosp | Probability that hospitalization is required | 0.5645 | 0.6044 | 0.6429 | −137.91 | −158.46 | 20.54 | 0.011 | 0.974 | - |
| COVID_prev | Prevalence of COVID-19 | 0.079 | 0.156 | 0.412 | −163.52 | −143.81 | 19.70 | 0.010 | 0.984 | - |
| cSavanna_COVID (€) | Costs of Savanna POCT in ER | 32 | 40 | 48 | −157.12 | −139.62 | 17.50 | 0.008 | 0.992 | - |
| cRev_day_ POCT (€) | Additional revenue per day due to POCT | 263.54 | 329.42 | 395.30 | −142.20 | −154.54 | 12.34 | 0.004 | 0.996 | - |
| cRT_PCR_ext (€) | Costs of PCR in external laboratory | 37.8 | 37.8 | 56.7 | −159.79 | −148.37 | 11.42 | 0.003 | 1.000 | - |
| sec_COVID_ HCW | Secondary cases in HCW due to one unknown COVID-19 case | 0.0085 | 0.0248 | 0.0704 | −150.43 | −147.63 | 2.80 | 0.000 | 1.000 | - |
| Savanna_COVID_ sens | Sensitivity of Savanna to detect SARS-CoV-2 | 0.9617 | 0.9931 | 0.9988 | −147.21 | −148.58 | 1.37 | 0.000 | 1.000 | - |
| sick_days | Number of days of HCW out of work due to COVID-19 | 15 | 15 | 27 | −149.27 | −148.37 | 0.90 | 0.000 | 1.000 | - |
| Clin_sens_ COVID | Sensitivity of clinically diagnosing COVID-19 if present | 0.729 | 0.806 | 0.869 | −148.83 | −147.99 | 0.84 | 0.000 | 1.000 | - |
| cAntithromb_day | Costs of enoxaparin per day | 5.53 | 6.91 | 8.3 | −148.59 | −148.15 | 0.45 | 0.000 | 1.000 | - |
| cPL_day (€) | Costs of productivity loss per day | 170.9 | 170.9 | 205.8 | −148.60 | −148.37 | 0.23 | 0.000 | 1.000 | - |
| cDexa_day | Costs of dexamethasone per day | 1.09 | 1.36 | 1.6284 | −148.41 | −148.32 | 0.09 | 0.000 | 1.000 | - |
| dHosp | Median length of hospital stay | 5 | 10 | 19 | −148.37 | −148.37 | 0.00 | 0.000 | 1.000 | - |
| Probabilistic Sensitivity Analysis | Comparators | Mean Cost Per Patient (€) | Standard Deviation (±SD) | Incremental Cost (€) * |
|---|---|---|---|---|
| COVID-19 patients prior to hospitalization | Savanna® Multiplex RT-PCR | −0.38 | 25.22 | −0.38 |
| Conventional approach | 106.40 | 24.95 | 106.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diel, R.; Nienhaus, A. Cost–Benefit of Real-Time Multiplex PCR Testing of SARS-CoV-2 in German Hospitals. Int. J. Environ. Res. Public Health 2023, 20, 3447. https://doi.org/10.3390/ijerph20043447
Diel R, Nienhaus A. Cost–Benefit of Real-Time Multiplex PCR Testing of SARS-CoV-2 in German Hospitals. International Journal of Environmental Research and Public Health. 2023; 20(4):3447. https://doi.org/10.3390/ijerph20043447
Chicago/Turabian StyleDiel, Roland, and Albert Nienhaus. 2023. "Cost–Benefit of Real-Time Multiplex PCR Testing of SARS-CoV-2 in German Hospitals" International Journal of Environmental Research and Public Health 20, no. 4: 3447. https://doi.org/10.3390/ijerph20043447
APA StyleDiel, R., & Nienhaus, A. (2023). Cost–Benefit of Real-Time Multiplex PCR Testing of SARS-CoV-2 in German Hospitals. International Journal of Environmental Research and Public Health, 20(4), 3447. https://doi.org/10.3390/ijerph20043447








