Recent Advances in Thallium Removal from Water Environment by Metal Oxide Material
Abstract
:1. Introduction
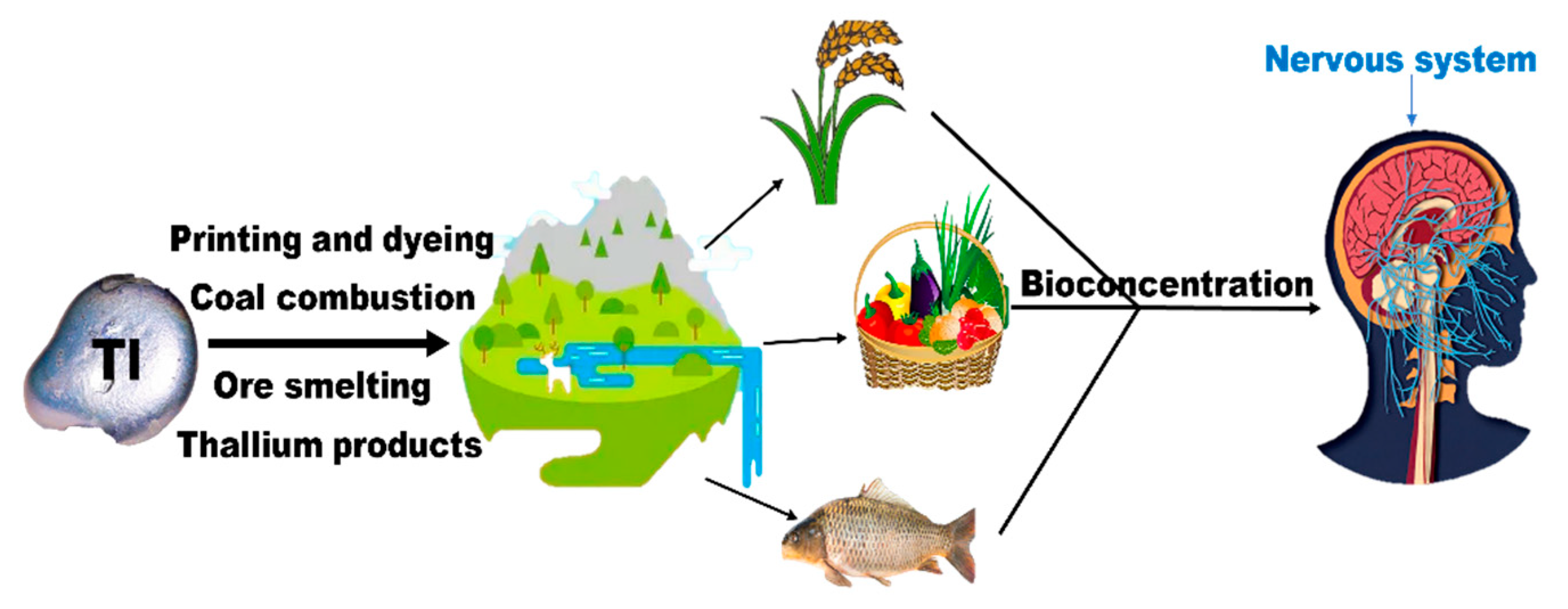
2. Environmental Behavior of Thallium in Aqueous Systems
3. The Synthesis and Environmental Applications of Metal Oxides
3.1. Synthesis Methods of Metal Oxides
3.1.1. Physical Synthesis
3.1.2. Chemical Synthesis
3.2. Removal of Thallium from Water/Wastewater by Metal Oxides
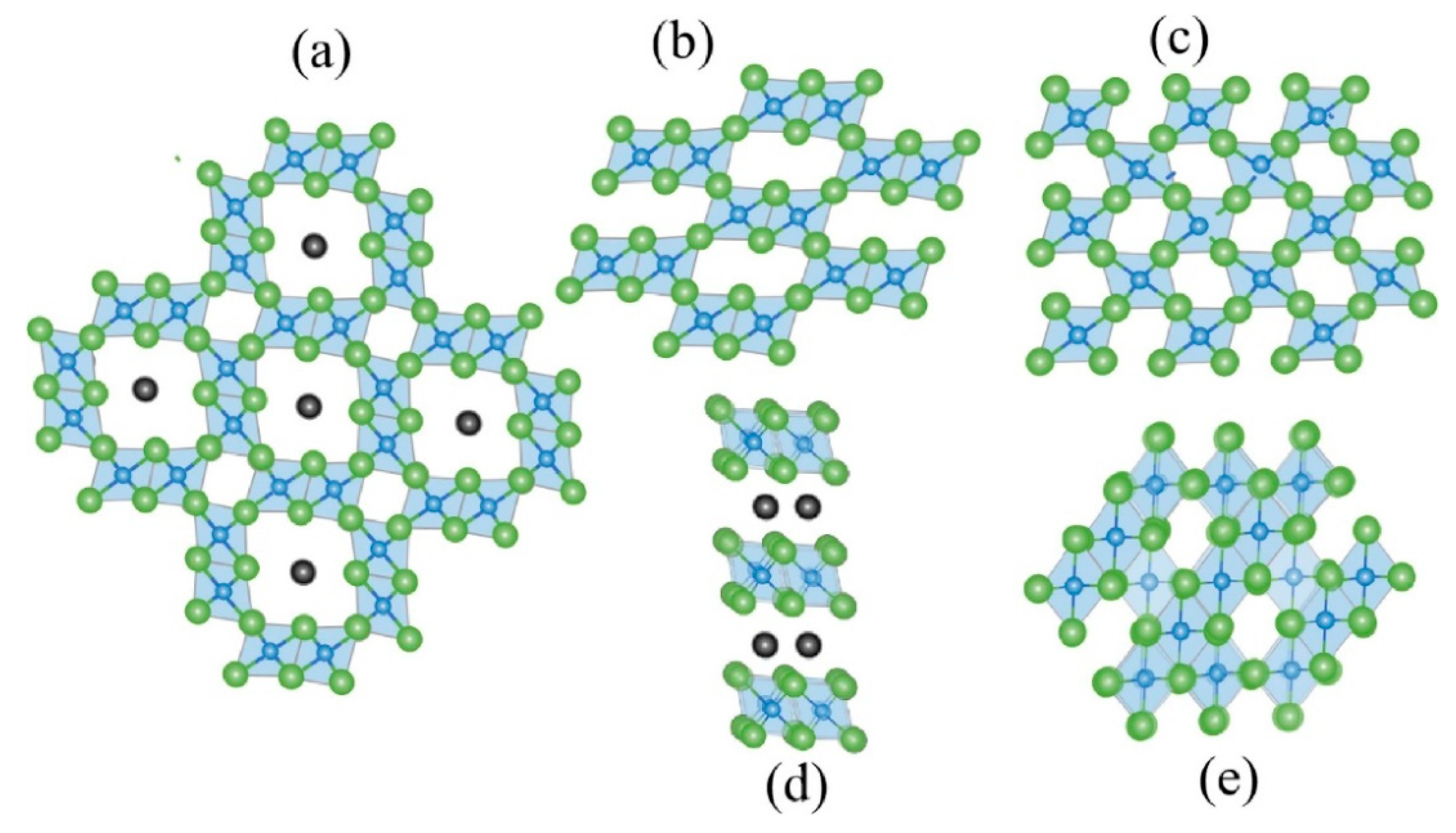
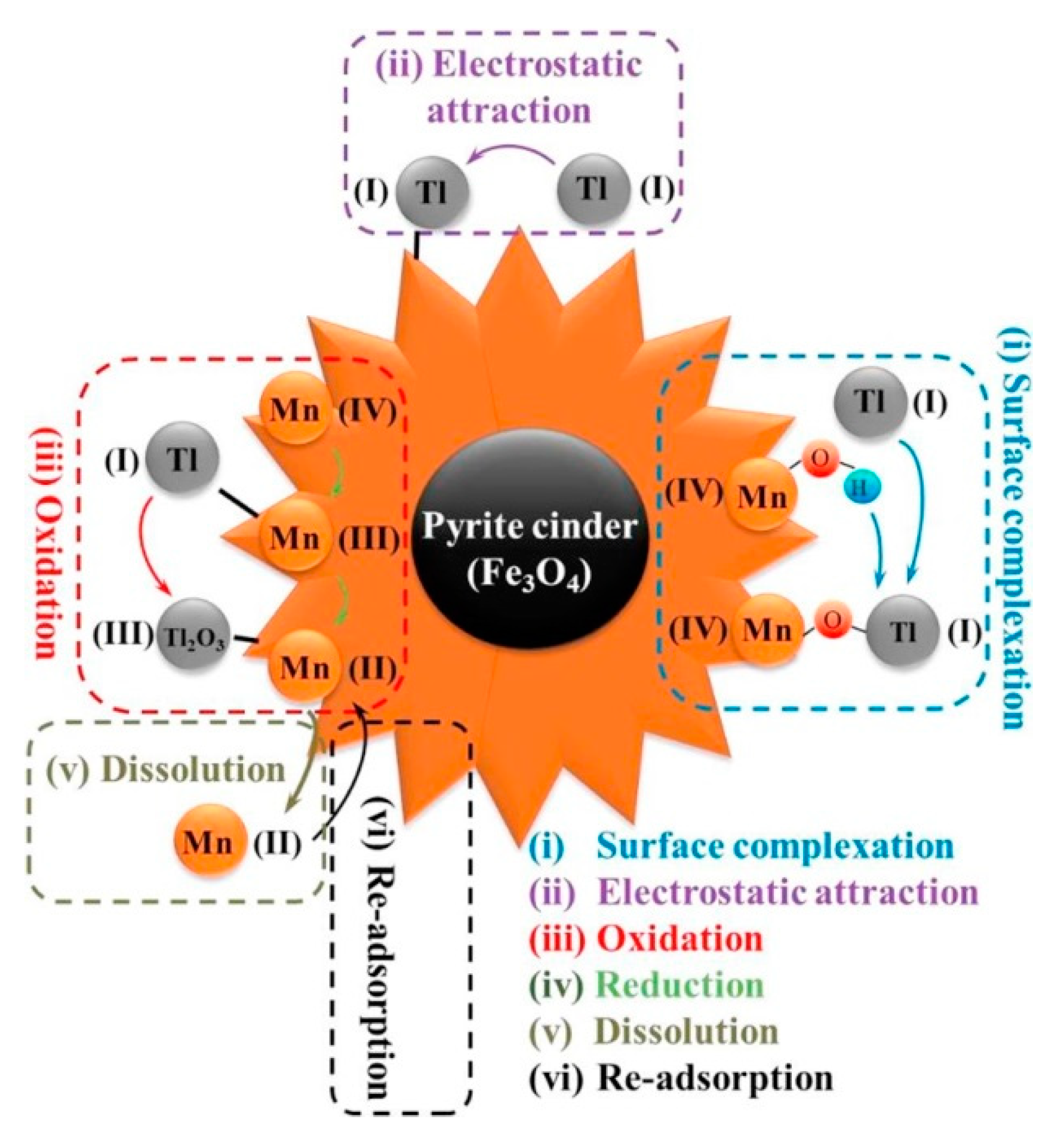
3.2.1. Thallium Removal by Manganese Oxides
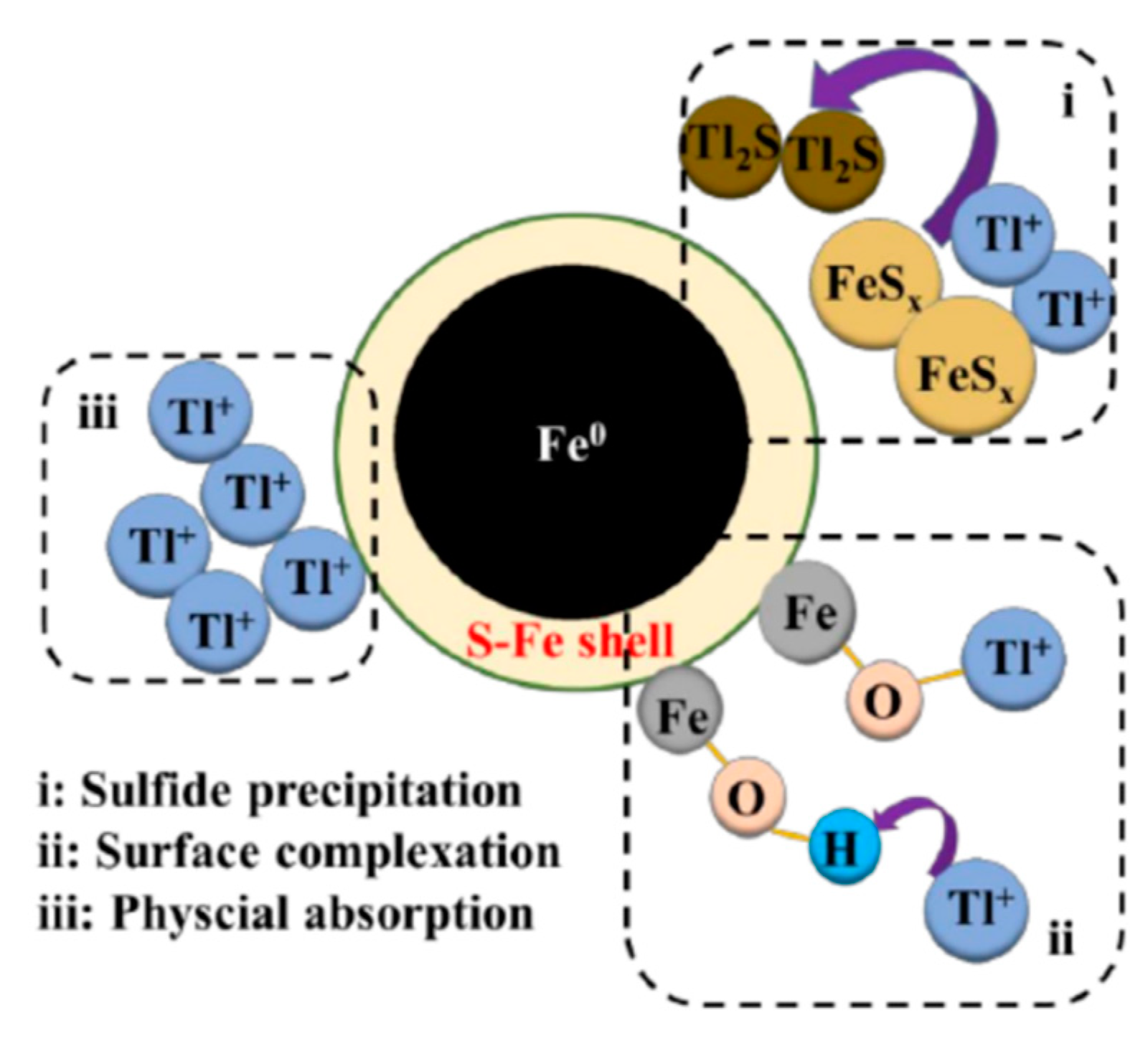
3.2.2. Thallium Removal by Iron Oxides
| Metal Oxides | Dosage | Thallium Concentration in the Tested Water | Performance | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Magnetic Prussian blue | 0.6 g/L | 1000 μg/L | 528 mg/L | High removal efficiency | Poor reproducibility | [53] |
| Fe@Fe2O3 Core–Shell | 0.75 g/L | 10 mg/L | 95.60% | Less influenced by environmental conditions | Poor reproducibility | [57] |
| 3-ZVIMn | 0.1 g/L | 200 mg/L | 990 mg/g | High removal efficiency | Highly influenced by environmental conditions | [59] |
| Fe-Mn binary oxides | 0.5 g/L | 10 mg/L | 197.6 mg/g | Less influenced by environmental conditions | Poor reproducibility | [60] |
| Titanium iron magnetic | 0.1 g/L | 12.5 mg/L | 111.3 mg/g | Less influenced by environmental conditions | Poor reproducibility | [58] |
| Fe-Mn binary oxides activated aluminosilicate mineral | 1.0 g/L | 10 mg/L | 78.06 mg/g | Less influenced by environmental conditions | Poor removal ability | [61] |
| Fe3O4@TiO2 decorated RGO nanosheets | 0.2 g/L | _ | 673.2 mg/g | High removal efficiency | Highly influenced by environmental conditions | [62] |
3.2.3. Thallium Removal by Aluminum Oxides
3.2.4. Thallium Removal by Titanium Oxides
3.3. Factors Affecting the Effectiveness of Thallium Removal by Metal Oxides
3.3.1. Effect of pH
3.3.2. Effect of Co-Existing Ions
3.3.3. Effect of Organic Matters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Luo, X.; Sun, Y.; Tsang, D.C.; Qi, J.; Zhang, W.; Li, N.; Yin, M.; Wang, J.; Lippold, H.; et al. Thallium pollution in China and removal technologies for waters: A review. Environ. Int. 2019, 126, 771–790. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Ye, J.; Ren, G.; Qi, Y.; Chen, Y.; Zhou, S. Metal-Free Semiconductor-Based Bio-Nano Hybrids for Sustainable CO2-to-CH4 Conversion with High Quantum Yield. Angew. Chem. Int. Ed. 2022, 61, e202206508. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, Y.; Wang, P.; Zhu, J.; Yang, Z.; Liu, Z. Removal of thallium in water/wastewater: A review. Water Res. 2019, 165, 114981. [Google Scholar] [CrossRef] [PubMed]
- Campanella, B.; Casiot, C.; Onor, M.; Perotti, M.; Petrini, R.; Bramanti, E. Thallium release from acid mine drainages: Speciation in river and tap water from Valdicastello mining district (northwest Tuscany). Talanta 2017, 171, 255–261. [Google Scholar] [CrossRef]
- Perotti, M.; Petrini, R.; D’Orazio, M.; Ghezzi, L.; Giannecchini, R.; Vezzoni, S. Thallium and Other Potentially Toxic Elements in the Baccatoio Stream Catchment (Northern Tuscany, Italy) Receiving Drainages from Abandoned Mines. Mine Water Environ. 2018, 37, 431–441. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; El-Naggar, A.; Wang, H.; Du Laing, G.; Alessi, D.S.; Ok, Y.S. Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Environ. Int. 2020, 140, 105754. [Google Scholar] [CrossRef]
- Tatsi, K.; Turner, A. Distributions and concentrations of thallium in surface waters of a region impacted by historical metal mining (Cornwall, UK). Sci. Total. Environ. 2014, 473–474, 139–146. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Z.; Wang, J. Geochemistry of trace elements and water quality assessment of natural water within the Tarim River Basin in the extreme arid region, NW China. J. Geochem. Explor. 2014, 136, 118–126. [Google Scholar] [CrossRef]
- Xiao, T. Environmental Impact of Thallium Related to the Mercury-Thallium-Gold Mineralization in Southwest Guizhou Province, China; Universite du Quebec a Chicoutimi: Saguenay, QU, Canada, 2001. [Google Scholar]
- Peacock, C.L.; Moon, E.M. Oxidative scavenging of thallium by birnessite: Explanation for thallium enrichment and stable isotope fractionation in marine ferromanganese precipitates. Geochim. Cosmochim. Acta 2012, 84, 297–313. [Google Scholar] [CrossRef]
- Khavidaki, H.D.; Aghaie, H. Adsorption of Thallium(I) Ions Using Eucalyptus Leaves Powder. Clean-Soil Air Water 2013, 41, 673–679. [Google Scholar] [CrossRef]
- Krasnodebska-Ostrega, B.; Dmowski, K.; Stryjewska, E.; Golimowski, J. Determination of Thallium and Other Elements (As, Cd, Cu, Mn, Pb, Se, Sb, and Zn) in Water and Sediment Samples from the Vicinity of the Zinc-Lead Smelter in Poland (3 pp). J. Soils Sediments 2005, 5, 71–73. [Google Scholar] [CrossRef]
- Yao, J.; Wang, H.; Ma, C.; Cao, Y.; Chen, W.; Gu, L.; He, Q.; Liu, C.; Xiong, J.; Ma, J.; et al. Cotransport of thallium(I) with polystyrene plastic particles in water-saturated porous media. J. Hazard. Mater. 2022, 422, 126910. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, J.; Tang, L.; Feng, C.; Liu, H.; Zhang, H.; Peng, B.; Chen, Z.; Xie, Q. Intimate coupling of photocatalysis and biodegradation for wastewater treatment: Mechanisms, recent advances and environmental applications. Water Res. 2020, 175, 115673. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.N.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Tereshatov, E.E.; Boltoeva, M.Y.; Folden, C.M. Resin Ion Exchange and Liquid-Liquid Extraction of Indium and Thallium from Chloride Media. Solvent Extr. Ion Exch. 2015, 33, 607–624. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, J.; Shen, Y.; Liu, X.; Li, W.; Wang, W.; Yang, Y. The efficient removal of thallium from sintering flue gas desulfurization wastewater in ferrous metallurgy using emulsion liquid membrane. Environ. Sci. Pollut. Res. 2017, 24, 24214–24222. [Google Scholar] [CrossRef]
- Ma, C.; Huang, R.; Huangfu, X.; Ma, J.; He, Q. Light- and H2O2-Mediated Redox Transformation of Thallium in Acidic Solutions Containing Iron: Kinetics and Mechanistic Insights. Environ. Sci. Technol. 2022, 56, 5530–5541. [Google Scholar] [CrossRef]
- Meng, C.; Hu, X.; Yu, S.; Co, D.S. Removal of thallium in raw water by powdered activated carbon. Water Technol. 2015, 9, 1–3. [Google Scholar]
- Ye, J.; Wang, C.; Gao, C.; Fu, T.; Yang, C.; Ren, G.; Lü, J.; Zhou, S.; Xiong, Y. Solar-driven methanogenesis with ultrahigh selectivity by turning down H2 production at biotic-abiotic interface. Nat. Commun. 2022, 13, 6612. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiong, Y.; Cheng, X.; Hou, X.; Yang, Y.; Tian, Y.; You, J.; Xu, L. Adsorptive removal of trace thallium(I) from wastewater: A review and new perspectives. J. Hazard. Mater. 2020, 393, 122378. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Wang, J.; Yu, J.; Feng, H.; Lu, Y.; Chen, Y.; Liu, Y.; Wang, J.; Xie, Q. Enhanced surface activation process of persulfate by modified bagasse biochar for degradation of phenol in water and soil: Active sites and electron transfer mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 599, 124904. [Google Scholar] [CrossRef]
- Soltani, R.; Marjani, A.; Shirazian, S. Facile one-pot synthesis of thiol-functionalized mesoporous silica submicrospheres for Tl(I) adsorption: Isotherm, kinetic and thermodynamic studies. J. Hazard. Mater. 2019, 371, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sabermahani, F.; Mahani, N.M.; Noraldiny, M. Removal of thallium (I) by activated carbon prepared from apricot nucleus shell and modified with rhodamine B. Toxin Rev. 2016, 36, 154–160. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Co-ord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chang, X.Y.; Tu, X.L. Thallium pollution and its countermeasures. Soils 2007, 39, 528–535. [Google Scholar]
- Nowicka, A.M.; Krasnodebska-Ostrega, B.; Wrzosek, B.; Jastrzebska, M.; Sadowska, M.; Maćkiewicz, M.; Stojek, Z. Detection of Oxidative Damage of Synthetic Oligonucleotides Caused by Thallium(III) Complexes. Electroanalysis 2013, 26, 340–350. [Google Scholar] [CrossRef]
- Lin, T.-S.; Nriagu, J. Revised Hydrolysis Constants for Thallium(I) and Thallium(III) and the Environmental Implications. J. Air Waste Manag. Assoc. 1998, 48, 151–156. [Google Scholar] [CrossRef]
- Liu, J.; Lippold, H.; Wang, J.; Lippmann-Pipke, J.; Chen, Y. Sorption of thallium(I) onto geological materials: Influence of pH and humic matter. Chemosphere 2011, 82, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y. The aqueous geochemistry of thallium: Speciation and solubility of thallium in low temperature systems. Environ. Chem. 2009, 6, 441–451. [Google Scholar] [CrossRef]
- Feng, H.-P.; Tang, L.; Zeng, G.-M.; Tang, J.; Deng, Y.-C.; Yan, M.; Liu, Y.-N.; Zhou, Y.-Y.; Ren, X.-Y.; Chen, S. Carbon-based core–shell nanostructured materials for electrochemical energy storage. J. Mater. Chem. A 2018, 6, 7310–7337. [Google Scholar] [CrossRef]
- Xiang, X.; Xia, F.; Zhan, L.; Xie, B. Preparation of zinc nano structured particles from spent zinc manganese batteries by vacuum separation and inert gas condensation. Sep. Purif. Technol. 2015, 142, 227–233. [Google Scholar] [CrossRef]
- Ni, J.; Kawabe, Y.; Morishita, M.; Watada, M.; Sakai, T. Improved electrochemical activity of LiMnPO4 by high-energy ball-milling. J. Power Sources 2011, 196, 8104–8109. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Xiao, T.; Zhang, G.; Liang, A.; Zhang, P.; Lin, L.; Chen, Z.; Cao, X.; Long, J. Zero-valent manganese nanoparticles coupled with different strong oxidants for thallium removal from wastewater. Front. Environ. Sci. Eng. 2020, 14, 34. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Xiao, T.; Chen, Y.; Long, J.; Zhang, G.; Zhang, P.; Li, C.; Zhuang, L.; Li, K. Efficient removal of thallium(I) from wastewater using flower-like manganese dioxide coated magnetic pyrite cinder. Chem. Eng. J. 2018, 353, 867–877. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, P.; Borthwick, A.G.; Chen, H.; Ni, J. Adsorption mechanisms of thallium(I) and thallium(III) by titanate nanotubes: Ion-exchange and co-precipitation. J. Colloid Interface Sci. 2014, 423, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Cheng, F.; Men, B.; Yi, H.E.; Wang, D. The water-sediment interfacial process of thallium. Environ. Chem. 2019, 38, 2047–2054. [Google Scholar]
- Chen, W.; Xiong, J.; Liu, J.; Wang, H.; Yao, J.; Liu, H.; Huangfu, X.; He, Q.; Ma, J.; Liu, C.; et al. Thermodynamic and kinetic coupling modeling for thallium(I) sorption at a heterogeneous titanium dioxide interface. J. Hazard. Mater. 2022, 428, 128230. [Google Scholar] [CrossRef]
- Liu, F.; Li, H.; Liao, D.; Xu, Y.; Yu, M.; Deng, S.; Zhang, G.; Xiao, T.; Long, J.; Zhang, H.; et al. Carbon quantum dots derived from the extracellular polymeric substance of anaerobic ammonium oxidation granular sludge for detection of trace Mn(vii) and Cr(vi). RSC Adv. 2020, 10, 32249–32258. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Hu, X.; Wang, H.; Zou, Y.; He, Q.; Ma, J.; Liu, C.; Chen, Y.; Huangfu, X. Effects of MnO2 crystal structure on the sorption and oxidative reactivity toward thallium(I). Chem. Eng. J. 2021, 416, 127919. [Google Scholar] [CrossRef]
- Song, L.; Duan, Y.; Liu, J.; Pang, H. Transformation between nanosheets and nanowires structure in MnO2 upon providing Co2+ ions and applications for microwave absorption. Nano Res. 2020, 13, 95–104. [Google Scholar] [CrossRef]
- Wan, S.; Ma, M.; Lv, L.; Qian, L.; Xu, S.; Xue, Y.; Ma, Z. Selective capture of thallium(I) ion from aqueous solutions by amorphous hydrous manganese dioxide. Chem. Eng. J. 2014, 239, 200–206. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Li, S.; Yang, S.; Lin, Z.; Dang, Z. The effects of interaction between vermiculite and manganese dioxide on the environmental geochemical process of thallium. Sci. Total. Environ. 2019, 669, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, P.; Yu, L.; Liu, S.; Ruan, B.; Hu, H.; Zhu, N.; Lin, Z. FeOOH-loaded MnO2 nano-composite: An efficient emergency material for thallium pollution incident. J. Environ. Manag. 2017, 192, 31–38. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Chenthamarakshan, C.R.; Rajeshwar, K.; Qasim, S.R. Photocatalytic reactivity of thallium(I) species in aqueous suspensions of titania. J. Electroanal. Chem. 2002, 519, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, X.; Huang, Z.; Xu, C.; Yang, T.; Zhao, X.; Qi, J.; Ma, J. Highly efficient removal of trace thallium from contaminated source waters with ferrate: Role of in situ formed ferric nanoparticle. Water Res. 2017, 124, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Coup, K.M.; Swedlund, P.J. Demystifying the interfacial aquatic geochemistry of thallium(I): New and old data reveal just a regular cation. Chem. Geol. 2015, 398, 97–103. [Google Scholar] [CrossRef]
- Yantasee, W.; Warner, C.L.; Sangvanich, T.; Addleman, R.S.; Carter, T.G.; Wiacek, R.J.; Fryxell, G.E.; Timchalk, C.; Warner, M.G. Removal of Heavy Metals from Aqueous Systems with Thiol Functionalized Superparamagnetic Nanoparticles. Environ. Sci. Technol. 2007, 41, 5114–5119. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yu, J.; Tang, J.; Tang, L.; Liu, Y.; Lu, Y.; Wang, J.; Ni, T.; Yang, Y.; Yi, Y. Enhanced electro-oxidation performance of FeCoLDH to organic pollutants using hydrophilic structure. J. Hazard. Mater. 2022, 430, 128464. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Tang, L.; Feng, C.; Zhu, X.; Yi, Y.; Feng, H.; Yu, J.; Ren, X. Preparation of floating porous g-C3N4 photocatalyst via a facile one-pot method for efficient photocatalytic elimination of tetracycline under visible light irradiation. Chem. Eng. J. 2022, 430, 132669. [Google Scholar] [CrossRef]
- Feng, H.; Yu, J.; Tang, L.; Wang, J.; Dong, H.; Ni, T.; Tang, J.; Tang, W.; Zhu, X.; Liang, C. Improved hydrogen evolution activity of layered double hydroxide by optimizing the electronic structure. Appl. Catal. B Environ. 2021, 297, 120478. [Google Scholar] [CrossRef]
- Feng, H.; Yu, J.; Tang, L.; Zeng, G.; Tang, W.; Wang, J.; Luo, T.; Peng, B.; Song, B.; Wang, L.; et al. Tuning Electron Density Endows Fe1–xCoxP with Exceptional Capability of Electrooxidation of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 13878–13887. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Wu, S.; Xia, F.; Han, X.; Xu, X.; Deng, S.; Jiang, Y. Insights into the synergistic removal mechanisms of thallium(I) by biogenic manganese oxides in a wide pH range. Sci. Total. Environ. 2022, 831, 154865. [Google Scholar] [CrossRef]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-X. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Feng, H.; Tang, L.; Zeng, G.; Yu, J.; Deng, Y.; Zhou, Y.; Wang, J.; Feng, C.; Luo, T.; Shao, B. Electron density modulation of Fe1-xCoxP nanosheet arrays by iron incorporation for highly efficient water splitting. Nano Energy 2020, 67, 104174. [Google Scholar] [CrossRef]
- Liu, S.; Feng, H.; Tang, L.; Dong, H.; Wang, J.; Yu, J.; Feng, C.; Liu, Y.; Luo, T.; Ni, T. Removal of Sb(III) by sulfidated nanoscale zerovalent iron: The mechanism and impact of environmental conditions. Sci. Total. Environ. 2020, 736, 139629. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, Z.; Xu, N.; Chen, Y.; Wu, H.; Liu, T.; Ye, H. Removal of Tl(I) Ions From Aqueous Solution Using Fe@Fe2O3Core-Shell Nanowires. CLEAN Soil Air Water 2016, 44, 1214–1224. [Google Scholar] [CrossRef]
- Tang, L.; Feng, H.; Tang, J.; Zeng, G.; Deng, Y.; Wang, J.; Liu, Y.; Zhou, Y. Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism. Water Res. 2017, 117, 175–186. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Liu, F.; Zhang, G.; Xu, Y.; Xiao, T.; Long, J.; Chen, Z.; Liao, D.; Zhang, J.; et al. Zero-valent iron-manganese bimetallic nanocomposites catalyze hypochlorite for enhanced thallium(I) oxidation and removal from wastewater: Materials characterization, process optimization and removal mechanisms. J. Hazard. Mater. 2020, 386, 121900. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Long, J.; Li, X.; Jiang, D.; Zhang, P.; Qi, J.; Huang, X.; Liu, J.; Xu, R.; et al. Removal of thallium from aqueous solutions using Fe-Mn binary oxides. J. Hazard. Mater. 2017, 338, 296–305. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Q.; Tao, T.; Ye, P.; Zhang, P.; Liu, Y. Fe-Mn binary oxides activated aluminosilicate mineral and its Tl(I) removal by oxidation, precipitation and adsorption in aqueous. J. Solid State Chem. 2021, 303, 122383. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Ma, R.; Yu, Y.; Chang, Z.; Zhang, X.; Yang, C.; Chen, D.; Yu, Y.; Li, W.; et al. Enhanced oxidative and adsorptive removal of thallium(I) using Fe3O4@TiO2 decorated RGO nanosheets as persulfate activator and adsorbent. Sep. Purif. Technol. 2021, 271, 118827. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gupta, A.K.; Ghosal, P.S. A review on Trihalomethanes and Haloacetic acids in drinking water: Global status, health impact, insights of control and removal technologies. J. Environ. Chem. Eng. 2021, 9, 106511. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, T.; Zhang, M.; Guo, X.; Yuan, Z. Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J. Hazard. Mater. 2008, 157, 352–357. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Ulusoy, U. Thallium adsorption onto polyacryamide–aluminosilicate composites: A Tl isotope tracer study. Chem. Eng. J. 2010, 162, 97–105. [Google Scholar] [CrossRef]
- Chen, W.; Huangfu, X.; Xiong, J.; Liu, J.; Wang, H.; Yao, J.; Liu, H.; He, Q.; Ma, J.; Liu, C.; et al. Retention of thallium(I) on goethite, hematite, and manganite: Quantitative insights and mechanistic study. Water Res. 2022, 221, 118836. [Google Scholar] [CrossRef]
- Biaduń, E.; Sadowska, M.; Ospina-Alvarez, N.; Krasnodębska-Ostręga, B. Direct speciation analysis of thallium based on solid phase extraction and specific retention of a Tl(III) complex on alumina coated with sodium dodecyl sulfate. Microchim. Acta 2015, 183, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, J.; Huang, H.; Huang, Z.; Xu, C.; Guo, G.; He, H.; Ma, J. Treatment of trace thallium in contaminated source waters by ferrate pre-oxidation and poly aluminium chloride coagulation. Sep. Purif. Technol. 2019, 227, 115663. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Chenthamarakshan, C.; Rajeshwar, K.; Qasim, S. Adsorption of Thallium(I) Ions on Titania Particle Surfaces in Aqueous Media. Adsorpt. Sci. Technol. 2003, 21, 217–228. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, T.; Liu, N.; Liu, X.; Li, H. Sorption of thallium(III) ions from aqueous solutions using titanium dioxide nanoparticles. Microchim. Acta 2009, 165, 73–78. [Google Scholar] [CrossRef]
- Ferro, S.; Donatoni, M.; De Battisti, A.; Andreev, V.N. Adsorption of thallium cations on RuO2–TiO2 electrodes. J. Appl. Electrochem. 2007, 37, 1389–1394. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, F.; Li, X.; Qi, J.; Chen, Y. Superior adsorption of thallium(I) on titanium peroxide: Performance and mechanism. Chem. Eng. J. 2018, 331, 471–479. [Google Scholar] [CrossRef]
- Murray, J.W. The surface chemistry of hydrous manganese dioxide. J. Colloid Interface Sci. 1974, 46, 357–371. [Google Scholar] [CrossRef]
- Huangfu, X.; Jiang, J.; Lu, X.; Wang, Y.; Liu, Y.; Pang, S.-Y.; Cheng, H.; Zhang, X.; Ma, J. Adsorption and Oxidation of Thallium(I) by a Nanosized Manganese Dioxide. Water Air Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- Huangfu, X.; Ma, C.; Ma, J.; He, Q.; Yang, C.; Jiang, J.; Wang, Y.; Wu, Z. Significantly improving trace thallium removal from surface waters during coagulation enhanced by nanosized manganese dioxide. Chemosphere 2017, 168, 264–271. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, J.; Liu, F.; Wang, Z.; Ma, X.; He, D. One-pot synthesis of magnetic Prussian blue for the highly selective removal of thallium(I) from wastewater: Mechanism and implications. J. Hazard. Mater. 2022, 423, 126972. [Google Scholar] [CrossRef]
- Wang, N.; Su, Z.; Deng, N.; Qiu, Y.; Ma, L.; Wang, J.; Chen, Y.; Hu, K.; Huang, C.; Xiao, T. Removal of thallium(I) from aqueous solutions using titanate nanomaterials: The performance and the influence of morphology. Sci. Total. Environ. 2020, 717, 137090. [Google Scholar] [CrossRef]
- Pan, B.; Wan, S.; Zhang, S.; Guo, Q.; Xu, Z.; Lv, L.; Zhang, W. Recyclable polymer-based nano-hydrous manganese dioxide for highly efficient Tl(I) removal from water. Sci. China Chem. 2014, 57, 763–771. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.H.; Liang, M.H. Reflectance Spectroscopic Characterization of Thallium Wastewater Treatment by Pyrite and Pyrite Slag. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2007, 27, 747–749. [Google Scholar]
- Marc, F.; Benedetti Chris, J.; Milne David, G.; Kinniburgh, W. Metal Ion Binding to Humic Substances: Application of the Non-Ideal Competitive Adsorption Model. Environ. Sci. Technol. 1995, 29, 446–457. [Google Scholar]
- Law, S.; Turner, A. Thallium in the hydrosphere of south west England. Environ. Pollut. 2011, 159, 3484–3489. [Google Scholar] [CrossRef] [PubMed]


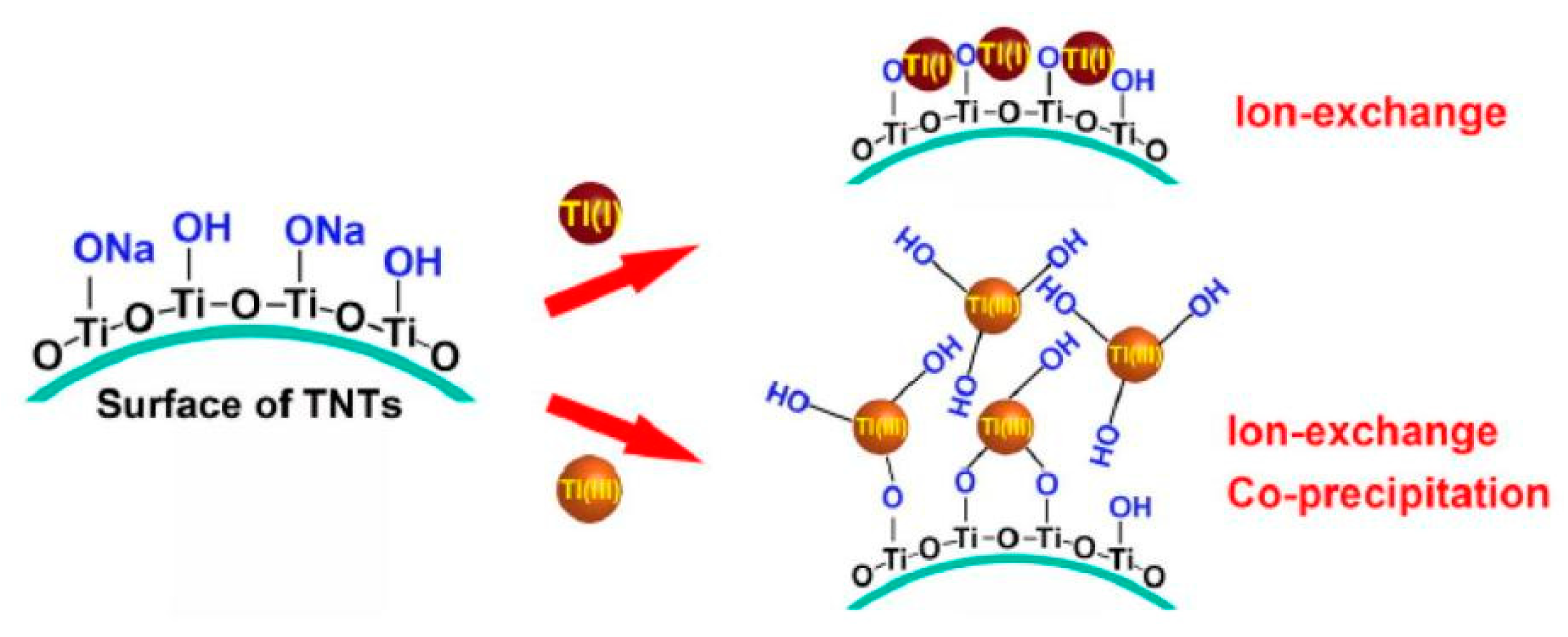
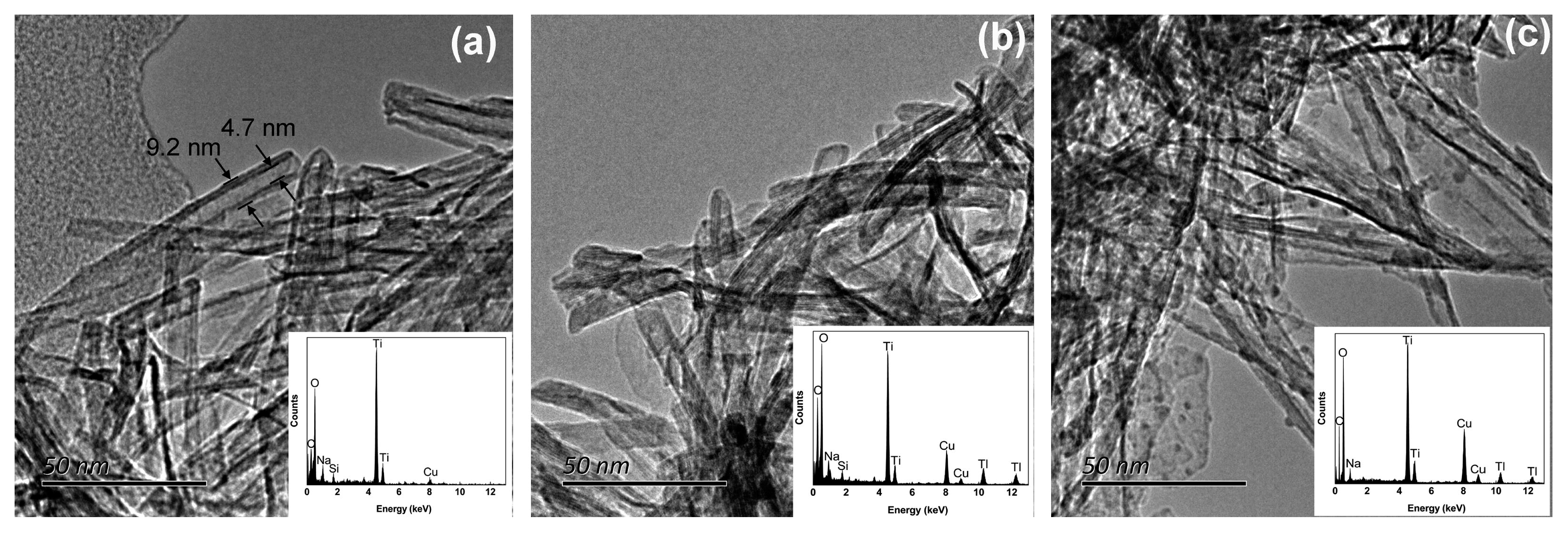
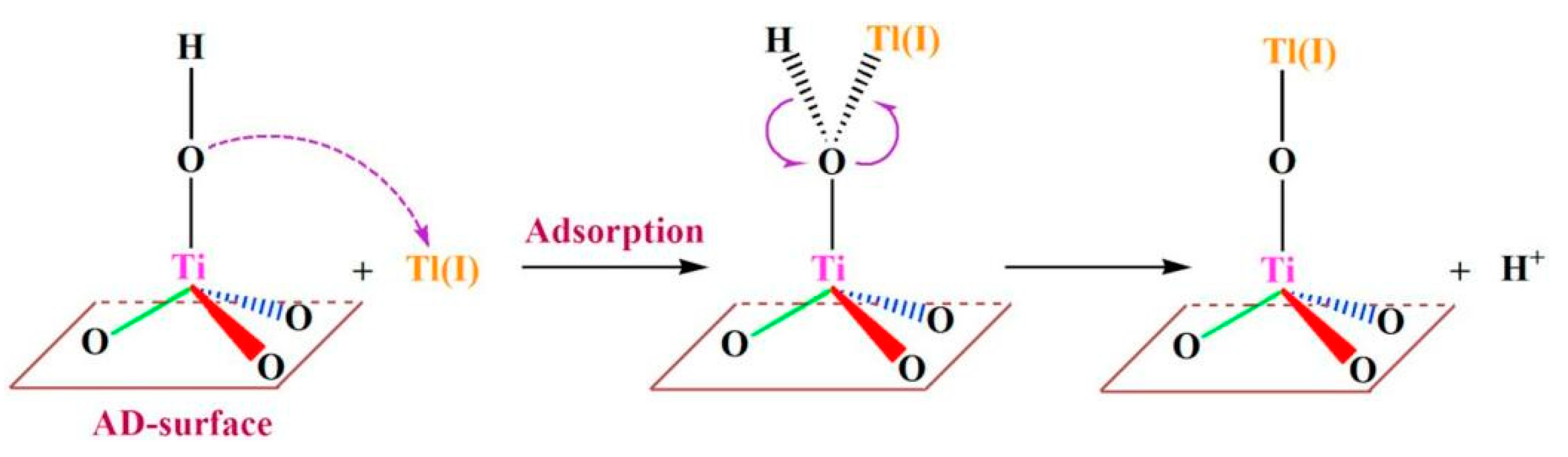

| Metal Oxides | Dosage | Thallium Concentration in the Tested Water | Performance | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| nMnO2 | 0.05 mmol·L−1 | 3.5 mg/L | 672 mg/g | High removal efficiency | Poor reproducibility | [3] |
| HMO | 50 mg | 0.5 mmol·L−1 | 348.84 mg/g | With reusable properties | Highly influenced by environmental conditions | [42] |
| VER-MnO2 | — | 20 μg/L | 144.29 mg/g | Less influenced by environmental conditions | Poor removal ability | [43] |
| Zero-valent manganese nanoparticles | 2 g/L | 10 mg/L | 95.7% | Less influenced by environmental conditions | Poor reproducibility | [34] |
| Flower-like manganese dioxide coated magnetic pyrite cinder | 0.5 g/L | 10 mg/L | 320 mg/g | With reusable properties | Highly influenced by environmental conditions | [35] |
| FeOOH-loaded MnO2 nano-composite | 10 mg | 0~150 mg/L | 450 mg/g | With reusable properties | Highly influenced by environmental conditions | [44] |
| δ-MnO2 | 500 μmol·L−1 | 25 mg/L | 581 mg/g | High removal efficiency | Highly influenced by environmental conditions | [41] |
| MnO2@slag | 1 g/L | 10 mg/L | 99.5% | High removal efficiency | Poor reproducibility | [35] |
| Metal Oxides | Dosage | Thallium Concentration in the Tested Water | Performance | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Nano-Al2O3 | 0.03 g | 10 mg/L | 6.28 mg/g | Less influenced by environmental conditions | Poor reproducibility | [66] |
| PAAm-B | 0.1 g | 0.005 mol·L−1 | 197.88 mg/g-Tl(I), 32.64 mg/g-Tl(III) | High removal efficiency | Highly influenced by environmental conditions | [67] |
| PAAm-Z | 0.1 g | 0.005 mol·L−1 | 337.4 mg/g-Tl(I), 73.44 mg/g-Tl(III) | High removal efficiency | Highly influenced by environmental conditions | [67] |
| Ferrate pre-oxidation and poly aluminum chloride coagulation | 0.5–4 mg | 0.76 μg/L | 87% | Less influenced by environmental conditions | Poor reproducibility | [70] |
| Metal Oxides | Dosage | Thallium Concentration in the Tested Water | Performance | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Titanium iron magnetic | 0.1 g/L | 12.5 mg/L | 111.3 mg/g | Less influenced by environmental conditions | Poor reproducibility | [58] |
| Fe3O4@TiO2 decorated RGO nanosheets | 0.2 g/L | _ | 673.2 mg/g | High removal efficiency | Highly influenced by environmental conditions | [62] |
| TNTs | 0.2 g/L | 100 mg/L | 709.2 mg/g | High removal efficiency | Highly influenced by environmental conditions | [36] |
| Titanium peroxide | 0.2 g/L | 0.046–20 mg/L | 412 mg/g | Less influenced by environmental conditions | Poor reproducibility | [74] |
| Metal Oxides | Effect of pH Value | Effect of Co-Existing Ions | Effect of Organic Matters | Ref. |
|---|---|---|---|---|
| nMnO2 | pH < 7, little influence | Ca2+, Mg2+ | HA of 3 mg/L | [3] |
| HMO | little influence | Ca2+, Mg2+ | NA | [42] |
| VER-MnO2 | little influence | Ca2+ | NA | [43] |
| Zero-valent manganese nanoparticles | little influence | NA | NA | [34] |
| Flower-like manganese dioxide coated magnetic pyrite cinder | pH < 12, little influence | Ca2+, Mg2+, Na+ | EDTA | [35] |
| FeOOH-loaded MnO2 nano-composite | little influence | Ca2+ | HA | [44] |
| δ-MnO2 | little influence | Ca2+, Na+ | NA | [41] |
| MnO2@slag | little influence | Ca2+ | HA, FA | [35] |
| Nano-Al2O3 | little influence | NA | HA, FA, EDTA and DTPA | [66] |
| PAAm-B | little influence | NA | NA | [67] |
| PAAm-Z | little influence | Cd(II), Cu(II), Pb(II) and Mn(II) | NA | [67] |
| Ferrate pre-oxidation and poly aluminum chloride coagulation | little influence | Fe2+, Pb2+, Zn2+ | NA | [70] |
| Magnetic Prussian blue | little influence | NA | NA | [53] |
| Fe@Fe2O3 Core–Shell | NA | Mg2+, PO43, Ca2+, Na+, Cl− | NA | [57] |
| 3-ZVIMn | little influence | Fe2+, Fe3+ | NA | [59] |
| Fe-Mn binary oxides | little influence | Cu2+, Zn2+ | NA | [60] |
| Titanium iron magnetic | little influence | Na+, NO3− | NA | [58] |
| Fe-Mn-binary-oxides-activated aluminosilicate mineral | little influence | Mg2+, Ca2+ | HA | [61] |
| Fe3O4@TiO2-decorated RGO nanosheets | little influence | no influence | EDTA and DTPA | [62] |
| TNT | little influence | Na+, K+, Ca2+ | NA | [36] |
| Titanium peroxide | little influence | NA | NA | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Feng, H.; Zhao, M.; Zhou, X.; Zhu, X.; Ouyang, X.; Tang, J.; Li, C.; Wang, J.; Tang, W.; et al. Recent Advances in Thallium Removal from Water Environment by Metal Oxide Material. Int. J. Environ. Res. Public Health 2023, 20, 3829. https://doi.org/10.3390/ijerph20053829
Ren X, Feng H, Zhao M, Zhou X, Zhu X, Ouyang X, Tang J, Li C, Wang J, Tang W, et al. Recent Advances in Thallium Removal from Water Environment by Metal Oxide Material. International Journal of Environmental Research and Public Health. 2023; 20(5):3829. https://doi.org/10.3390/ijerph20053829
Chicago/Turabian StyleRen, Xiaoyi, Haopeng Feng, Mengyang Zhao, Xin Zhou, Xu Zhu, Xilian Ouyang, Jing Tang, Changwu Li, Jiajia Wang, Wangwang Tang, and et al. 2023. "Recent Advances in Thallium Removal from Water Environment by Metal Oxide Material" International Journal of Environmental Research and Public Health 20, no. 5: 3829. https://doi.org/10.3390/ijerph20053829




