Species Composition, Seasonal Abundance, and Biting Behavior of Malaria Vectors in Rural Conhane Village, Southern Mozambique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Meteorological Data
2.3. Mosquito Collection
2.4. Laboratory Processing
2.4.1. Morphological Identification
2.4.2. Molecular Identification
2.4.3. Circumsporozoite Protein Detection
2.5. Data Analysis
3. Results
3.1. Composition of Anopheles Species in Conhane Village
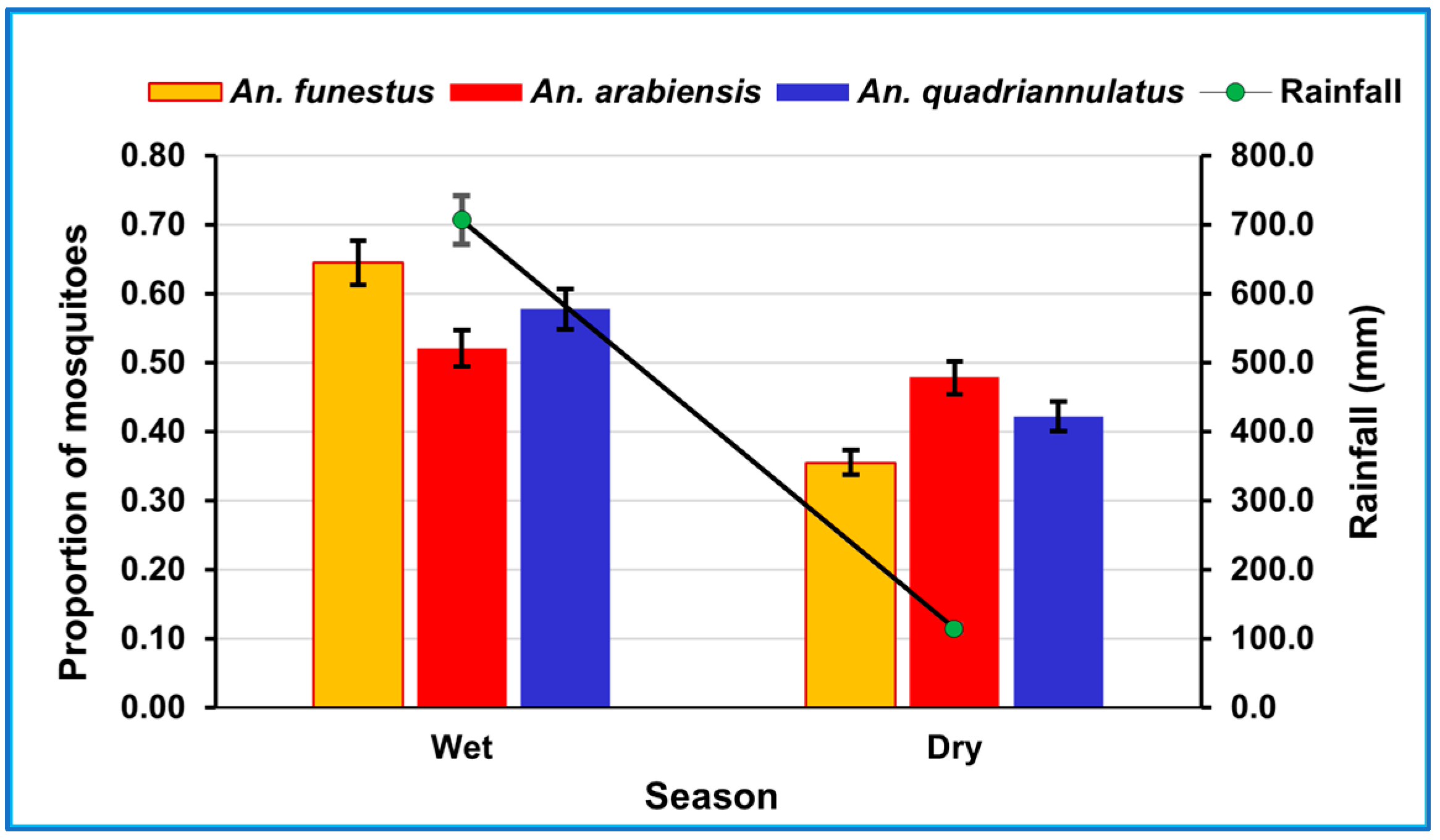
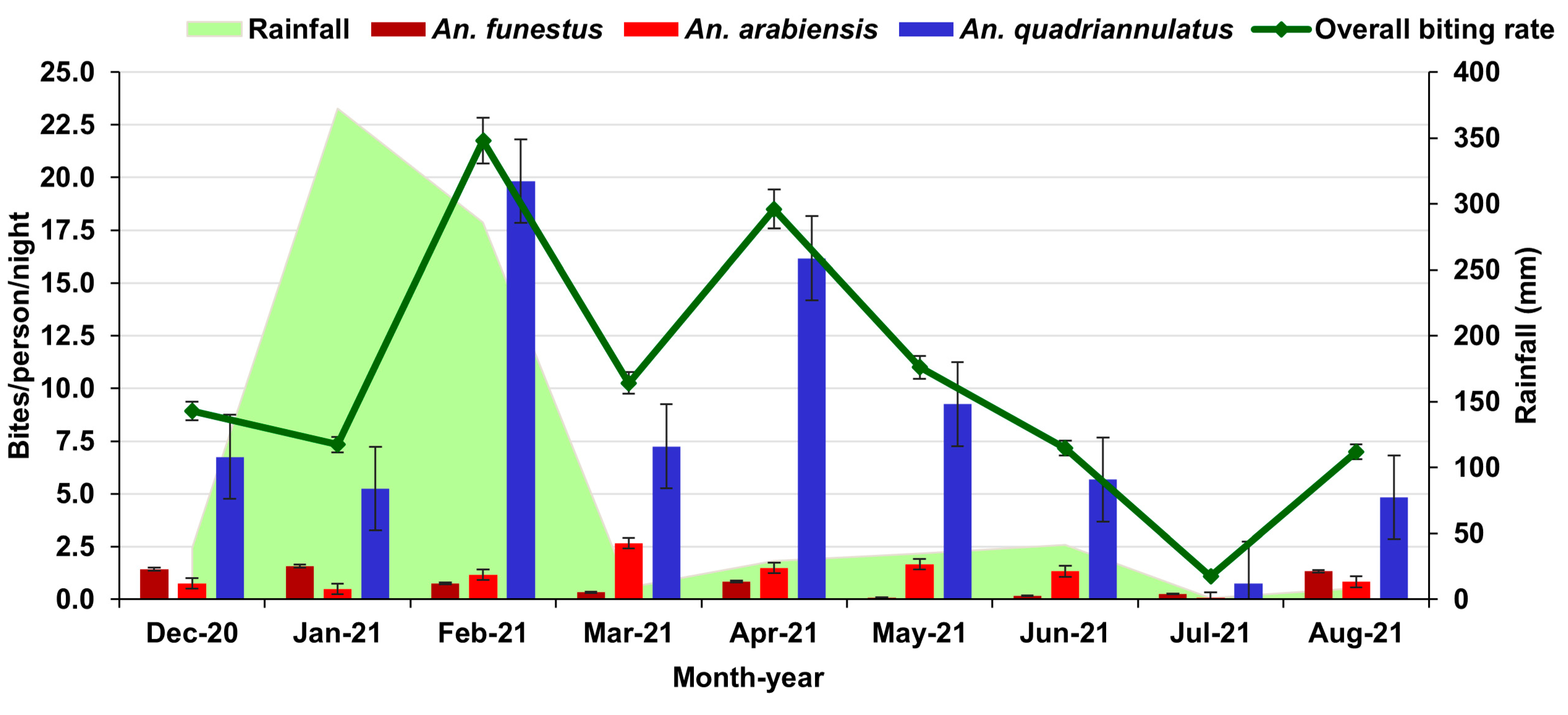
3.2. Rainfall and Seasonal Abundance of Mosquitoes
3.3. Human Biting Rates
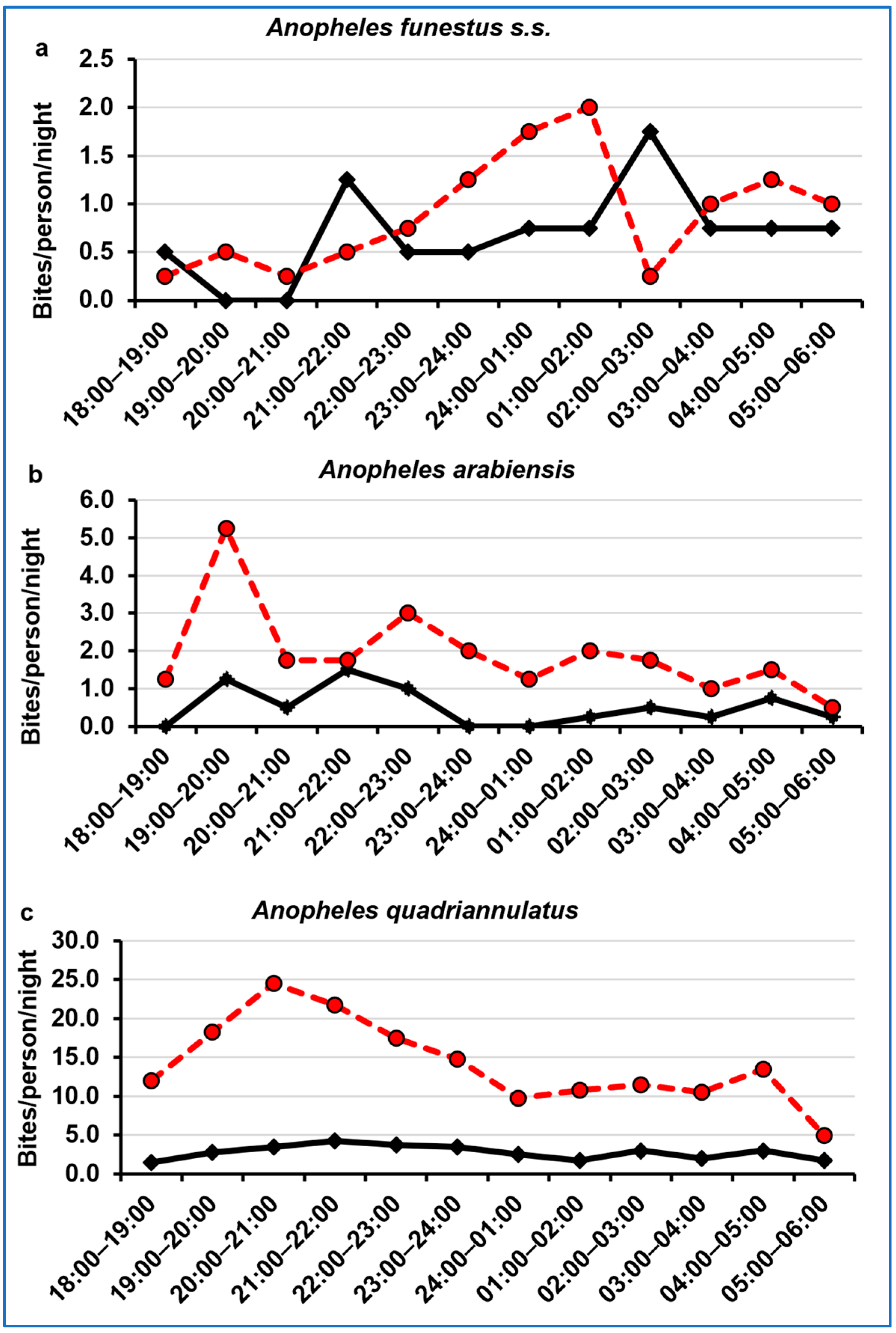
3.4. Night-Biting Cycle
3.5. Sporozoite Rate and Entomologic Inoculation Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guideline for Malaria, 13 July 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Durnez, L.; Coosemans, M. Residual Transmission of Malaria: An old issue for new approaches. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killeen, G.F.; Kiware, S.S.; Okumu, F.O.; Sinka, M.E.; Moyes, C.L.; Massey, N.C.; Gething, P.W.; Marshall, J.M.; Chaccour, C.J.; Tusting, L.S. Going beyond personal protection against mosquito bites to eliminate malaria transmission: Population suppression of malaria vectors that exploit both human and animal blood. BMJ Global Health 2017, 2, e000198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degefa, T.; Yewhalaw, D.; Zhou, G.; Lee, M.-c.; Atieli, H.; Githeko, A.K.; Yan, G. Indoor and outdoor malaria vector surveillance in western Kenya: Implications for better understanding of residual transmission. Malar. J. 2017, 16, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finda, M.F.; Moshi, I.R.; Monroe, A.; Limwagu, A.J.; Nyoni, A.P.; Swai, J.K.; Ngowo, H.S.; Minja, E.G.; Toe, L.P.; Kaindoa, E.W.; et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE 2019, 14, e0217414. [Google Scholar] [CrossRef]
- World Health Organization. Malaria Surveillance, Monitoring and Evaluation: A Reference Manual; World Health Organization: Geneva, Switzerland, 2018; ISBN 9789241565578. [Google Scholar]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasit Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [Green Version]
- Sharp, B.L.; Ridl, F.C.; Govender, D.; Kuklinski, J.; Kleinschmidt, I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar. J. 2007, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Russell, T.L.; Lwetoijera, D.W.; Maliti, D.; Chipwaza, B.; Kihonda, J.; Charlwood, J.D.; Smith, T.A.; Lengeler, C.; Mwanyangala, M.A.; Nathan, R.; et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar. J. 2010, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Bayoh, M.N.; Mathias, D.K.; Odiere, M.R.; Mutuku, F.M.; Kamau, L.; Gimnig, J.E.; Vulule, J.M.; Hawley, W.A.; Hamel, M.J.; Walker, E.D. Anopheles gambiae: Historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 2010, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). S. Afr. Inst. Med. Res. 1968, 54, 343. [Google Scholar]
- Kitau, J.; Oxborough, R.M.; Tungu, P.K.; Matowo, J.; Malima, R.C.; Magesa, S.M.; Bruce, J.; Mosha, F.W.; Rowland, M.W. Species shifts in the Anopheles gambiae complex: Do LLINs successfully control Anopheles arabiensis? PLoS ONE 2012, 7, e31481. [Google Scholar] [CrossRef] [Green Version]
- Tirados, I.; Costantini, C.; Gibson, G.; Torr, S.J. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: Implications for vector control. Med. Vet. Entomol. 2006, 20, 425–437. [Google Scholar] [CrossRef]
- Russell, T.L.; Govella, N.J.; Azizi, S.; Drakeley, C.J.; Kachur, S.P.; Killeen, G.F. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Moiroux, N.; Gomez, M.B.; Pennetier, C.; Elanga, E.; Djenontin, A.; Chandre, F.; Djegbe, I.; Guis, H.; Corbel, V. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 2012, 206, 1622–1629. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K.; et al. Escalation of pyrethroid resistance in the malaria vector Anopheles funestus induces a loss of efficacy of Piperonyl Butoxide–based insecticide-treated nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.; Koekemoer, L.L.; Brooke, B.D.; Hunt, R.H.; Mthembu, J.; Coetzee, M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med. Vet. Entomol. 2000, 14, 181–189. [Google Scholar] [CrossRef]
- Russell, T.L.; Beebe, N.W.; Cooper, R.D.; Lobo, N.F.; Burkot, T.R. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar. J. 2013, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.; Lines, J.; Fuseini, G.; Schwabe, C.; Monti, F.; Slotman, M.; Vargas, D.; Garcia, G.; Hergott, D.; Kleinschmidt, I. Outdoor biting by Anopheles mosquitoes on Bioko Island does not currently impact on malaria control. Malar. J. 2015, 14, 170. [Google Scholar] [CrossRef] [Green Version]
- Cuamba, N.; Mendis, C. The role of Anopheles merus in malaria transmission in an area of southern Mozambique. J. Vector Borne Dis. 2009, 46, 157–159. [Google Scholar]
- Lobo, N.F.; Laurent, B.S.; Sikaala, C.H.; Hamainza, B.; Chanda, J.; Chinula, D.; Krishnankutty, S.M.; Mueller, J.D.; Deason, N.A.; Hoang, Q.T.; et al. Unexpected diversity of Anopheles species in eastern Zambia: Implications for evaluating vector behavior and interventions using molecular tools. Sci. Rep. 2015, 5, 17952. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, J.C.; Simubali, L.; Mbambara, S.; Musonda, M.; Mweetwa, S.; Mudenda, T.; Pringle, J.C.; Jones, C.M.; Norris, D.E. Detection of Plasmodium in Anopheles squamosus (Diptera: Culicidae) in anaArea targeted for malaria elimination, southern Zambia. J. Med. Entomol. 2016, 53, 1482–1487. [Google Scholar] [CrossRef] [Green Version]
- Fillinger, U.; Ndenga, B.; Githeko, A.; Lindsay, S.W. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: A controlled trial. Bull. World Health Organ. 2009, 87, 655–665. [Google Scholar] [CrossRef]
- Massebo, F.; Lindtjørn, B. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: A randomized trial. Malar. J. 2013, 12, 319. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, V.; Kioko, E.; Kasili, S.; Ngumbi, P.; Hollingdale, M.R. Field trial of five repellent formulations against mosquitoes in Ahero, Kenya. US Army Med. Dep. J. 2009, 60–65. [Google Scholar]
- Sluydts, V.; Durnez, L.; Heng, S.; Gryseels, C.; Canier, L.; Kim, S.; Van Roey, K.; Kerkhof, K.; Khim, N.; Mao, S.; et al. Efficacy of topical mosquito repellent (picaridin) plus long-lasting insecticidal nets versus long-lasting insecticidal nets alone for control of malaria: A cluster randomised controlled trial. Lancet Infect. Dis. 2016, 16, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Saúde (INS); ICF. Inquérito Nacional Sobre Indicadores de Malária em Moçambique 2018; INS e ICF: Maputo, Mozambique; EUA: Rockville, MD, USA, 2019. [Google Scholar]
- Sibanda, M.; Focke, W.; Braack, L.; Leuteritz, A.; Brünig, H.; Tran, N.H.A.; Wieczorek, F.; Trümper, W. Bicomponent fibres for controlled release of volatile mosquito repellents. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Mapossa, A.B.; Sibanda, M.M.; Sitoe, A.; Focke, W.W.; Braack, L.; Ndonyane, C.; Mouatcho, J.; Smart, J.; Muaimbo, H.; Androsch, R.; et al. Microporous polyolefin strands as controlled-release devices for mosquito repellents. Chem. Eng. J. 2019, 360, 435–444. [Google Scholar] [CrossRef]
- Ministério da Administração Estatal. Perfil do Distrito de Chókwè Província de Gaza; Ministério da Administração Estatal: Maputo, Mozambique, 2005.
- Instituto Nacional de Estatística—Delegação Provincial de Gaza. Anuário Estatístico da Província de Gaza; Instituto Nacional de Estatística: Gaza, Mozambique, 2021. [Google Scholar]
- Brito, R.; Famba, S.; Munguambe, P.; Ibraimo, N.; Julaia, C. Profile of the Limpopo Basin in Mozambique a Contribution to the Challenge Program on Water and Food Project 17 “Integrated Water Resource Management for Improved Rural Livelihoods: Managing Risk, Mitigating Drought and Improving Water Productivity in the Water Scarce Limpopo Basin”; WaterNet: Harare, Zimbabwe, 2009. [Google Scholar]
- Gimnig, J.E.; Walker, E.D.; Otieno, P.; Kosgei, J.; Olang, G.; Ombok, M.; Williamson, J.; Marwanga, D.; Abong’o, D.; Desai, M.; et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am. J. Trop. Med. Hyg. 2013, 88, 301–308. [Google Scholar] [CrossRef]
- Gillies, M.T.; Coetzee, M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). S. Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Koekemoer, L.L.; Kamau, L.; Hunt, R.H.; Coetzee, M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002, 66, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Burkot, T.R.; Williams, J.L.; Schneider, I. Identification of Plasmodium falciparum-infected mosquitoes by a double antibody enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 1984, 33, 783–788. [Google Scholar] [CrossRef]
- Wirtz, R.A.; Burkot, T.R.; Andre, R.G.; Rosenberg, R.; Collins, W.E.; Roberts, D.R. Identification of Plasmodium Vivax sporozoites in mosquitoes using an Enzyme-Linked Immunosorbent Assay. Am. J. Trop. Med. Hyg. 1985, 34, 1048–1054. [Google Scholar] [CrossRef]
- Wirtz, R.A.; Sattabongkot, J.; Hall, T.; Burkot, T.R.; Rosenberg, R. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax-VK247 sporozoites. J. Med. Entomol. 1992, 29, 854–857. [Google Scholar] [CrossRef] [Green Version]
- Afrane, Y.A.; Bonizzoni, M.; Yan, G. Secondary malaria vectors of sub-Saharan Africa: Threat to malaria elimination on the continent? In Current Topics in Malaria; Rodriguez-Morales, A.J., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Burke, A.; Dahan-Moss, Y.; Duncan, F.; Qwabe, B.; Coetzee, M.; Koekemoer, L.; Brooke, B. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar. J. 2019, 18, 257. [Google Scholar] [CrossRef]
- Burke, A.; Dandalo, L.; Munhenga, G.; Dahan-Moss, Y.; Mbokazi, F.; Ngxongo, S.; Coetzee, M.; Koekemoer, L.; Brooke, B. A new malaria vector mosquito in South Africa. Sci. Rep. 2017, 7, 43779. [Google Scholar] [CrossRef] [Green Version]
- Alafo, C.; Martí-Soler, H.; Máquina, M.; Malheia, A.; Aswat, A.S.; Koekemoer, L.L.; Colborn, J.; Lobo, N.F.; Tatarsky, A.; Williams, Y.A.; et al. To spray or target mosquitoes another way: Focused entomological intelligence guides the implementation of indoor residual spraying in southern Mozambique. Malar. J. 2022, 21, 215. [Google Scholar] [CrossRef]
- Beier, J.C. Vector incrimination and entomological inoculation rates. In Malaria Methods and Protocols: Methods and Protocols; Doolan, D.L., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 3–11. [Google Scholar]
- Coetzee, M. Distribution of the African malaria vectors of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 2004, 70, 103–104. [Google Scholar] [CrossRef] [Green Version]
- Kiware, S.S.; Chitnis, N.; Devine, G.J.; Moore, S.J.; Majambere, S.; Killeen, G.F. Biologically meaningful coverage indicators for eliminating malaria transmission. Biol. Lett. 2012, 8, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Abílio, A.P.; Kleinschmidt, I.; Rehman, A.M.; Cuamba, N.; Ramdeen, V.; Mthembu, D.S.; Coetzer, S.; Maharaj, R.; Wilding, C.S.; Steven, A.; et al. The emergence of insecticide resistance in central Mozambique and potential threat to the successful indoor residual spraying malaria control programme. Malar. J. 2011, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.; Begtrup, K.; Cuamba, N.; Dgedge, M.; Mendis, C.; Gamage-Mendis, A.; Enosse, S.M.; Barreto, J.; Sinden, R.E.; Hogh, B. The Matola malaria project: A temporal and spatial study of malaria transmission and disease in a suburban area of Maputo, Mozambique. Am. J. Trop. Med. Hyg. 1997, 57, 550–559. [Google Scholar] [CrossRef]
- Echodu, R.; Okello-Onen, J.; Lutwama, J.; Enyaru, J.C.K.; Ocan, R.; Asaba, R.; Ajuga, F.; Rubaire-Akiiki, C.; Bradley, D.; Mutero, C.; et al. Heterogeneity of Anopheles mosquitoes in Nyabushozi county, Kiruhura district, Uganda. J. Parasit. Vector Biol. 2010, 2, 28–34. [Google Scholar]
- Wagman, J.M.; Varela, K.; Zulliger, R.; Saifodine, A.; Muthoni, R.; Magesa, S.; Chaccour, C.; Gogue, C.; Tynuv, K.; Seyoum, A.; et al. Reduced exposure to malaria vectors following indoor residual spraying of pirimiphos-methyl in a high-burden district of rural Mozambique with high ownership of long-lasting insecticidal nets: Entomological surveillance results from a cluster-randomized trial. Malar. J. 2021, 20, 54. [Google Scholar] [PubMed]
- Cuamba, N.J.B. The Bionomics, Population Structure and the Role of Malaria Transmission of Vectors in Mozambique and Angola. Ph.D. Thesis, The University of Liverpool, Liverpool, UK, 2003. [Google Scholar]
- Dandalo, L.C.; Brooke, B.D.; Munhenga, G.; Lobb, L.N.; Zikhali, J.; Ngxongo, S.P.; Zikhali, P.M.; Msimang, S.; Wood, O.R.; Mofokeng, M.; et al. Population Dynamics and Plasmodium falciparum (Haemosporida: Plasmodiidae) infectivity rates for the Malaria vector Anopheles arabiensis (Diptera: Culicidae) at Mamfene, KwaZulu-Natal, South Africa. J. Med. Entomol. 2017, 54, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Mzilahowa, T.; Hastings, I.M.; Molyneux, M.E.; McCall, P.J. Entomological indices of malaria transmission in Chikhwawa district, Southern Malawi. Malar. J. 2012, 11, 380. [Google Scholar] [CrossRef] [Green Version]
- Seyoum, A.; Sikaala, C.H.; Chanda, J.; Chinula, D.; Ntamatungiro, A.J.; Hawela, M.; Miller, J.M.; Russell, T.L.; Briet, O.J.; Killeen, G.F. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, south-east Zambia. Parasit. Vectors 2012, 5, 101. [Google Scholar] [CrossRef]
- Sharp, B.L.; Kleinschmidt, I.; Streat, E.; Maharaj, R.; Barnes, K.I.; Durrheim, D.N.; Ridl, F.C.; Morris, N.; Seocharan, I.; Kunene, S.; et al. Seven years of regional malaria control collaboration-Mozambique, South Africa, and Swaziland. Am. J. Trop. Med. Hyg. 2007, 76, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Tawe, L.; Ramatlho, P.; Waniwa, K.; Muthoga, C.W.; Makate, N.; Ntebela, D.S.; Quaye, I.K.; Pombi, M.; Paganotti, G.M. Preliminary survey on Anopheles species distribution in Botswana shows the presence of Anopheles gambiae and Anopheles funestus complexes. Malar. J. 2017, 16, 106. [Google Scholar] [CrossRef] [Green Version]
- Charlwood, J.D. Some like it hot: A differential response to changing temperatures by the malaria vectors Anopheles funestus and An. gambiae s.l. PeerJ 2017, 5, e3099. [Google Scholar] [CrossRef] [Green Version]
- Charlwood, J.D.; Macia, G.A.; Manhaca, M.; Sousa, B.; Cuamba, N.; Braganca, M. Population dynamics and spatial structure of human-biting mosquitoes, inside and outside of houses, in the Chockwe irrigation scheme, southern Mozambique. Geospat. Health 2013, 7, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Casimiro, S. Susceptibility and Resistance to Insecticides among Malaria Vector Mosquitoes in Mozambique. Ph.D. Thesis, University of Kwazulu-Natal, Durban, South Africa, 2003. [Google Scholar]
- Irish, S.; Kyalo, D.; Snow, R.; Coetzee, M. Anopheles species present in countries in sub-Saharan Africa and associated islands. Zootaxa 2019, 4747, 401–449. [Google Scholar] [CrossRef]
- Abílio, A.P.; Marrune, P.; De Deus, N.; Mbofana, F.; Muianga, P.; Kampango, A. Bio-efficacy of new long-lasting insecticide-treated bed nets against Anopheles funestus and Anopheles gambiae from central and northern Mozambique. Malar. J. 2015, 14, 352. [Google Scholar] [CrossRef] [Green Version]
- Aranda, C.; Aponte, J.J.; Saute, F.; Casimiro, S.; Pinto, J.; Sousa, C.; Rosário, V.D.; Petrarca, V.; Dgedge, M.; Alonso, P. Entomological characteristics of malaria transmission in Manhiça, a rural area in southern Mozambique. J. Med. Entomol. 2005, 42, 180–186. [Google Scholar] [CrossRef]
- Amaechi, E.C.; Mkpola Ukpai, O.; Chima Ohaeri, C.; Blessing Ejike, U.; Irole-Eze, O.P.; Egwu, O.; Comfort Nwadike, C. Distribution and seasonal abundance of anopheline mosquitoes and their association with rainfall around irrigation and non-irrigation areas in Nigeria. URJ 2018, 10, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Gage, K.L.; Burkot, T.R.; Eisen, R.J.; Hayes, E.B. Climate and vectorborne diseases. Am. J. Prev. Med. 2008, 35, 436–450. [Google Scholar] [CrossRef]
- Abiodun, G.J.; Maharaj, R.; Witbooi, P.; Okosun, K.O. Modelling the influence of temperature and rainfall on the population dynamics of Anopheles arabiensis. Malar. J. 2016, 15, 364. [Google Scholar] [CrossRef] [Green Version]
- Diakité, N.R.; Guindo-Coulibaly, N.; Adja, A.M.; Ouattara, M.; Coulibaly, J.T.; Utzinger, J.; N’Goran, E.K. Spatial and temporal variation of malaria entomological parameters at the onset of a hydro-agricultural development in central Côte d’Ivoire. Malar. J. 2015, 14, 340. [Google Scholar] [CrossRef] [Green Version]
- Keating, J.; Macintyre, K.; Mbogo, C.; Githeko, A.; Regens, J.L.; Swalm, C.; Ndenga, B.; Steinberg, L.J.; Kibe, L.; Githure, J.I.; et al. A geographic sampling strategy for studying relationships between human activity and malaria vectors in urban Africa. Am. J. Trop. Med. Hyg. 2003, 68, 357–365. [Google Scholar] [CrossRef]
- Coluzzi, M. Heterogeneities of the malaria vectorial system in tropical Africa and their significance in malaria epidemiology and control. Bull. World Health Organ. 1984, 62, 107–113. [Google Scholar]
- Gimnig, J.E.; Ombok, M.; Kamau, L.; Hawley, W.A. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J. Med. Entomol. 2001, 38, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Nambunga, I.H.; Ngowo, H.S.; Mapua, S.A.; Hape, E.E.; Msugupakulya, B.J.; Msaky, D.S.; Mhumbira, N.T.; McHwembo, K.R.; Tamayamali, G.Z.; Mlembe, S.V.; et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar. J. 2020, 19, 219. [Google Scholar] [CrossRef]
- Mendis, C.; Jacobsen, J.L.; Gamage-Mendis, A.; Bule, E.; Dgedge, M.; Thompson, R.; Cuamba, N.; Barreto, J.; Begtrup, K.; Sinden, R.E.; et al. Anopheles arabiensis and An. funestus are equally important vectors of malaria in Matola coastal suburb of Maputo, southern Mozambique. Med. Vet. Entomol. 2000, 14, 171–180. [Google Scholar] [CrossRef] [PubMed]
- White, G.B. Anopheles gambiae complex and disease transmission in Africa. Trans. R. Soc. Trop. Med. Hyg. 1974, 68, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, M.; Boelee, E. Early biting rhythm in the afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 2012, 26, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Thiaw, O.; Wotodjo, A.N.; Bouganali, C.; Diagne, N.; Parola, P.; Sokhna, C. Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low level exposure to malaria vectors. Malar. J. 2020, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.L.; Quicke, F.C.; Jansen, E.J. Aspects of the behaviour of five anopheline species in the endemic malaria area of Natal. J. Entomol. Soc. S. Afr. 1984, 47, 251–258. [Google Scholar]
- Pates, H.V.; Takken, W.; Curtis, C.F.; Huisman, P.W.; Akinpelu, O.; Gill, G.S. Unexpected anthropophagic behaviour in Anopheles quadriannulatus. Med. Vet. Entomol. 2001, 15, 293–298. [Google Scholar] [CrossRef]
- Torr, S.J.; Della Torre, A.; Calzetta, M.; Costantini, C.; Vale, G.A. Towards a fuller understanding of mosquito behaviour: Use of electrocuting grids to compare the odour-orientated responses of Anopheles arabiensis and An. quadriannulatus in the field. Med. Vet. Entomol. 2008, 22, 93–108. [Google Scholar] [CrossRef]
- Pates, H.; Curtis, C. Mosquito behavior and vector control. Annu. Rev. Entomol. 2005, 50, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Sande, S.; Zimba, M.; Chinwada, P.; Masendu, H.T.; Makuwaza, A. Biting behaviour of Anopheles funestus populations in Mutare and Mutasa districts, Manicaland province, Zimbabwe: Implications for the malaria control programme. J. Vector Borne Dis. 2016, 53, 118–126. [Google Scholar]
- Kenea, O.; Balkew, M.; Tekie, H.; Gebre-Michael, T.; Deressa, W.; Loha, E.; Lindtjørn, B.; Overgaard, H.J. Human-biting activities of Anopheles species in south-central Ethiopia. Parasit. Vectors 2016, 9, 527. [Google Scholar] [CrossRef] [Green Version]
- Adugna, T.; Getu, E.; Yewhalaw, D. Species diversity and distribution of Anopheles mosquitoes in Bure district, Northwestern Ethiopia. Heliyon 2020, 6, e05063. [Google Scholar] [CrossRef]
- Kent, R.J.; Thuma, P.E.; Mharakurwa, S.; Norris, D.E. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am. J. Trop. Med. Hyg. 2007, 76, 267–274. [Google Scholar] [CrossRef]
- Munhenga, G.; Brooke, B.D.; Spillings, B.; Essop, L.; Hunt, R.H.; Midzi, S.; Govender, D.; Braack, L.; Koekemoer, L.L. Field study site selection, species abundance and monthly distribution of anopheline mosquitoes in the northern Kruger National Park, South Africa. Malar. J. 2014, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Mwanziva, C.E.; Kitau, J.; Tungu, P.K.; Mweya, C.N.; Mkali, H.; Ndege, C.M.; Sangas, A.; Mtabho, C.; Lukwaro, C.; Azizi, S.; et al. Transmission intensity and malaria vector population structure in Magugu, Babati district in northern Tanzania. Tanzan. J. Health Res. 2011, 13, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Shililu, J.; Ghebremeskel, T.; Seulu, F.; Mengistu, S.; Fekadu, H.; Zerom, M.; Asmelash, G.E.; Sintasath, D.; Mbogo, C.; Githure, J.; et al. Seasonal abundance, vector behavior, and malaria parasite transmission in Eritrea. J. Am. Mosq. Control Assoc. 2004, 20, 155–164. [Google Scholar]
- Lwetoijera, D.W.; Harris, C.; Kiware, S.S.; Dongus, S.; Devine, G.J.; McCall, P.J.; Majambere, S. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar. J. 2014, 13, 331. [Google Scholar] [CrossRef] [Green Version]
- Simard, F.; Lehmann, T.; Lemasson, J.J.; Diatta, M.; Fontenille, D. Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: An indirect approach using microsatellite loci. Insect Mol. Biol. 2000, 9, 467–479. [Google Scholar] [CrossRef]
- Animut, A.; Negash, Y. Dry season occurrence of Anopheles mosquitoes and implications in Jabi Tehnan District, West Gojjam Zone, Ethiopia. Malar. J. 2018, 17, 445. [Google Scholar] [CrossRef]
- Mala, A.O.; Irungu, L.W.; Shililu, J.I.; Muturi, E.J.; Mbogo, C.C.; Njagi, J.K.; Githure, J.I. Dry season ecology of Anopheles gambiae complex mosquitoes at larval habitats in two traditionally semi-arid villages in Baringo, Kenya. Parasit. Vectors 2011, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Githeko, A.K.; Adungo, N.I.; Karanja, D.M.; Hawley, W.A.; Vulule, J.M.; Seroney, I.K.; Ofulla, A.V.; Atieli, F.K.; Ondijo, S.O.; Genga, I.O.; et al. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp. Parasitol. 1996, 82, 306–315. [Google Scholar] [CrossRef]
- Huho, B.; Briet, O.; Seyoum, A.; Sikaala, C.; Bayoh, N.; Gimnig, J.; Okumu, F.; Diallo, D.; Abdulla, S.; Smith, T.; et al. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int. J. Epidemiol. 2013, 42, 235–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbale, F.G.; Akol, A.M.; Kaddu, J.B.; Onapa, A.W. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District, Uganda. Parasit. Vectors 2013, 6, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakilla, C.; Manjurano, A.; Nelwin, K.; Martin, J.; Mashauri, F.; Kinung’Hi, S.M.; Lyimo, E.; Mangalu, D.; Bernard, L.; Iwuchukwu, N.; et al. Malaria vector species composition and entomological indices following indoor residual spraying in regions bordering Lake Victoria, Tanzania. Malar. J. 2020, 19, 383. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.C.; Oster, C.N.; Onyango, F.K.; Bales, J.D.; Sherwood, J.A.; Perkins, P.V.; Chumo, D.K.; Koech, D.V.; Whitmire, R.E.; Roberts, C.R.; et al. Plasmodium falciparum Incidence Relative to Entomologic Inoculation Rates at a Site Proposed for Testing Malaria Vaccines in Western Kenya. Am. J. Trop. Med. Hyg. 1994, 50, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Mosha, J.F.; Lukole, E.; Charlwood, J.D.; Wright, A.; Rowland, M.; Bullock, O.; Manjurano, A.; Kisinza, W.; Mosha, F.W.; Kleinschmidt, I.; et al. Risk factors for malaria infection prevalence and household vector density between mass distribution campaigns of long-lasting insecticidal nets in North-western Tanzania. Malar. J. 2020, 19, 297. [Google Scholar] [CrossRef]
- Beier, J.C.; Killeen, G.F.; Githure, J.I. Short report: Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999, 61, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Ebenezer, A.; Noutcha, A.E.M.; Okiwelu, S.N. Relationship of annual entomological inoculation rates to malaria transmission indices, Bayelsa State, Nigeria. J. Vector Borne Dis. 2016, 53, 46–53. [Google Scholar]
- Mbogo, C.N.; Snow, R.W.; Khamala, C.P.; Kabiru, E.W.; Ouma, J.H.; Githure, J.I.; Marsh, K.; Beier, J.C. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am. J. Trop. Med. Hyg. 1995, 52, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Graumans, W.; Jacobs, E.; Bousema, T.; Sinnis, P. When Is a Plasmodium-Infected Mosquito an Infectious Mosquito? Trends Parasitol. 2020, 36, 705–716. [Google Scholar] [CrossRef]
- Takken, W.; Eling, W.; Hooghof, J.; Dekker, T.; Hunt, R.; Coetzee, M. Susceptibility of Anopheles quadriannulatus Theobald (Diptera: Culicidae) to Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 578–580. [Google Scholar] [CrossRef]
- Habtewold, T.; Povelones, M.; Blagborough, A.M.; Christophides, G.K. Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. PLoS Pathog. 2008, 4, e1000070. [Google Scholar] [CrossRef] [Green Version]
- Sikaala, C.H.; Killeen, G.F.; Chanda, J.; Chinula, D.; Miller, J.M.; Russell, T.L.; Seyoum, A. Evaluation of alternative mosquito sampling methods for malaria vectors in lowland south-east Zambia. Parasit. Vectors 2013, 6, 91. [Google Scholar] [CrossRef] [Green Version]

| Genus | Conhane N (%) | Cotsuane N (%) | Total N (%) |
|---|---|---|---|
| Mansonia | 1202 (19.6) | 4922 (80.4) | 6124 (46.9) |
| Culex | 1995 (55.1) | 1624 (44.9) | 3619 (27.7) |
| Anopheles | 681 (37.7) | 1124 (62.3) | 1805 (13.8) |
| Aedes | 42 (62.7) | 25 (37.3) | 67 (0.5) |
| All other genera | 581 (40.2) | 863 (59.8) | 1444 (11.1) |
| Total | 4501 (34.5) | 8558 (65.5) | 13,059 (100.0) |
| Anopheles Species | Conhane N (%) | Cotsuane N (%) | Total N (%) |
|---|---|---|---|
| funestus s.s. | 7 (2.5) | 69 (9.6) | 76 (7.6) |
| arabiensis | 51 (18.0) | 66 (9.1) | 117 (11.6) |
| quadriannulatus | 225 (79.5) | 587 (81.3) | 812 (80.8) |
| Total | 283 | 722 | 1005 |
| Anopheles Species | Indoor N (%) | Outdoor N (%) | Total N (%) |
|---|---|---|---|
| funestus s.s. | 33 (17.3) | 43 (5.3) | 76 (7.6) |
| arabiensis | 25 (13.1) | 92 (11.3) | 117 (11.6) |
| quadriannulatus | 133 (69.6) | 679 (83.4) | 812 (80.8) |
| Total | 191 | 814 | 1005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomé, G.; Riddin, M.; Braack, L. Species Composition, Seasonal Abundance, and Biting Behavior of Malaria Vectors in Rural Conhane Village, Southern Mozambique. Int. J. Environ. Res. Public Health 2023, 20, 3597. https://doi.org/10.3390/ijerph20043597
Salomé G, Riddin M, Braack L. Species Composition, Seasonal Abundance, and Biting Behavior of Malaria Vectors in Rural Conhane Village, Southern Mozambique. International Journal of Environmental Research and Public Health. 2023; 20(4):3597. https://doi.org/10.3390/ijerph20043597
Chicago/Turabian StyleSalomé, Graça, Megan Riddin, and Leo Braack. 2023. "Species Composition, Seasonal Abundance, and Biting Behavior of Malaria Vectors in Rural Conhane Village, Southern Mozambique" International Journal of Environmental Research and Public Health 20, no. 4: 3597. https://doi.org/10.3390/ijerph20043597





