Disparities in Cutaneous T-Cell Lymphoma Incidence by Race/Ethnicity and Area-Based Socioeconomic Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Socioeconomic Data
2.3. Population Data
2.4. Statewide Incidence
2.5. Geographic Modeling
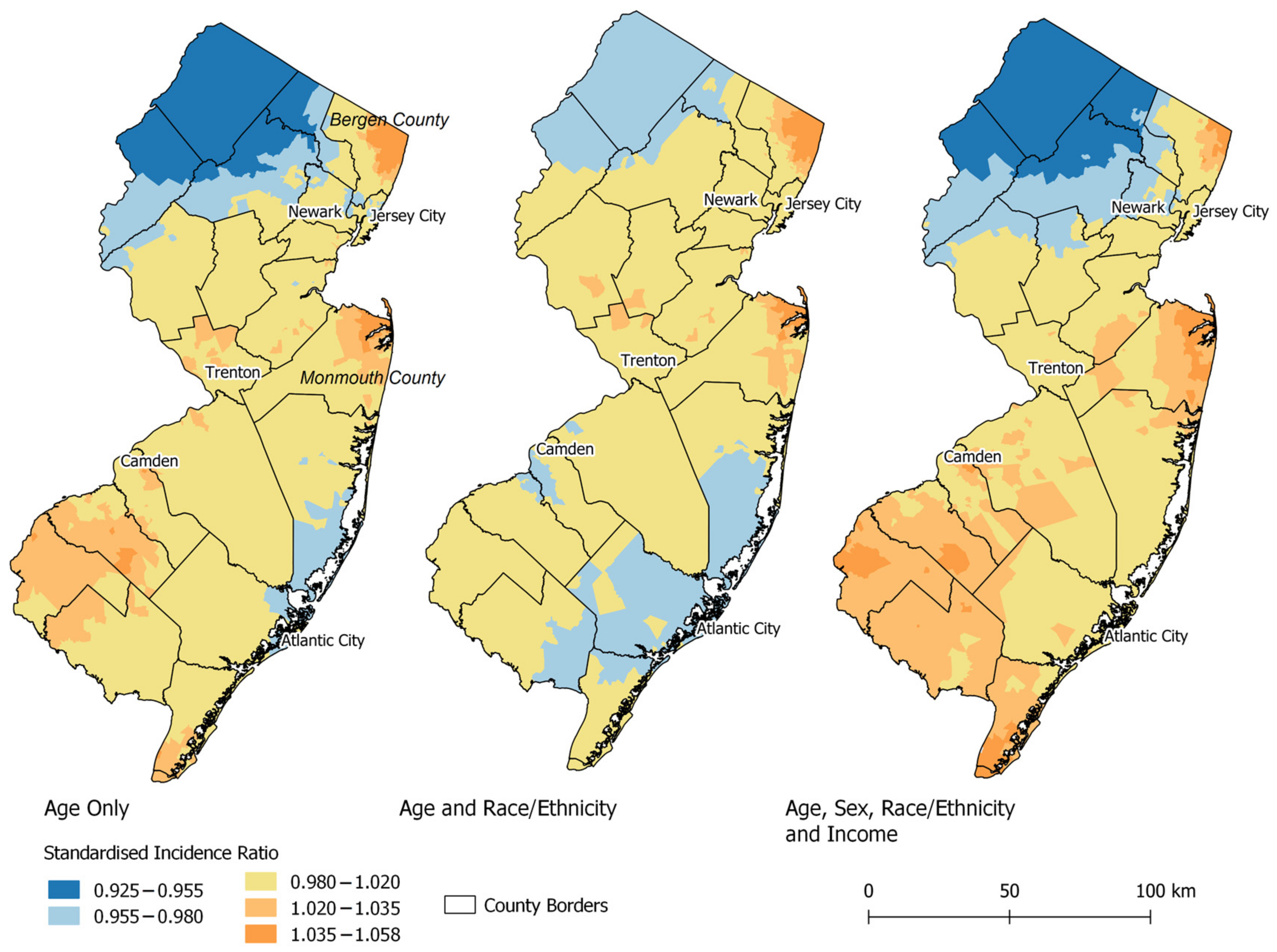
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.; Duncan, L.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, J.A.; Yag-Howard, C.; Messina, J.L.; Glass, L.F. Cutaneous T-cell lymphoma. Int. J. Dermatol. 1997, 36, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, E. End Results (SEER) Program. Data from: Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence—SEER Research Plus Data (Specialized with Census Tract SES/Rurality), 18 Registries (excl AK), Nov 2020 Sub (2006–2018) <Vintage 2019 Pops by Tract 2010 Geographies>—Linked To Census Tract Attributes SEER 18 excl AK, Census 2010 Geographies Time Dependent (2006–2018; 2008 copied to 06, 07; 2017 copied to 18), National Cancer Institute, DCCPS, Surveillance Research Program, released December 2021, Based on the November 2020 Submission. 2021. Available online: www.seer.cancer.gov (accessed on 1 June 2022).
- Wilson, L.D.; Hinds, G.A.; James, B.Y. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin. Lymphoma Myeloma Leuk. 2012, 12, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobrowicz, M.; Fassnacht, C.; Ignatova, D.; Chang, Y.-T.; Dimitriou, F.; Guenova, E. Pathogenesis and Therapy of Primary Cutaneous T-Cell Lymphoma: Collegium Internationale Allergologicum (CIA) Update 2020. Int. Arch. Allergy Immunol. 2020, 181, 733–745. [Google Scholar] [CrossRef]
- Dupin, M.; Darie, H.; Jumbou, O.; Veran, Y.; Gros, P.; Dreno, B.; Dormont, D.; Millet, P. Conjugal mycosis fungoides. Ann Derm. Venereol 1995, 122, 595–598. [Google Scholar]
- Schmidt, A.N.; Robbins, J.B.; Greer, J.P.; Zic, J.A. Conjugal transformed mycosis fungoides: The unknown role of viral infection and environmental exposures in the development of cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2006, 54, S202–S205. [Google Scholar] [CrossRef]
- Lozano, A.; Duvic, M. Cutaneous T-cell lymphoma in non-blood-related family members: Report of an additional case. J. Am. Acad. Dermatol. 2007, 56, 521. [Google Scholar] [CrossRef]
- Baykal, C.; Büyükbabani, N.; Kaymaz, R. Familial mycosis fungoides. Br. J. Dermatol. 2002, 146, 1108–1109. [Google Scholar] [CrossRef]
- Shelley, W.B. Familial Mycosis Fungoides Revisited. Arch. Dermatol. 1980, 116, 1177–1178. [Google Scholar] [CrossRef]
- Vassallo, C.; BRAzzELLI, V.; Cestone, E.; Castello, M.; Ciocca, O.; Borroni, R.G.; Martinetti, M.; Borroni, G. Mycosis fungoides in childhood: Description and study of two siblings. Acta Derm. -Venereol. 2007, 87, 529–532. [Google Scholar] [CrossRef] [Green Version]
- Morales-Suárez-Varela, M.M.; Olsen, J.; Johansen, P.; Kaerlev, L.; Guénel, P.; Arveux, P.; Wingren, G.; Hardell, L.; Ahrens, W.; Stang, A. Occupational risk factors for mycosis fungoides: A European multicenter case-control study. J. Occup. Environ. Med. 2004, 46, 205–211. [Google Scholar] [CrossRef]
- Morales-Suarez-Varela, M.M.; Olsen, J.; Johansen, P.; Kaerlev, L.; Guenel, P.; Arveux, P.; Wingren, G.; Hardell, L.; Ahrens, W.; Stang, A.; et al. Occupational exposures and mycosis fungoides. A European multicentre case-control study (Europe). Cancer Causes Control 2005, 16, 1253–1259. [Google Scholar] [CrossRef]
- Greene, M.; Dalager, N.; Lamberg, S.I.; Argyropoulos, C.; Fraumeni, J., Jr. Mycosis fungoides: Epidemiologic observations. Cancer Treat. Rep. 1979, 63, 597–606. [Google Scholar]
- Wohl, Y.; Tur, E. Environmental risk factors for mycosis fungoides. In Environmental Factors in Skin Diseases; Karger Publishers: Basel, Switzerland, 2007; Volume 35, pp. 52–64. [Google Scholar]
- Fischmann, A.; Bunn, P., Jr.; Guccion, J.; Matthews, M.; Minna, J. Exposure to chemicals, physical agents, and biologic agents in mycosis fungoides and the Sézary syndrome. Cancer Treat. Rep. 1979, 63, 591–596. [Google Scholar]
- Morales-Suárez-Varela, M.M.; Olsen, J.; Johansen, P.; Kaerlev, L.; Guénel, P.; Arveux, P.; Wingren, G.; Hardell, L.; Ahrens, W.; Stang, A. Occupational sun exposure and mycosis fungoides: A European multicenter case–control study. J. Occup. Environ. Med. 2006, 48, 390–393. [Google Scholar] [CrossRef]
- Morton, L.M.; Slager, S.L.; Cerhan, J.R.; Wang, S.S.; Vajdic, C.M.; Skibola, C.F.; Bracci, P.M.; de Sanjosé, S.; Smedby, K.E.; Chiu, B.C. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 130–144. [Google Scholar] [CrossRef] [Green Version]
- Biggar, R.J.; Engels, E.A.; Frisch, M.; Goedert, J.J. Risk of T-cell lymphomas in persons with AIDS. J. Acquir. Immune Defic. Syndr. (1999) 2001, 26, 371–376. [Google Scholar] [CrossRef]
- Pomerantz, R.G.; Campbell, L.S.; Jukic, D.M.; Geskin, L.J. Posttransplant cutaneous T-cell lymphoma: Case reports and review of the association of calcineurin inhibitor use with posttransplant lymphoproliferative disease risk. Arch. Dermatol. 2010, 146, 513–516. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Netchiporouk, E.; Rahme, E.; Tsang, M.; Moreau, L.; Glassman, S.; Provost, N.; Gilbert, M.; Jean, S.-E.; Roshdy, O. Distribution and clustering of cutaneous T-cell lymphoma (CTCL) cases in Canada during 1992 to 2010. J. Cutan. Med. Surg. 2018, 22, 154–165. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Glassman, S.J.; Sasseville, D.; Litvinov, I.V. Using patient registries to identify triggers of rare diseases. Ehealth—Mak. Health Care Smarter 2018, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Ghazawi, F.M.; Alghazawi, N.; Le, M.; Netchiporouk, E.; Glassman, S.; Sasseville, D.; Litvinov, I.V. Environmental and other extrinsic risk factors contributing to the pathogenesis of cutaneous T cell lymphoma (ctcl). Front. Oncol. 2019, 9, 300. [Google Scholar] [CrossRef]
- Moreau, J.F.; Buchanich, J.M.; Geskin, J.Z.; Akilov, O.E.; Geskin, L.J. Non-random geographic distribution of patients with cutaneous T-cell lymphoma in the Greater Pittsburgh Area. Dermatol. Online J. 2014, 20, 1. [Google Scholar] [CrossRef]
- Clough, L.; Bayakly, A.R.; Ward, K.C.; Khan, M.K.; Chen, S.C.; Lechowicz, M.J.; Flowers, C.R.; Allen, P.B.; Switchenko, J.M. Clustering of cutaneous T-cell lymphoma is associated with increased levels of the environmental toxins benzene and trichloroethylene in the state of Georgia. Cancer 2020, 126, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Tetzlaff, M.T.; Rahme, E.; Habel, Y.; Risser, D.R.; Gangar, P.; Jennings, M.A.; Pehr, K.; Prieto, V.G.; Sasseville, D.; et al. Identification of geographic clustering and regions spared by cutaneous T-cell lymphoma in Texas using 2 distinct cancer registries. Cancer 2015, 121, 1993–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghfour, J.; Gill, F.; Olson, J.; Guido, N.; Echuri, H.; Murina, A. Association of airborne toxins with geographic clustering of cutaneous T-cell lymphoma in Louisiana. J. Am. Acad. Dermatol. 2022, 87, 1184–1186. [Google Scholar] [CrossRef]
- Malachowski, S.J.; Moy, A.; Messina, J.; Chen, Y.A.; Sun, J.; Sokol, L.; Seminario-Vidal, L. Mapping Cutaneous T-Cell Lymphoma in the State of Florida: A Retrospective Exploratory Spatial Analysis of Incidence Patterns. J. Am. Acad. Dermatol. 2022, 86, 186–188. [Google Scholar] [CrossRef]
- Allen, P.B.; Clough, L.; Bayakly, A.R.; Ward, K.C.; Khan, M.K.; Chen, S.; Flowers, C.; Switchenko, J.M. Association of Geographic Clustering of Cutaneous T-Cell Lymphoma in the State of Georgia with Environmental Exposure to Benzene and Trichloroethylene; American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar]
- Texas A&M NaN. NAACCR Geocoder Data Dictionary. 2016. Available online: http://www.naaccr.org/wp-content/uploads/2016/11/NAACCR-Geocoder-Data-Dictionary.pdf (accessed on 1 June 2022).
- Sahar, L.; Foster, S.L.; Sherman, R.L.; Henry, K.A.; Goldberg, D.W.; Stinchcomb, D.G.; Bauer, J.E. GIScience and cancer: State of the art and trends for cancer surveillance and epidemiology. Cancer 2019, 125, 2544–2560. [Google Scholar] [CrossRef]
- Umlauf, N.; Adler, D.; Kneib, T.; Lang, S.; Zeileis, A. Structured additive regression models: An R interface to BayesX. J. Stat. Softw. 2015, 63, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.; Brezger, A. Bayesian P-splines. J. Comput. Graph. Stat. 2004, 13, 183–212. [Google Scholar] [CrossRef] [Green Version]
- BayesXsrc: R Package Distribution of the BayesX C++ Sources. 2012. Available online: http://CRAN.R-project.org/package=BayesXsrc (accessed on 1 June 2022).
- R2BayesX: Estimate Structured Additive Regression Models with ’BayesX’; Version 0.1-1; R Foundation for Statistical Computing: Innsbruck, Austria, 2012.
- Jin, X.; Carlin, B.P.; Banerjee, S. Generalized Hierarchical Multivariate CAR Models for Areal Data. Biometrics 2005, 61, 950–961. [Google Scholar] [CrossRef]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2017, 92, 1085–1102. [Google Scholar] [CrossRef] [Green Version]
- Scarisbrick, J.J.; Prince, H.M.; Vermeer, M.H.; Quaglino, P.; Horwitz, S.; Porcu, P.; Stadler, R.; Wood, G.S.; Beylot-Barry, M.; Pham-Ledard, A.; et al. Cutaneous Lymphoma International Consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: Effect of specific prognostic markers on survival and development of a prognostic model. J. Clin. Oncol. 2015, 33, 3766. [Google Scholar] [CrossRef]
- Weed, J.; Girardi, M. The difficult—And often delayed—Diagnosis of CTCL. Sci. Transl. Med. 2015, 7, 308fs41. [Google Scholar] [CrossRef]
- Ben-Gashir, M.; Seed, P.; Hay, R. Clinical and Laboratory Investigations. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br. J. Dermatol. 2002, 147, 920–925. [Google Scholar] [CrossRef]
- Vachiramon, V.; Tey, H.L.; Thompson, A.E.; Yosipovitch, G. Atopic dermatitis in African American children: Addressing unmet needs of a common disease. Pediatr. Dermatol. 2012, 29, 395–402. [Google Scholar] [CrossRef]
- Silverberg, J.I. Racial and Ethnic Disparities in Atopic Dermatitis. Curr. Dermatol. Rep. 2015, 4, 44–48. [Google Scholar] [CrossRef]
- Mei-Yen Yong, A.; Tay, Y.-K. Atopic Dermatitis: Racial and Ethnic Differences. Dermatol. Clin. 2017, 35, 395–402. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population–based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef]
| Age at Diagnosis, Years | n | % | Crude IR * |
|---|---|---|---|
| <30 | 60 | 5.5 | 1.22 |
| 30–39 | 94 | 8.7 | 1.80 |
| 40–49 | 153 | 14.1 | 2.53 |
| 50–59 | 232 | 21.4 | 4.23 |
| 60–69 | 242 | 22.3 | 6.62 |
| 70–79 | 197 | 18.2 | 8.99 |
| ≥80 | 106 | 9.8 | 6.64 |
| Characteristic | n | % | Age-Adjusted IR *†‡ | 95% CI |
|---|---|---|---|---|
| Sex | ||||
| Female | 631 | 58.21 | 0.98 | 0.89–1.08 |
| Male | 453 | 41.79 | 1.58 | 1.46–1.71 |
| Race/Ethnicity | ||||
| Non-Hispanic White | 733 | 67.62 | 1.24 | 1.14–1.33 |
| Non-Hispanic Black | 166 | 15.31 | 1.63 | 1.39–1.91 |
| Hispanic | 125 | 11.53 | 1.14 | 0.94–1.37 |
| Asian/Pacific Islander | 60 | 5.54 | 0.91 | 0.68–1.18 |
| CT Median Income | ||||
| Very High (≥$109,665) | 271 | 25.0 | 1.51 | 1.33–1.70 |
| High ($80,382–109,665) | 270 | 25.0 | 1.17 | 1.05–1.33 |
| Medium ($58,927–80,382) | 271 | 25.0 | 1.25 | 1.10–1.41 |
| Low (<$58,927) | 272 | 25.0 | 1.17 | 1.04–1.32 |
| CT Poverty | ||||
| Very Low (<2.89%) | 270 | 25.0 | 1.37 | 1.20–1.54 |
| Low (2.89–5.48%) | 271 | 25.0 | 1.26 | 1.11–1.43 |
| Medium (5.48–10.34%) | 271 | 25.0 | 1.23 | 1.09–1.39 |
| High (10.34%+) | 272 | 25.0 | 1.17 | 1.03–1.32 |
| Characteristic | Crude RR (95% CI) | Age-Adjusted RR (95% CI) † | Multivariable-Adjusted RR (95% CI) ‡ |
|---|---|---|---|
| Sex | |||
| Male | Referent | Referent | Referent |
| Female | 0.68 (0.61–0.76) | 0.59 (0.52–0.66) | 0.59 (0.52–0.67) |
| Race/Ethnicity | |||
| Non-Hispanic White | Referent | Referent | Referent |
| Non-Hispanic Black | 1.02 (0.86–1.2) | 1.39 (1.17–1.63) | 1.56 (1.29–1.89) |
| Hispanic, Any Race | 0.57 (0.47–0.69) | 0.94 (0.76–1.17) | 1.06 (0.86–1.29) |
| Asian/Pacific Islander | 0.56 (0.43–0.72) | 0.8 (0.61–1.04) | 0.8 (0.6–1.04) |
| CT Median Income | |||
| Very High (≥$109,665) | 1.39 (1.17–1.67) | 1.30 (1.10–1.55) | 1.47 (1.22–1.78) |
| High ($80,382–109,665) | 1.12 (0.95–1.34) | 1.03 (0.86–1.24) | 1.14 (0.96–1.38) |
| Medium ($58,927–80,382) | 1.17 (0.98–1.4) | 1.10 (0.93–1.32) | 1.19 (1.00–1.43) |
| Low (<$58,927) | Referent | Referent | Referent |
| CT Poverty | |||
| Very Low (<2.89%) | 1.38 (1.15–1.64) | 1.18 (1.00–1.40) | 1.31 (1.08–1.58) |
| Low (2.89–5.48%) | 1.29 (1.09–1.54) | 1.12 (0.94–1.33) | 1.23 (1.01–1.47) |
| Medium (5.48–10.34%) | 1.21 (1.02–1.43) | 1.07 (0.91–1.26) | 1.16 (0.97–1.37) |
| High (10.34%+) | Referent | Referent | Referent |
| CT Median Household Income Quartile | ||||||||
|---|---|---|---|---|---|---|---|---|
| Race/Ethnicity | Very High (≥$109,665) | High ($80,382–109,665) | Medium ($58,927–80,382) | Low (<$58,927) | ||||
| n (%) | RR ‡ (95% CI) | n (%) | RR ‡ (95% CI) | n (%) | RR ‡ (95% CI) | n (%) | RR ‡ (95% CI) | |
| Non-Hispanic White | 221 (30) | 1.39 (1.1–1.74) | 216 (29) | 1.11 (0.88–1.4) | 185 (25) | 1.12 (0.88–1.42) | 111 (15) | Referent |
| Non-Hispanic Black | 18 (11) | 2.61 (1.58–4.29) | 23 (16) | 1.57 (1–2.46) | 39 (22) | 1.76(1.22–2.54) | 86 (52) | 1.4 (1.06–1.86) |
| Hispanic | 9 (7) | 1.11 (0.56–2.2) | 13 (10) | 0.83 (0.47–1.47) | 35 (28) | 1.33 (0.91–1.95) | 68 (54) | 1.01 (0.75–1.38) |
| Asian/Pacific Islander | 23 (38) | 1.16 (0.74–1.82) | 18 (30) | 0.85 (0.51–1.4) | 12 (20) | 0.75 (0.42–1.37) | 7 (12) | 0.61 (0.28–1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiese, D.; Stroup, A.M.; Shevchenko, A.; Hsu, S.; Henry, K.A. Disparities in Cutaneous T-Cell Lymphoma Incidence by Race/Ethnicity and Area-Based Socioeconomic Status. Int. J. Environ. Res. Public Health 2023, 20, 3578. https://doi.org/10.3390/ijerph20043578
Wiese D, Stroup AM, Shevchenko A, Hsu S, Henry KA. Disparities in Cutaneous T-Cell Lymphoma Incidence by Race/Ethnicity and Area-Based Socioeconomic Status. International Journal of Environmental Research and Public Health. 2023; 20(4):3578. https://doi.org/10.3390/ijerph20043578
Chicago/Turabian StyleWiese, Daniel, Antoinette M. Stroup, Alina Shevchenko, Sylvia Hsu, and Kevin A. Henry. 2023. "Disparities in Cutaneous T-Cell Lymphoma Incidence by Race/Ethnicity and Area-Based Socioeconomic Status" International Journal of Environmental Research and Public Health 20, no. 4: 3578. https://doi.org/10.3390/ijerph20043578




