Impact of Textile Industries on Surface Water Contamination by Sb and Other Potential Toxic Elements: A Case Study in Taihu Lake Basin, China
Abstract
:1. Introduction
2. Materials and Methods
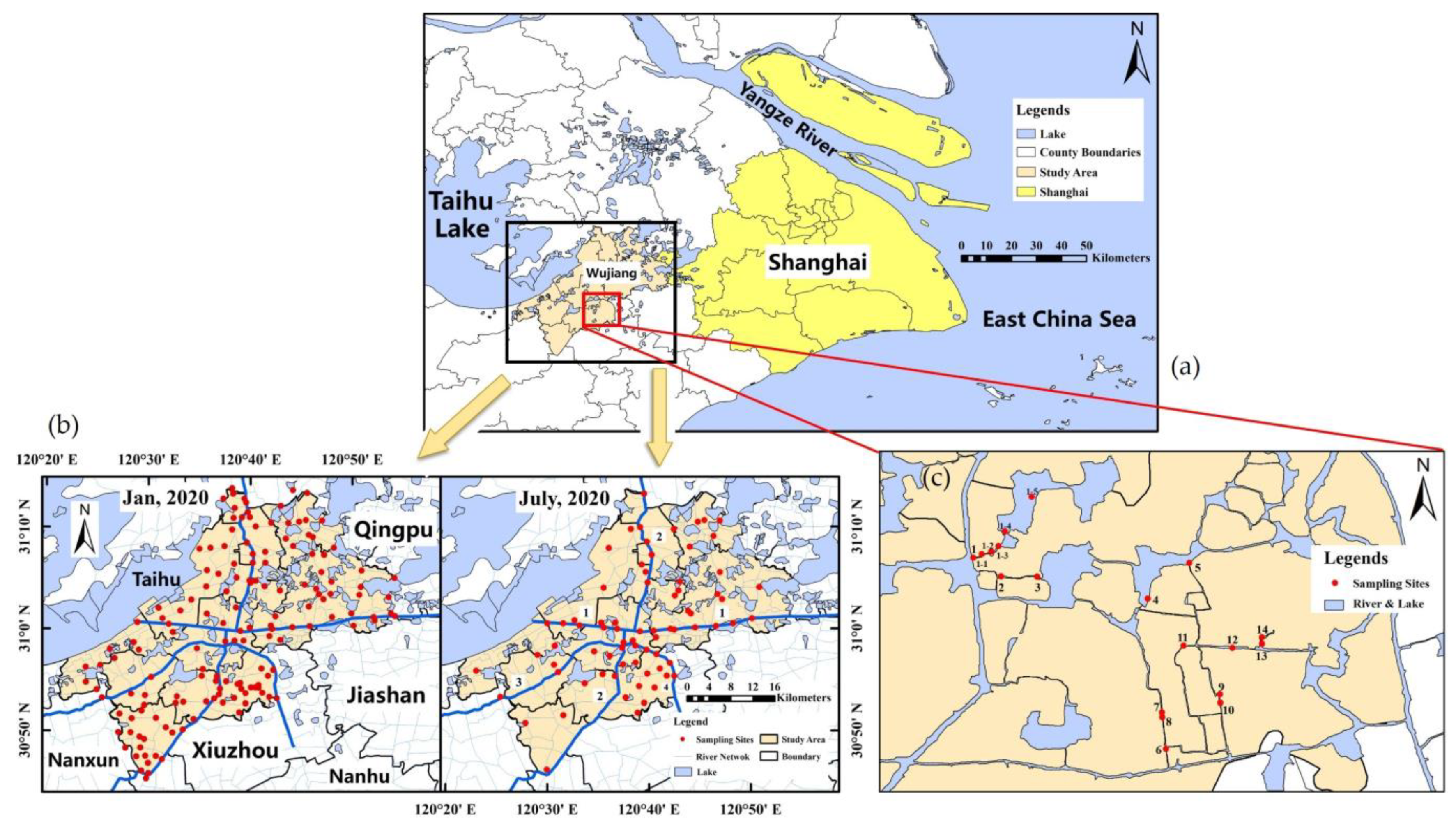
2.1. Study Area
2.2. Sampling and Experimental Methods
2.3. Water Analysis
2.4. Assessment of Water Quality and Ecological Risk
2.5. Statistical Analysis
2.6. Quality Assurance and Control
3. Results
3.1. Physicochemical Properties of Surface Water Samples
3.2. Spatial and Temporal Distribution of the Nine PTEs in Surface Water
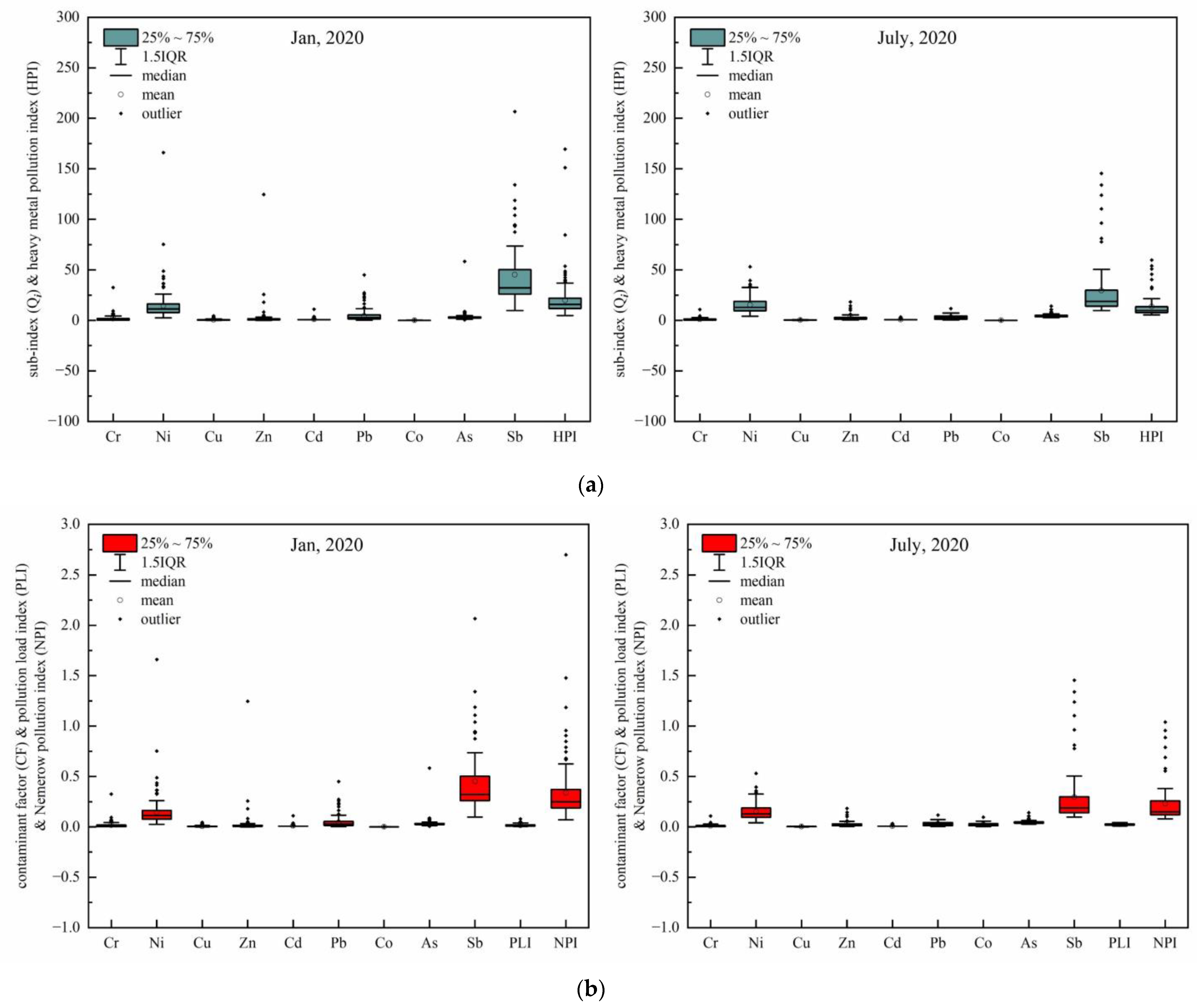
3.3. Water Quality and Ecological Risk Assessment
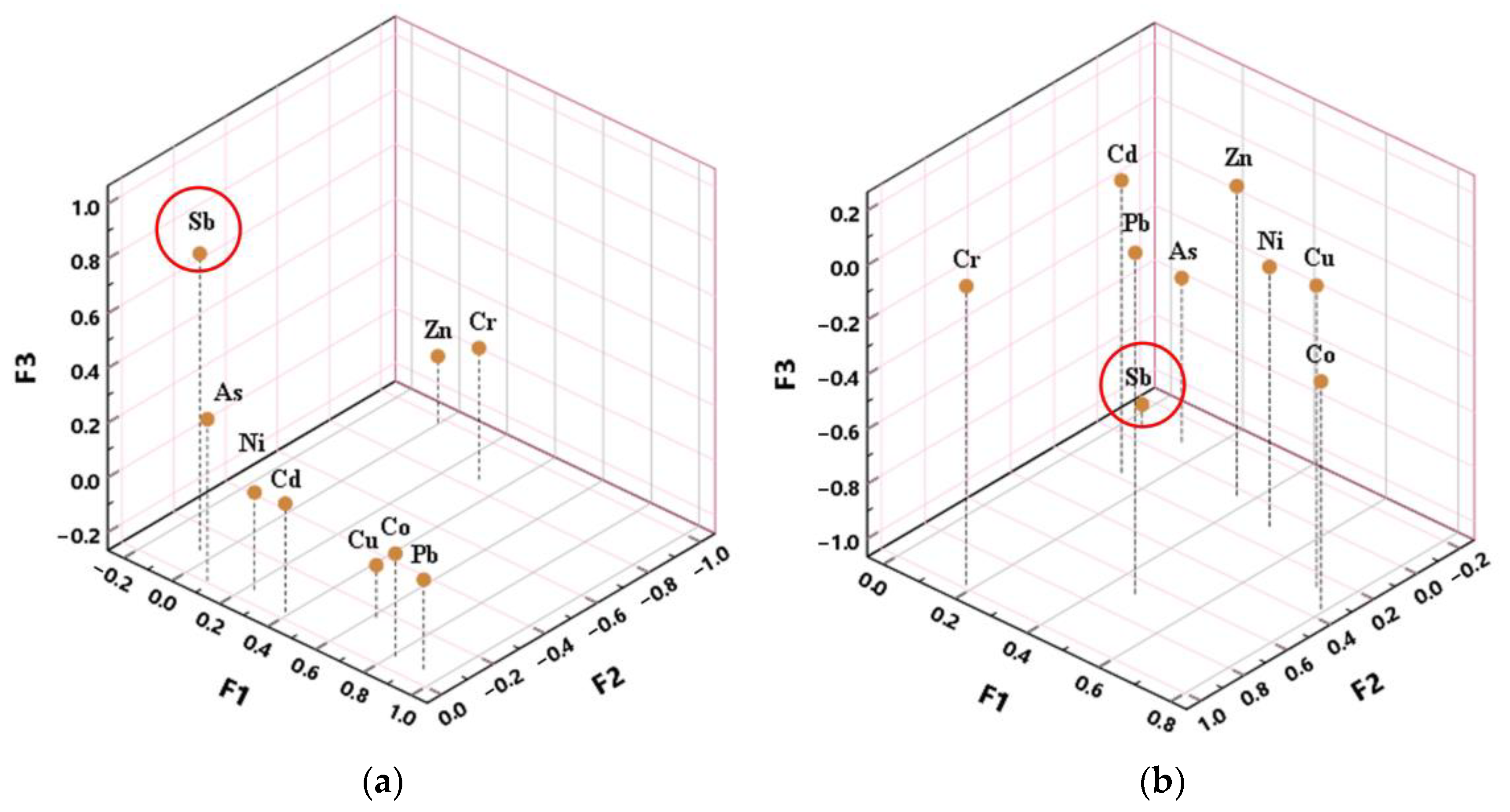
3.4. Statistical Analysis
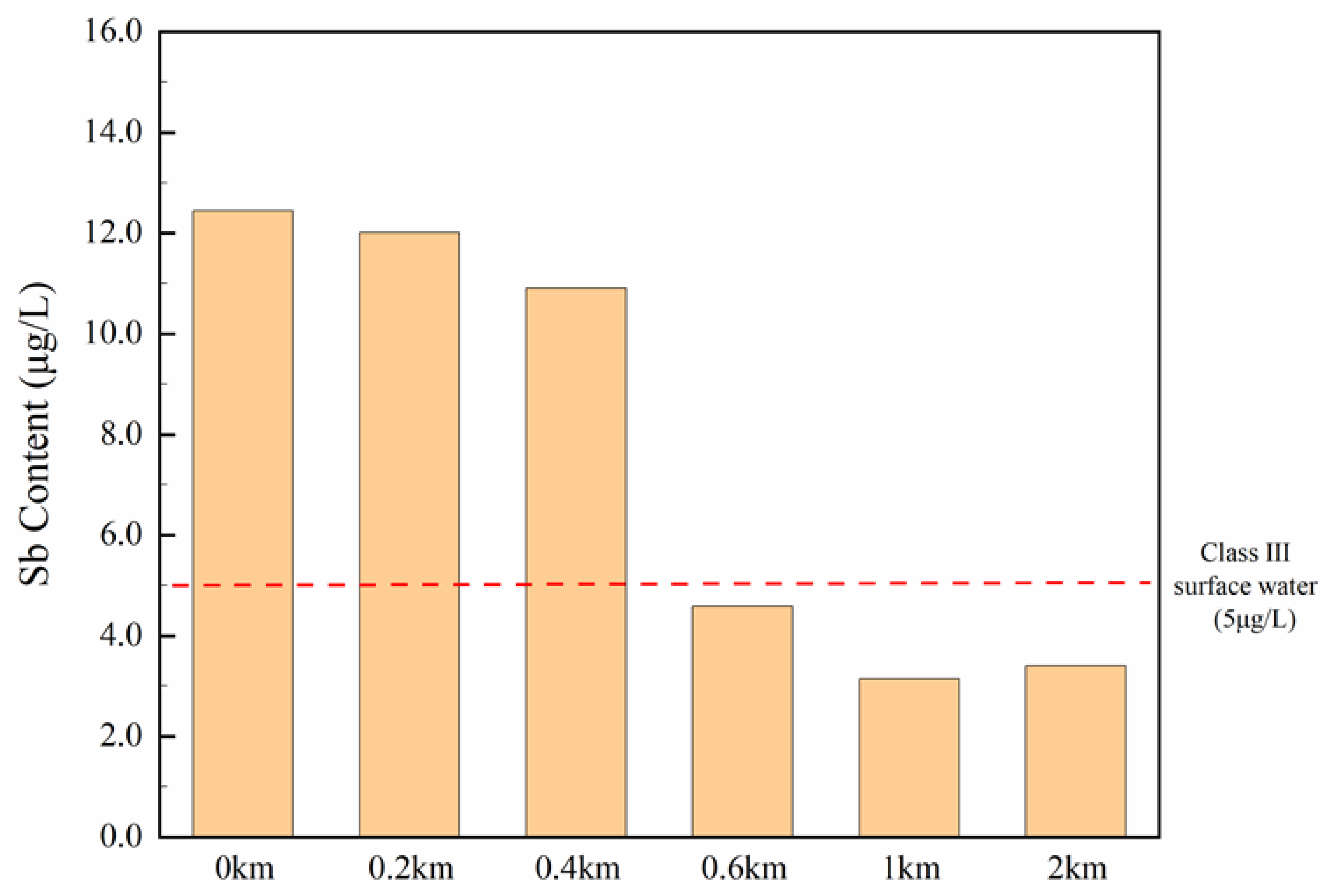
3.5. PTEs Concentration in Water Directly Collected from Drainage Outlets and Spatial Distribution of Sb Downstream
4. Discussion
4.1. Physicochemical Characteristics of Surface Water and Potential Toxic Element Concentrations in Dry Period and Wet Period
4.2. Impact of Textile Industries on Sb Distribution in Surface Water
4.3. Contribution of Sb to the Heavy Metal Pollution Risk Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imtiazuddin, S.; Mumtaz, M.; Khalil, A. Mallick Pollutants of Wastewater Characteristics in Textile Industries. J. Basic Appl. Sci. 2012, 8, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.L.; Abdullah, A.Z. Current Status of Textile Industry Wastewater Management and Research Progress in Malaysia: A Review. Clean Soil Air Water 2013, 41, 751–764. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and Health Concerns of Persistent Coloring Pollutants of Textile Industry Wastewater and Treatment Approaches for Environmental Safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Dey, S.; Islam, A. A Review on Textile Wastewater Characterization in Bangladesh. Resour. Environ. 2015, 5, 15–44. [Google Scholar] [CrossRef]
- El-Gendy, A.H.; Augustyniak, M.; Toto, N.A.; Al Farraj, S.; El-Samad, L.M. Oxidative Stress Parameters, DNA Damage and Expression of HSP70 and MT in Midgut of Trachyderma Hispida (Forskål, 1775) (Coleoptera: Tenebrionidae) from a Textile Industry Area. Environ. Pollut. 2020, 267, 115661. [Google Scholar] [CrossRef]
- Colin, N.; Maceda-Veiga, A.; Flor-Arnau, N.; Mora, J.; Fortuño, P.; Vieira, C.; Prat, N.; Cambra, J.; de Sostoa, A. Ecological Impact and Recovery of a Mediterranean River after Receiving the Effluent from a Textile Dyeing Industry. Ecotoxicol. Environ. Saf. 2016, 132, 295–303. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Hua, Z.; Du, G. Research and Application of Biotechnology in Textile Industries in China. Enzym. Microb. Technol. 2007, 40, 1651–1655. [Google Scholar] [CrossRef]
- Lin, B.; Zhao, H. Technology Gap and Regional Energy Efficiency in China’s Textile Industry: A Non-Parametric Meta-Frontier Approach. J. Clean. Prod. 2016, 137, 21–28. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Tan, Y.; Wang, L.; Shen, M. Decoupling Water Consumption and Environmental Impact on Textile Industry by Using Water Footprint Method: A Case Study in China. Water 2017, 9, 124. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Z.; Gu, C.; Shen, C.; Ma, C.; Liu, Y.; Yin, S.; Xu, C. Metals Pollution from Textile Production Wastewater in Chinese Southeastern Coastal Area: Occurrence, Source Identification, and Associated Risk Assessment. Environ. Sci. Pollut. Res. 2021, 28, 38689–38697. [Google Scholar] [CrossRef]
- You, S.; Cheng, S.; Yan, H. The Impact of Textile Industry on China’s Environment. Int. J. Fash. Des. Technol. Educ. 2009, 2, 33–43. [Google Scholar] [CrossRef]
- Agrawal, S.; Tipre, D.; Patel, B.; Dave, S. Bacterial Decolourization, Degradation and Detoxification of Azo Dyes: An Eco-Friendly Approach. In Microbial Applications Vol.1; Kalia, V.C., Kumar, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 91–124. ISBN 978-3-319-52665-2. [Google Scholar]
- Chen, F.; Zhu, J.; Yang, Y.; Wang, L. Assessing Environmental Impact of Textile Production with Water Alkalization Footprint. Sci. Total Environ. 2020, 719, 137522. [Google Scholar] [CrossRef]
- Jadhav, J.P.; Kalyani, D.C.; Telke, A.A.; Phugare, S.S.; Govindwar, S.P. Evaluation of the Efficacy of a Bacterial Consortium for the Removal of Color, Reduction of Heavy Metals, and Toxicity from Textile Dye Effluent. Bioresour. Technol. 2010, 101, 165–173. [Google Scholar] [CrossRef]
- Basha, S.A.; Rajaganesh, K. Microbial Bioremediation of Heavy Metals from Textile Industry Dye Effluents Using Isolated Bacterial Strains. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 785–794. [Google Scholar]
- Chandanshive, V.; Kadam, S.; Rane, N.; Jeon, B.-H.; Jadhav, J.; Govindwar, S. In Situ Textile Wastewater Treatment in High Rate Transpiration System Furrows Planted with Aquatic Macrophytes and Floating Phytobeds. Chemosphere 2020, 252, 126513. [Google Scholar] [CrossRef]
- Meng, L.; Wu, M.; Chen, H.; Xi, Y.; Huang, M.; Luo, X. Rejection of Antimony in Dyeing and Printing Wastewater by Forward Osmosis. Sci. Total Environ. 2020, 745, 141015. [Google Scholar] [CrossRef]
- Wang, N.; Wang, N.; Tan, L.; Zhang, R.; Zhao, Q.; Wang, H. Removal of Aqueous As(III) Sb(III) by Potassium Ferrate (K2FeO4): The Function of Oxidation and Flocculation. Sci. Total Environ. 2020, 726, 138541. [Google Scholar] [CrossRef]
- Xue, G.; Wang, Q.; Qian, Y.; Gao, P.; Su, Y.; Liu, Z.; Chen, H.; Li, X.; Chen, J. Simultaneous Removal of Aniline, Antimony and Chromium by ZVI Coupled with H2O2: Implication for Textile Wastewater Treatment. J. Hazard. Mater. 2019, 368, 840–848. [Google Scholar] [CrossRef]
- Fazio, F.; Piccione, G.; Tribulato, K.; Ferrantelli, V.; Giangrosso, G.; Arfuso, F.; Faggio, C. Bioaccumulation of Heavy Metals in Blood and Tissue of Striped Mullet in Two Italian Lakes. J. Aquat. Anim. Health 2014, 26, 278–284. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on Seasonal Pollution of Heavy Metals in Water, Sediment, Fish and Oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a Global Dilemma: Geochemistry, Mobility, Fate and Transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, Z.; Qiao, H.; Liu, F. Assessment of Eutrophication and Water Quality in the Estuarine Area of Lake Wuli, Lake Taihu, China. Sci. Total Environ. 2019, 650, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhou, C.; Li, C.; Dou, S.; Li, X.; Wang, X.; Xu, X. Efficient Removal of Sb(V) in Textile Wastewater through Novel Amorphous Si-Doped Fe Oxide Composites: Phase Composition, Stability and Adsorption Mechanism. Chem. Eng. J. 2021, 407, 127217. [Google Scholar] [CrossRef]
- Yao, C.; Jiang, X.; Che, F.; Wang, K.; Zhao, L. Antimony Speciation and Potential Ecological Risk of Metal(Loid)s in Plain Wetlands in the Lower Yangtze River Valley, China. Chemosphere 2019, 218, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, Z.; Wei, M.; Xiaona, H. Characterization of Heavy Metals in Water and Sediments in Taihu Lake, China. Environ. Monit. Assess 2012, 184, 4367–4382. [Google Scholar] [CrossRef]
- Yang, M.; Yang, K.; Li, G.; Niu, X. The Cooperation Mechanism of Water Resources Protection in Trans-Boundary River Based on Game Theory: A Case Study of the Taipu River in the Taihu Lake Basin. J. Nat. Resour. 2019, 34, 1232–1244. [Google Scholar] [CrossRef]
- He, Y. Drinking Water Source Quality Safety and Collaborative Legislation on Related Substantial System in Yangtze River Delta Demonstration Area. Environ. Pollut. Control 2022, 44, 278–284. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Mao, L.; Chen, M.; Tao, H.; Li, J. Spatial Distribution and Pollution Assessment of Potentially Toxic Elements (PTEs) in Surface Sediments at the Drinking Water Source Channel of Taipu River in China. Minerals 2021, 11, 1202. [Google Scholar] [CrossRef]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk Analysis of Heavy Metal Concentration in Surface Waters across the Rural-Urban Interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Nithila, P.; Reddy, S.J. Estimation of Heavy Metals in Drinking Water and Development of Heavy Metal Pollution Index. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxic Hazard. Subst. Control 1996, 31, 283–289. [Google Scholar] [CrossRef]
- Sheykhi, V.; Moore, F. Geochemical Characterization of Kor River Water Quality, Fars Province, Southwest Iran. Water Qual. Expo. Health 2012, 4, 25–38. [Google Scholar] [CrossRef]
- Reza, R.; Singh, G. Heavy Metal Contamination and Its Indexing Approach for River Water. Int. J. Environ. Sci. Technol. 2010, 7, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Ogbeibu, A.E.; Omoigberale, M.O.; Ezenwa, I.M.; Eziza, J.O.; Igwe, J.O. Using Pollution Load Index and Geoaccumulation Index for the Assessment of Heavy Metal Pollution and Sediment Quality of the Benin River, Nigeria. Nat. Environ. 2014, 2, 1. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgol. Meeresunters 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Nemerow, N.L. Stream, Lake, Estuary, and Ocean Pollution, 2nd ed; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1991.
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy Metal Contamination Status in Greek Surface Waters: A Review with Application and Evaluation of Pollution Indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A Review of Its Sources and Environmental Toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Filella, M.; Williams, P.A.; Belzile, N. Antimony in the Environment: Knowns and Unknowns. Environ. Chem. 2009, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhou, S.; Zou, J.; Fang, R.; Jiang, T.; Bao, Y.; Shi, H. Analysis on the Origin of Antimony Pollution in Urban Sewage Treatment Plant Mainly Engaged in Printing and Dyeing Wastewater. J. Zhejiang Univ. 2018, 45, 569–575. [Google Scholar] [CrossRef]
- Sivakumar, K.K.; Balamurugan, C.; Ramakrishnan, D.; Bhai, L.H. Assessment studies on wastewater pollution by textile dyeing and bleaching industries at Karur, Tamil Nadu. Rasayan J. Chem. 2011, 4, 264–269. [Google Scholar]
- Correia, V.M.; Stephenson, T.; Judd, S.J. Characterisation of Textile Wastewaters—A Review. Environ. Technol. 1994, 15, 917–929. [Google Scholar] [CrossRef]
- Xue, F.; Tang, B.; Bin, L.; Ye, J.; Huang, S.; Fu, F.; Li, P.; Cui, J. Residual Micro Organic Pollutants and Their Biotoxicity of the Effluent from the Typical Textile Wastewater Treatment Plants at Pearl River Delta. Sci. Total Environ. 2019, 657, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and Antimony in Water and Wastewater: Overview of Removal Techniques with Special Reference to Latest Advances in Adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pang, Y.; Han, Y.; Zhan, X.; Wang, H.; Liu, J.; Gao, R.; Liu, H.; Shi, H. Removal of Trace Concentration Sb(V) in Textile Wastewater by Mn-Doped Fe3O4: The Mechanisms of Mn Affect Adsorption Performance. Microporous Mesoporous Mater. 2022, 343, 112150. [Google Scholar] [CrossRef]

| Physicochemical Property | Dry Period (January, 2020; n = 154) | Wet Period (July, 2020; n = 74) | ||||||
|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | CV * (%) | Max | Min | Mean | CV * (%) | |
| Temperature (°C) | 11.29 | 7.73 | 8.81 | 7.38 | 32.85 | 26.19 | 28.57 | 5.59 |
| pH | 8.03 | 6.94 | 7.43 | 2.83 | 7.35 | 6.19 | 7.05 | 3.98 |
| DO (mg/L) | 12.56 | 4.18 | 9.09 | 20.06 | 16.09 | 0.86 | 6.48 | 50.48 |
| SPC (μs/cm) | 995.00 | 361.40 | 566.19 | 19.78 | 838.10 | 197.10 | 387.40 | 33.78 |
| TDS (mg/L) | 979.49 | 42.15 | 370.87 | 29.45 | 545.00 | 128.00 | 251.82 | 33.77 |
| Turbidity (NTU) | 240.30 | 2.38 | 53.79 | 92.75 | 59.70 | 3.40 | 18.45 | 84.73 |
| COD (mg/L) | 49.50 | 0.10 | 19.89 | 44.53 | 77.60 | 10.70 | 31.66 | 37.73 |
| fDOM (RFU) | 445.52 | 2.75 | 102.82 | 70.06 | 26.39 | 4.67 | 11.66 | 42.29 |
| Chl (μg/L) | 76.05 | 0.46 | 7.02 | 144.29 | 43.98 | 1.76 | 11.83 | 88.81 |
| Potential Toxic Elements | Dry Period (January, 2020; n = 154) | Wet Period (July, 2020; n = 74) | ||||||
|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | CV 1 (%) | Max | Min | Mean | CV 1 (%) | |
| Cr | 16.30 | N 2 | 1.17 | 151.13 | 5.40 | N 2 | 0.82 | 110.47 |
| Ni | 33.21 | 0.49 | 2.89 | 107.31 | 10.62 | 0.82 | 3.04 | 58.71 |
| Cu | 44.67 | N | 5.78 | 120.03 | 7.75 | 0.60 | 2.70 | 59.63 |
| Zn | 1245.05 | N | 24.68 | 433.74 | 183.20 | 3.52 | 27.69 | 110.86 |
| Cd | 0.55 | N | 0.10 | 98.75 | 0.17 | N | 0.08 | 52.78 |
| Pb | 22.48 | N | 2.42 | 121.44 | 5.88 | 0.21 | 1.49 | 69.90 |
| Co | 4.27 | 0.06 | 0.52 | 106.56 | 1.91 | 0.06 | 0.48 | 66.74 |
| As | 29.15 | 0.26 | 1.65 | 140.46 | 7.06 | 1.37 | 2.43 | 43.64 |
| Sb | 21.37 | 0.48 | 2.26 | 107.19 | 7.28 | 0.49 | 1.48 | 99.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Guo, Z.; Mao, L.; Feng, J.; Huang, J.; Tao, H. Impact of Textile Industries on Surface Water Contamination by Sb and Other Potential Toxic Elements: A Case Study in Taihu Lake Basin, China. Int. J. Environ. Res. Public Health 2023, 20, 3600. https://doi.org/10.3390/ijerph20043600
Li F, Guo Z, Mao L, Feng J, Huang J, Tao H. Impact of Textile Industries on Surface Water Contamination by Sb and Other Potential Toxic Elements: A Case Study in Taihu Lake Basin, China. International Journal of Environmental Research and Public Health. 2023; 20(4):3600. https://doi.org/10.3390/ijerph20043600
Chicago/Turabian StyleLi, Feipeng, Ziyi Guo, Lingchen Mao, Junyi Feng, Jiong Huang, and Hong Tao. 2023. "Impact of Textile Industries on Surface Water Contamination by Sb and Other Potential Toxic Elements: A Case Study in Taihu Lake Basin, China" International Journal of Environmental Research and Public Health 20, no. 4: 3600. https://doi.org/10.3390/ijerph20043600
APA StyleLi, F., Guo, Z., Mao, L., Feng, J., Huang, J., & Tao, H. (2023). Impact of Textile Industries on Surface Water Contamination by Sb and Other Potential Toxic Elements: A Case Study in Taihu Lake Basin, China. International Journal of Environmental Research and Public Health, 20(4), 3600. https://doi.org/10.3390/ijerph20043600







