Associations of Mutually Exclusive Categories of Physical Activity and Sedentary Behavior with Body Composition and Fall Risk in Older Women: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Measurements
2.2.1. Fall Risk Assessment
2.2.2. PA Assessment
2.2.3. Body Composition Assessment
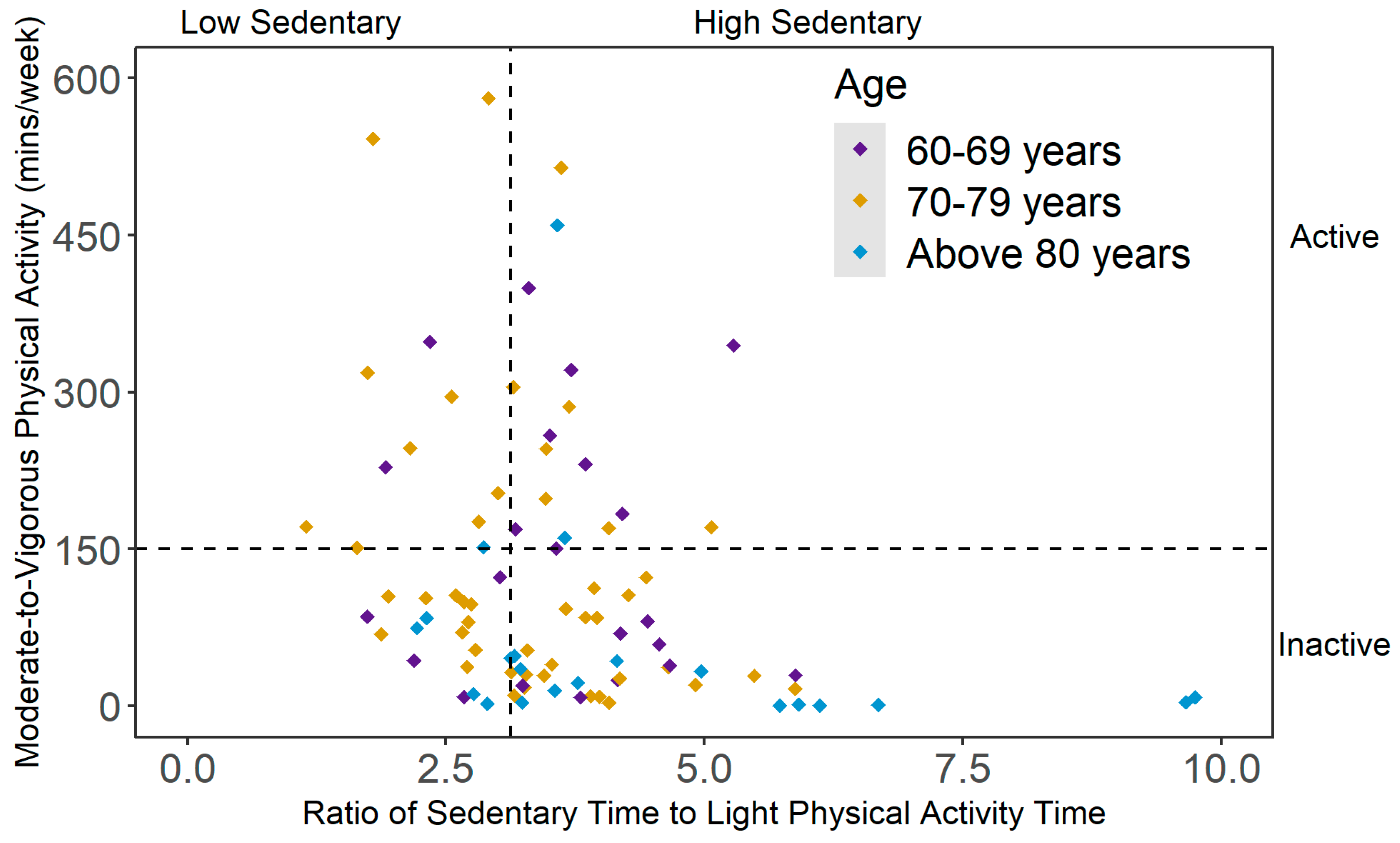
2.3. Mutually Exclusive Categories of PA and SB
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauman, A.; Merom, D.; Bull, F.C.; Buchner, D.M.; Fiatarone Singh, M.A. Updating the evidence for physical activity: Summative reviews of the epidemiological evidence, prevalence, and interventions to promote “active aging”. Gerontologist 2016, 56, S268–S280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sport. Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Etman, A.; Pierik, F.H.; Kamphuis, C.; Burdorf, A.; van Lenthe, F.J. The role of high-intensity physical exercise in the prevention of disability among community-dwelling older people. BMC Geriatr. 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantunen, H.; Wasenius, N.; Salonen, M.K.; Perälä, M.-M.; Osmond, C.; Kautiainen, H.; Simonen, M.; Pohjolainen, P.; Kajantie, E.; Rantanen, T. Objectively measured physical activity and physical performance in old age. Age Ageing 2017, 46, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Sugiyama, T.; Takamiya, T.; Oka, K.; Owen, N.; Shimomitsu, T. Television viewing time is associated with overweight/obesity among older adults, independent of meeting physical activity and health guidelines. J. Epidemiol. 2012, 22, 1112070273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennuso, K.P.; Gangnon, R.E.; Matthews, C.E.; Thraen-Borowski, K.M.; Colbert, L.H. Sedentary behavior, physical activity, and markers of health in older adults. Med. Sci. Sport. Exerc. 2013, 45, 1493. [Google Scholar] [CrossRef] [Green Version]
- Gianoudis, J.; Bailey, C.; Daly, R. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos. Int. 2015, 26, 571–579. [Google Scholar] [CrossRef]
- Pavey, T.G.; Peeters, G.G.; Brown, W.J. Sitting-time and 9-year all-cause mortality in older women. Br. J. Sport. Med. 2015, 49, 95–99. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.; Altenburg, T.M.; Chinapaw, M.J. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Chastin, S.F.M.; Ferriolli, E.; Stephens, N.A.; Fearon, K.C.; Greig, C. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing 2012, 41, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gába, A.; Pelclová, J.; Štefelová, N.; Přidalová, M.; Zając-Gawlak, I.; Tlučáková, L.; Pechová, J.; Svozilová, Z. Prospective study on sedentary behaviour patterns and changes in body composition parameters in older women: A compositional and isotemporal substitution analysis. Clin. Nutr. 2021, 40, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.G.S.V.; Verlengia, R.; de Oliveira, M.R.M.; Oliveira, M.V.A.; Pellegrinotti, I.L.; Crisp, A.H. Compliance of the Physical Activity Guidelines Accumulated in Bouts ≥ 10 Min and Nonbouts and Its Association with Body Composition and Physical Function: A Cross-Sectional Study in Brazilian Older Adults. J. Aging Phys. Act. 2020, 29, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.E.; Bellettiere, J.; Gardiner, P.A.; Villarreal, V.N.; Crist, K.; Kerr, J. Independent Associations Between Sedentary Behaviors and Mental, Cognitive, Physical, and Functional Health Among Older Adults in Retirement Communities. J. Gerontol. Ser. A 2015, 71, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, D.A.; Silva, A.M.; Baptista, F.; Santos, R.; Vale, S.; Mota, J.; Sardinha, L.B. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp. Gerontol 2012, 47, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Savikangas, T.; Tirkkonen, A.; Alen, M.; Rantanen, T.; Fielding, R.A.; Rantalainen, T.; Sipilä, S. Associations of physical activity in detailed intensity ranges with body composition and physical function. a cross-sectional study among sedentary older adults. Eur. Rev. Aging Phys. Act. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Swartz, A.M.; Tarima, S.; Miller, N.E.; Hart, T.L.; Grimm, E.K.; Rote, A.E.; Strath, S.J. Prediction of body fat in older adults by time spent in sedentary behavior. J. Aging Phys. Act. 2012, 20, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical costs of fatal and nonfatal falls in older adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Lee, H.; Cardinal, B.J. Daily movement patterns and biological markers among adults in the United States. Prev. Med. 2014, 60, 128–130. [Google Scholar] [CrossRef]
- Bakrania, K.; Edwardson, C.L.; Bodicoat, D.H.; Esliger, D.W.; Gill, J.M.R.; Kazi, A.; Velayudhan, L.; Sinclair, A.J.; Sattar, N.; Biddle, S.J.H.; et al. Associations of mutually exclusive categories of physical activity and sedentary time with markers of cardiometabolic health in English adults: A cross-sectional analysis of the Health Survey for England. BMC Public Health 2015, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Master, H.; Thoma, L.M.; Dunlop, D.D.; Christiansen, M.B.; Voinier, D.; White, D.K. Joint Association of Moderate-to-vigorous Intensity Physical Activity and Sedentary Behavior With Incident Functional Limitation: Data From the Osteoarthritis Initiative. J. Rheumatol. 2021, 48, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A.; Pardos-Sevilla, A.I.; Jiménez-Fuente, A.; Hubler-Figueiró, T.; d’Orsi, E.; Rech, C.R. Associations of mutually exclusive categories of physical activity and sedentary time with metabolic syndrome in older adults: An isotemporal substitution approach. J. Aging Phys. Act. 2021, 1, 1–9. [Google Scholar]
- Jefferis, B.J.; Parsons, T.J.; Sartini, C.; Ash, S.; Lennon, L.T.; Papacosta, O.; Morris, R.W.; Wannamethee, S.G.; Lee, I.-M.; Whincup, P.H. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: Does volume of activity matter more than pattern of accumulation? Br. J. Sport. Med. 2019, 53, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Thiamwong, L.; Stout, J.R.; Park, J.-H.; Yan, X. Technology-based fall risk assessments for older adults in low-income settings: Protocol for a cross-sectional study. JMIR Res. Protoc. 2021, 10, e27381. [Google Scholar] [CrossRef] [PubMed]
- Kuslansky, G.; Buschke, H.; Katz, M.; Sliwinski, M.; Lipton, R.B. Screening for Alzheimer’s disease: The memory impairment screen versus the conventional three-word memory test. J. Am. Geriatr. Soc. 2002, 50, 1086–1091. [Google Scholar] [CrossRef]
- Buschke, H.; Kuslansky, G.; Katz, M.; Stewart, W.F.; Sliwinski, M.J.; Eckholdt, H.M.; Lipton, R.B. Screening for dementia with the memory impairment screen. Neurology 1999, 52, 231. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.S.; Thralls, K.J.; Kviatkovsky, S.A. Validity and reliability of a portable balance tracking system, BTrackS, in older adults. J. Geriatr. Phys. Ther. 2018, 41, 102–107. [Google Scholar] [CrossRef]
- Goble, D.J.; Hearn, M.C.; Baweja, H.S. Combination of BTrackS and Geri-Fit as a targeted approach for assessing and reducing the postural sway of older adults with high fall risk. Clin. Interv. Aging 2017, 12, 351. [Google Scholar] [CrossRef] [Green Version]
- Yee, X.S.; Ng, Y.S.; Allen, J.C.; Latib, A.; Tay, E.L.; Abu Bakar, H.M.; Ho, C.Y.J.; Koh, W.C.C.; Kwek, H.H.T.; Tay, L. Performance on sit-to-stand tests in relation to measures of functional fitness and sarcopenia diagnosis in community-dwelling older adults. Eur. Rev. Aging Phys. Act. 2021, 18, 1. [Google Scholar] [CrossRef]
- Kempen, G.I.J.M.; Yardley, L.; Van Haastregt, J.C.M.; Zijlstra, G.A.R.; Beyer, N.; Hauer, K.; Todd, C. The Short FES-I: A shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing 2008, 37, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Clevenger, K.A.; Pfeiffer, K.A.; Montoye, A.H. Cross-generational comparability of raw and count-based metrics from ActiGraph GT9X and wGT3X-BT accelerometers during free-living in youth. Meas. Phys. Educ. Exerc. Sci. 2020, 24, 194–204. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Marín, P.J.; Torres-Luque, G.; Ruiz, J.R.; Lucía, A.; Garatachea, N. Technical variability of the GT3X accelerometer. Med. Eng. Phys. 2012, 34, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.A.; McMillan, D.G.; Anderson, A.; Fippinger, M.; Fillerup, G.; Rider, J. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med. Phys. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, V.T.; Fang, Z.; Langford, J.; Assah, F.; Mohammad, A.; Da Silva, I.C.; Trenell, M.I.; White, T.; Wareham, N.J.; Brage, S. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. J. Appl. Physiol. 2014, 117, 738–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakrania, K.; Yates, T.; Rowlands, A.V.; Esliger, D.W.; Bunnewell, S.; Sanders, J.; Davies, M.; Khunti, K.; Edwardson, C.L. Intensity thresholds on raw acceleration data: Euclidean norm minus one (ENMO) and mean amplitude deviation (MAD) approaches. PLoS ONE 2016, 11, e0164045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suorsa, K.; Pulakka, A.; Leskinen, T.; Pentti, J.; Holtermann, A.; Heinonen, O.J.; Sunikka, J.; Vahtera, J.; Stenholm, S. Comparison of Sedentary Time Between Thigh-Worn and Wrist-Worn Accelerometers. J. Meas. Phys. Behav. 2020, 3, 234–243. [Google Scholar] [CrossRef]
- Meier, N.F.; Bai, Y.; Wang, C.; Lee, D.-c. Validation of a multielectrode bioelectrical impedance analyzer with a dual-energy x-ray absorptiometer for the assessment of body composition in older adults. J. Aging Phys. Act. 2020, 28, 598–604. [Google Scholar] [CrossRef]
- Mañas, A.; del Pozo-Cruz, B.; Rodríguez-Gómez, I.; Leal-Martín, J.; Losa-Reyna, J.; Rodríguez-Mañas, L.; García-García, F.J.; Ara, I. Dose-response association between physical activity and sedentary time categories on ageing biomarkers. BMC Geriatr. 2019, 19, 270. [Google Scholar] [CrossRef]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Tremblay, M.S. Physical activity of Canadian adults: Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011, 22, 7. [Google Scholar] [PubMed]
- Hagströmer, M.; Troiano, R.P.; Sjöström, M.; Berrigan, D. Levels and patterns of objectively assessed physical activity—A comparison between Sweden and the United States. Am. J. Epidemiol. 2010, 171, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 2007, 30, 1384–1389. [Google Scholar] [CrossRef] [Green Version]
- LaMonte, M.J.; Buchner, D.M.; Rillamas-Sun, E.; Di, C.; Evenson, K.R.; Bellettiere, J.; Lewis, C.E.; Lee, I.M.; Tinker, L.F.; Seguin, R.; et al. Accelerometer-Measured Physical Activity and Mortality in Women Aged 63 to 99. J. Am. Geriatr. Soc. 2018, 66, 886–894. [Google Scholar] [CrossRef]
- Cooper, A.J.; Brage, S.; Ekelund, U.; Wareham, N.J.; Griffin, S.J.; Simmons, R.K. Association between objectively assessed sedentary time and physical activity with metabolic risk factors among people with recently diagnosed type 2 diabetes. Diabetologia 2014, 57, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.; Yates, T.; Edwardson, C.L.; Khunti, K.; Talbot, D.; Gray, L.J.; Leigh, T.M.; Carter, P.; Davies, M.J. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS ONE 2013, 8, e78350. [Google Scholar] [CrossRef] [Green Version]
- Rosique-Esteban, N.; Babio, N.; Díaz-López, A.; Romaguera, D.; Martínez, J.A.; Sanchez, V.M.; Schröder, H.; Estruch, R.; Vidal, J.; Buil-Cosiales, P. Leisure-time physical activity at moderate and high intensity is associated with parameters of body composition, muscle strength and sarcopenia in aged adults with obesity and metabolic syndrome from the PREDIMED-Plus study. Clin. Nutr. 2019, 38, 1324–1331. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, Z.; Howard, V.J.; Judd, S.E.; Blair, S.N.; Sun, Y.; Hooker, S.P. Is adiposity associated with objectively measured physical activity and sedentary behaviors in older adults? BMC Geriatr. 2020, 20, 257. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosisReport of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Tomita, Y.; Arima, K.; Tsujimoto, R.; Kawashiri, S.-y.; Nishimura, T.; Mizukami, S.; Okabe, T.; Tanaka, N.; Honda, Y.; Izutsu, K. Prevalence of fear of falling and associated factors among Japanese community-dwelling older adults. Medicine 2018, 97, e9721. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, V.; Brach, J.S.; Wert, D.M.; Hile, E.; Studenski, S.; VanSwearingen, J.M. What is the relation between fear of falling and physical activity in older adults? Arch. Phys. Med. Rehabil. 2013, 94, 2529–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, R.; Park, J.-H.; Thiamwong, L.; Xie, R.; Stout, J.R. Objectively Measured Physical Activity Levels and Associated Factors in Older US Women During the COVID-19 Pandemic: Cross-sectional Study. JMIR Aging 2022, 5, e38172. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Kim, K.I.; Kim, J.H.; Park, Y.S. Association between sleep duration and body composition measures in Korean adults: The Korea National Health and Nutrition Examination Survey 2010. Korean J. Fam. Med. 2018, 39, 219. [Google Scholar] [CrossRef]
- Tan, X.; Titova, O.E.; Lindberg, E.; Elmståhl, S.; Lind, L.; Schiöth, H.B.; Benedict, C. Association between self-reported sleep duration and body composition in middle-aged and older adults. J. Clin. Sleep Med. 2019, 15, 431–435. [Google Scholar] [CrossRef] [Green Version]




| Variables | All | Active-Low Sedentary | Active-High Sedentary | Inactive-Low Sedentary | Inactive-High Sedentary | p |
|---|---|---|---|---|---|---|
| N = 91 | n = 17 (19%) | n = 12 (13%) | n = 29 (32%) | n = 33 (36%) | ||
| Age (years) | 74.9 (7.35) | 73.4 (4.82) | 71.8 (6.90) | 76.2 (7.68) | 75.7 (8.10) | 0.259 |
| Race, White, % (n) 1 | 75 (68) | 88 (15) | 75 (9) | 76 (22) | 67 (22) | 0.424 2 |
| BMI (kg/m2) | 26.8 (5.5) | 24.5 (3.5) | 25.1 (5.5) | 26.8 (5.1) | 28.6 (6.2) | 0.046 |
| SB time (min/day) | 742 (130) | 634 (87) | 759 (71) | 701 (103) | 829 (131) | <0.001 |
| LPA (min/day) | 219 (63) | 263 (61) | 193 (28) | 258 (47) | 172 (45) | <0.001 |
| MVPA (min/day) | 17.4 (21.2) | 42.1 (27.9) | 38.1 (17.8) | 7.9 (5.0) | 5.6 (5.3) | <0.001 |
| Variables | All | Active-Low Sedentary | Active-High Sedentary | Inactive-Low Sedentary | Inactive-High Sedentary | p |
|---|---|---|---|---|---|---|
| Body composition | ||||||
| Extracellular-to-intracellular water ratio (kg/kg) | 0.631 (0.03) | 0.630 (0.03) | 0.632 (0.02) | 0.623 (0.03) | 0.636 (0.04) | 0.630 |
| Extracellular-to-total body water ratio (kg/kg) | 0.386 (0.01) | 0.386 (0.01) | 0.387 (0.01) | 0.384 (0.01) | 0.387 (0.01) | 0.593 |
| Lean mass (kg) | 46.9 (10.1) | 46.0 (3.0) | 47.5 (16.3) | 47.5 (8.7) | 45.7 (11.5) | 0.802 |
| Lean mass index (LMI, kg/m2) | 17.3 (4.0) | 16.7 (2.9) | 17.5 (4.4) | 17.2 (3.7) | 17.4 (5.2) | 0.966 |
| Body fat mass (kg) 1 | 23.5 (12.8) | 18.2 (12.6) | 21.4 (10.1) | 21.6 (10.8) | 28.7 (13.9) | 0.021 |
| Body fat mass index (kg/m2) 1 | 8.9 (4.7) | 6.7 (4.6) | 7.9 (3.8) | 8.0 (3.7) | 11.1 (5.2) | 0.006 |
| Skeletal muscle mass (kg) | 25.4 (6.0) | 26.4 (6.6) | 24.9 (2.6) | 27.1 (9.1) | 24.3 (5.2) | 0.041 |
| Skeletal muscle mass index (SMI, kg/m2) | 9.6 (1.6) | 9.9 (1.8) | 9.2 (1.6) | 10.3 (1.5) | 9.0 (1.6) | 0.020 |
| Appendicular lean mass (kg) | 19.6 (5.5) | 22.2 (6.4) | 19.4 (1.9) | 20.9 (7.0) | 18.4 (4.0) | 0.057 |
| Appendicular lean mass index (ALMI, kg/m2) | 7.3 (1.7) | 7.9 (1.8) | 7.0 (1.2) | 7.9 (1.3) | 7.1 (1.3) | 0.028 |
| Appendicular fat mass (kg) | 21.6 (18.5) | 18.1 (21.4) | 21.3 (10.5) | 19.8 (19.4) | 25.6 (17.4) | 0.068 |
| Appendicular fat mass index (AFMI, kg/m2) | 3.9 (2.7) | 3.1 (3.5) | 3.7 (1.6) | 3.5 (2.6) | 4.4 (3.4) | 0.024 |
| Fall risk | ||||||
| Perceived fall risk: Fear of falling score | 9 (5) | 8 (2) | 9 (2.5) | 9 (4) | 10 (5) | 0.421 |
| Static balance score: Centre of pressure path length (cm) | 26 (14) | 22 (9) | 24 (13) | 25 (10) | 30 (14) | 0.111 |
| Dynamic balance score: Sit-to-stand performance (reps) | 15 (5) | 17 (4) | 15 (5) | 15 (6) | 13 (5) | 0.035 |
| Variables | Active-Low Sedentary | Active-High Sedentary | Inactive-Low Sedentary | Inactive-High Sedentary | |||
|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | ||
| Extracellular-to-intracellular water ratio (%) | −0.01 (1.1) | 0.994 | −0.09 (1.3) | 0.941 | −1.65 (1.0) | 0.088 | Ref. |
| Extracellular-to-total body water ratio (%) | −0.01 (0.9) | 0.990 | −0.01 (1.1) | 0.986 | −0.69 (0.8) | 0.091 | Ref. |
| Lean mass (kg) | −0.84 (2.6) | 0.747 | 0.79 (2.9) | 0.786 | 0.32 (2.2) | 0.883 | Ref. |
| Lean mass index (LMI, kg/m2) | −1.41 (1.1) | 0.190 | −0.62 (1.2) | 0.604 | −0.34 (0.9) | 0.705 | Ref. |
| Body fat mass (kg) | −10.46 (3.7) | 0.006 | −6.64 (4.1) | 0.112 | −7.40 (3.1) | 0.019 | Ref. |
| Body fat mass index (kg/m2) | −4.37 (1.3) | 0.002 | −2.96 (1.5) | 0.054 | −3.14 (1.1) | 0.007 | Ref. |
| Skeletal muscle mass (kg) | 4.79 (1.6) | 0.004 | 1.00 (1.8) | 0.579 | 3.64 (1.3) | 0.008 | Ref. |
| Skeletal muscle mass index (SMI, kg/m2) | 1.23 (0.5) | 0.017 | −0.04 (0.6) | 0.947 | 1.05 (0.4) | 0.014 | Ref. |
| Appendicular lean mass (kg) | 6.38 (1.9) | 0.001 | 0.83 (2.1) | 0.697 | 2.24 (1.6) | 0.162 | Ref. |
| Appendicular lean mass index (ALMI, kg/m2) | 1.89 (0.6) | 0.003 | −0.004 (0.7) | 0.994 | 0.60 (0.5) | 0.249 | Ref. |
| Appendicular fat mass (kg) | −11.67 (4.3) | 0.008 | −8.69 (4.8) | 0.072 | −9.27 (3.6) | 0.011 | Ref. |
| Appendicular fat mass index (AFMI, kg/m2) | −2.19 (0.7) | 0.003 | −1.69 (0.8) | 0.037 | −1.74 (0.6) | 0.005 | Ref. |
| Perceived fall risk: Fear of falling score | −1.54 (1.19) | 0.197 | −1.13 (1.32) | 0.397 | −0.17 (0.99) | 0.862 | Ref. |
| Static balance score: Centre of pressure path length (cm) | −6.35 (4.4) | 0.152 | −4.67 (4.9) | 0.341 | −5.96 (3.7) | 0.107 | Ref. |
| Dynamic balance score: Sit-to-stand performance (reps) | 4.52 (1.8) | 0.014 | 1.51 (2.0) | 0.452 | 3.28 (1.5) | 0.031 | Ref. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, R.; Park, J.-H.; Banarjee, C.; Thiamwong, L.; Xie, R.; Stout, J.R. Associations of Mutually Exclusive Categories of Physical Activity and Sedentary Behavior with Body Composition and Fall Risk in Older Women: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 3595. https://doi.org/10.3390/ijerph20043595
Choudhury R, Park J-H, Banarjee C, Thiamwong L, Xie R, Stout JR. Associations of Mutually Exclusive Categories of Physical Activity and Sedentary Behavior with Body Composition and Fall Risk in Older Women: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3595. https://doi.org/10.3390/ijerph20043595
Chicago/Turabian StyleChoudhury, Renoa, Joon-Hyuk Park, Chitra Banarjee, Ladda Thiamwong, Rui Xie, and Jeffrey R. Stout. 2023. "Associations of Mutually Exclusive Categories of Physical Activity and Sedentary Behavior with Body Composition and Fall Risk in Older Women: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 20, no. 4: 3595. https://doi.org/10.3390/ijerph20043595
APA StyleChoudhury, R., Park, J. -H., Banarjee, C., Thiamwong, L., Xie, R., & Stout, J. R. (2023). Associations of Mutually Exclusive Categories of Physical Activity and Sedentary Behavior with Body Composition and Fall Risk in Older Women: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 20(4), 3595. https://doi.org/10.3390/ijerph20043595








