The Effects of Acute Sleep Curtailment on Salt Taste Measures and Relationships with Energy-Corrected Sodium Intake: A Randomized Cross-Over Trial with Methodology Validation
Abstract
:1. Introduction
- The adapted forced-choice paired-comparison tracking test could serve as a valid and reliable tool to determine salt taste preference.
- Salt taste liking, measured by the slope of liking ratings of five salty solutions across different concentrations would be steeper after a night of curtailed sleep compared to a night of habitual sleep; however, salt taste function, measured by the slope of intensity ratings would not be affected. Additionally, preferred salt concentration, measured by the newly developed test, would increase.
- Hedonic measures of salt taste (liking slope and preferred salt concentration) and salt taste function (intensity slope) would be positively associated with energy-corrected Na intake after both the habitual and the curtailed night of sleep.
- Distinguishable salt-liking phenotypes would be identified.
2. Materials and Methods
2.1. Participants
2.2. Study Protocol and Timeline
2.3. Compliance to Sleep Protocol
2.4. Consent Visit
2.5. Lab Visits
2.6. Taste Testing
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Demographic Information
3.2. Validation of the Adapted Forced-Choice Paired-Comparison Tracking Test in Determining Salt Taste Preference
3.3. Compliance of the Sleep Protocol
3.4. No Difference in Salt Taste Hedonic Measures and Function, Sodium and Macronutrient Intake, and Food Cravings between the Curtailed and Habitual Nights
3.5. Correlations between Hedonic Measures, Salt Taste Function, and Energy-Corrected Sodium Intake under the Habitual and the Curtailed Sleep Condition
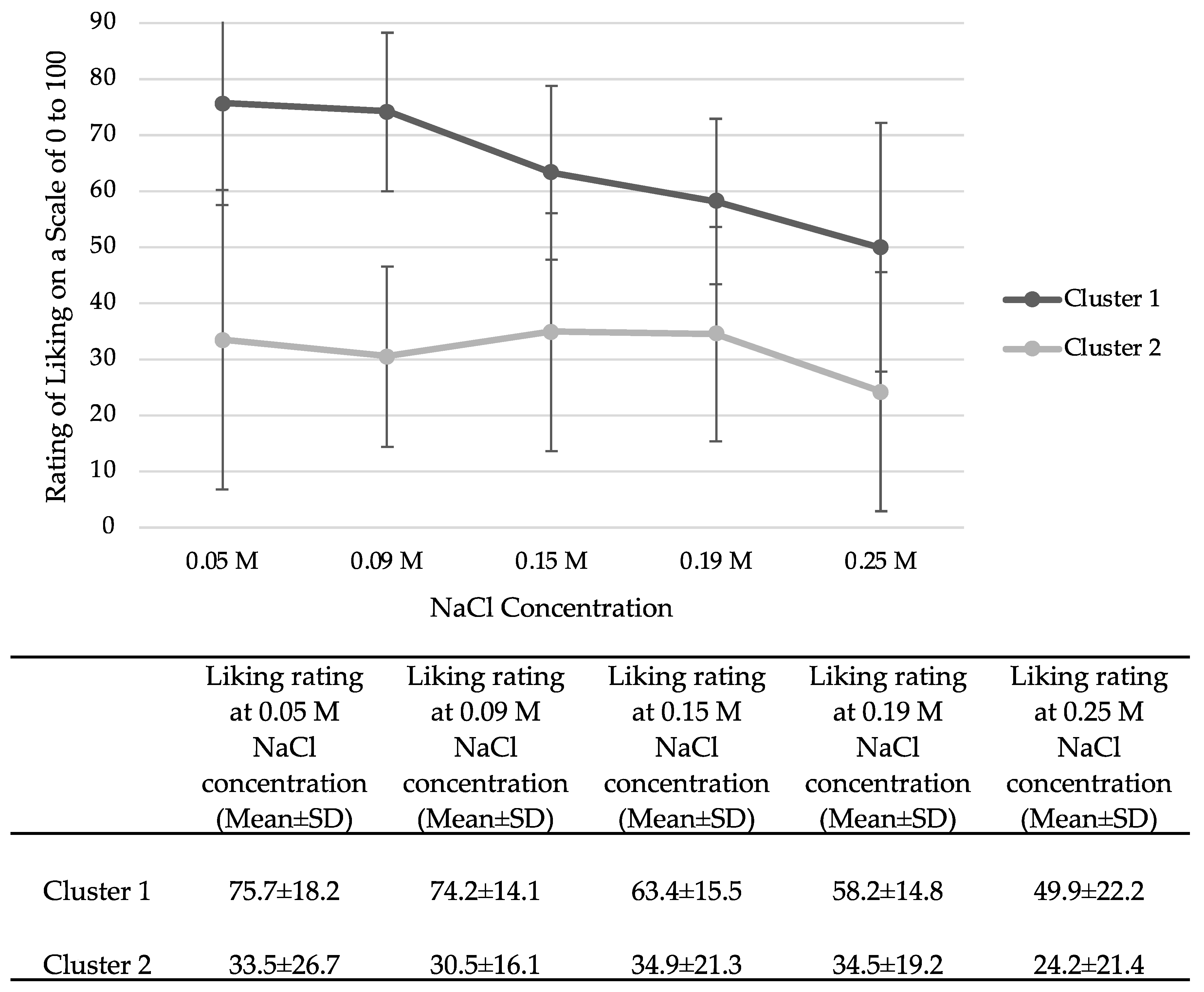
3.6. Exploration of Salt-Liking Phenotypes
4. Discussion
4.1. Validity of the Adapted Forced-Choice, Paired-Comparison Tracking Procedure in Determining Salt Taste Preference
4.2. The Effects of Sleep on Salt Taste Function
4.3. The Effects of Sleep on Hedonic Measures of Salt Taste
4.4. Associations between Salt Taste Measures and Dietary Intake
4.5. Identification of Salt-Liking Phenotypes
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining Variability in Sodium Intake through Oral Sensory Phenotype, Salt Sensation and Liking. Physiol. Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteleone, E.; Spinelli, S.; Dinnella, C.; Endrizzi, I.; Laureati, M.; Pagliarini, E.; Sinesio, F.; Gasperi, F.; Torri, L.; Aprea, E.; et al. Exploring Influences on Food Choice in a Large Population Sample: The Italian Taste Project. Food Qual. Prefer. 2017, 59, 123–140. [Google Scholar] [CrossRef]
- van Langeveld, A.W.B.; Gibbons, S.; Koelliker, Y.; Civille, G.V.; de Vries, J.H.M.; de Graaf, C.; Mars, M. The Relationship between Taste and Nutrient Content in Commercially Available Foods from the United States. Food Qual. Prefer. 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Sotirelis, E.; Bojeh, R.; Maan, I.; Medalle, M.; Chik, X.S.F.; Keast, R.; Tucker, R.M. Is Dietary Intake Associated with Salt Taste Function and Perception in Adults? A Systematic Review. Food Qual. Prefer. 2021, 92, 104174. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Tucker, R.M. Sweet Taste as a Predictor of Dietary Intake: A Systematic Review. Nutrients 2019, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Cornelis, M.C.; Tordoff, M.G.; El-Sohemy, A.; van Dam, R.M. Recalled Taste Intensity, Liking and Habitual Intake of Commonly Consumed Foods. Appetite 2017, 109, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Pilic, L.; Lubasinski, N.J.; Berk, M.; Ward, D.; Graham, C.A.-M.; Da Silva Anastacio, V.; King, A.; Mavrommatis, Y. The Associations between Genetics, Salt Taste Perception and Salt Intake in Young Adults. Food Qual. Prefer. 2020, 84, 103954. [Google Scholar] [CrossRef]
- Stone, L.J.; Pangborn†, R.M. Preferences and Intake Measures of Salt and Sugar, and Their Relation to Personality Traits. Appetite 1990, 15, 63–79. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Pecore, S.D. Taste Perception of Sodium Chloride in Relation to Dietary Intake of Salt. Am. J. Clin. Nutr. 1982, 35, 510–520. [Google Scholar] [CrossRef]
- Shepherd, R.; Farleigh, C.A.; Land, D.G. Preference and Sensitivity to Salt Taste as Determinants of Salt-Intake. Appetite 1984, 5, 187–197. [Google Scholar] [CrossRef]
- Inoue, H.; Kuwano, T.; Yamakawa-Kobayashi, K.; Waguri, T.; Nakano, T.; Suzuki, Y. Perceived 6-n-Propylthiouracil (PROP) Bitterness Is Associated with Dietary Sodium Intake in Female Japanese College Students. J. Nutr. Sci. Vitam. 2017, 63, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, H.; Takahashi, H.; Suzuki, H.; Hongo, T.; Suzuki, T.; Shidoji, Y.; Yoon, K.H. Interrelationship of Some Selected Nutritional Parameters Relevant to Taste for Salt in a Group of College-Aged Women. J Nutr Sci Vitam. 1985, 31, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, J.; Conde, S.R.; de Araújo, A.R.; Marcadenti, A. Association between Salt Taste Sensitivity Threshold and Blood Pressure in Healthy Individuals: A Cross-Sectional Study. Sao Paulo Med. J. 2020, 138, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Veček, N.N.; Mucalo, L.; Dragun, R.; Miličević, T.; Pribisalić, A.; Patarčić, I.; Hayward, C.; Polašek, O.; Kolčić, I. The Association between Salt Taste Perception, Mediterranean Diet and Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2020, 12, 1164. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Cho, H.-J.; Bae, E.; Kim, Y.C.; Kim, S.; Chin, H.J. Not Salt Taste Perception but Self-Reported Salt Eating Habit Predicts Actual Salt Intake. J. Korean Med. Sci. 2014, 29, S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Leong, C.S.-F.; Forde, C.G.; Tey, S.L.; Henry, C.J. Taste Perception and Diet in People of Chinese Ancestry. Asia Pac. J. Clin. Nutr. 2018, 27, 478–486. [Google Scholar] [CrossRef]
- Azinge, E.C.; Sofola, O.A.; Silva, B.O. Relationship between Salt Intake, Salt-Taste Threshold and Blood Pressure in Nigerians. West Afr. J. Med. 2011, 30, 373–376. [Google Scholar]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Keast, R.S.J.; Roura, E. Salivary Leptin and TAS1R2/TAS1R3 Polymorphisms Are Related to Sweet Taste Sensitivity and Carbohydrate Intake from a Buffet Meal in Healthy Young Adults. Br. J. Nutr. 2017, 118, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.; Keast, R.S.J. The Association between Sweet Taste Function, Anthropometry, and Dietary Intake in Adults. Nutrients 2016, 8, 241. [Google Scholar] [CrossRef] [Green Version]
- Szczygiel, E.J.; Cho, S.; Snyder, M.K.; Tucker, R.M. Associations between Chemosensory Function, Sweet Taste Preference, and the Previous Night’s Sleep in Non-Obese Males. Food Qual. Prefer. 2019, 75, 105–112. [Google Scholar] [CrossRef]
- Szczygiel, E.J.; Cho, S.; Tucker, R.M. Multiple Dimensions of Sweet Taste Perception Altered after Sleep Curtailment. Nutrients 2019, 11, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brondel, L.; Romer, M.A.; Nougues, P.M.; Touyarou, P.; Davenne, D. Acute Partial Sleep Deprivation Increases Food Intake in Healthy Men. Am. J. Clin. Nutr. 2010, 91, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvin, A.D.; Carter, R.E.; Adachi, T.; Macedo, P.G.; Albuquerque, F.N.; van der Walt, C.; Bukartyk, J.; Davison, D.E.; Levine, J.A.; Somers, V.K. Effects of Experimental Sleep Restriction on Caloric Intake and Activity Energy Expenditure. Chest 2013, 144, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Impact of Insufficient Sleep on Total Daily Energy Expenditure, Food Intake, and Weight Gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [Green Version]
- Szczygiel, E.J.; Cho, S.; Tucker, R.M. The Effect of Sleep Curtailment on Hedonic Responses to Liquid and Solid Food. Foods 2019, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.L.; Field, J.; Miller, L.E.; DiFrancesco, M.; Beebe, D.W. Sweet/Dessert Foods Are More Appealing to Adolescents after Sleep Restriction. PLoS ONE 2015, 10, e0115434. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep 2013, 36, 981–990. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Roberts, A.L.; Chen, J.; Kelleman, M.; O’Keeffe, M.; RoyChoudhury, A.; Jones, P.J. Short Sleep Duration Increases Energy Intakes but Does Not Change Energy Expenditure in Normal-Weight Individuals. Am. J. Clin. Nutr. 2011, 94, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-L.; Schnepp, J.; Tucker, R.M. Increased Hunger, Food Cravings, Food Reward, and Portion Size Selection after Sleep Curtailment in Women Without Obesity. Nutrients 2019, 11, 663. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, J.; Cheng, F.W.; Cui, L.; Shu, R.; Wu, S.; Gao, X. Poor Sleep Quality Is Associated with Altered Taste Perception in Chinese Adults. J. Acad. Nutr. Diet. 2021, 121, 435–445. [Google Scholar] [CrossRef]
- Lv, W.; Finlayson, G.; Dando, R. Sleep, food cravings and taste. Appetite 2018, 125, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Furchtgott, E.; Willingham, W.W. The Effect of Sleep-Deprivation upon the Thresholds of Taste. Am. J. Psychol. 1956, 69, 111–112. [Google Scholar] [CrossRef]
- Smith, S.L.; Ludy, M.-J.; Tucker, R.M. Changes in Taste Preference and Steps Taken after Sleep Curtailment. Physiol. Behav. 2016, 163, 228–233. [Google Scholar] [CrossRef] [PubMed]
- CDC-Data and Statistics-Sleep and Sleep Disorders. Available online: https://www.cdc.gov/sleep/data_statistics.html (accessed on 22 March 2022).
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Szczygiel, E.J.; Cho, S.; Tucker, R.M. Characterization of the Relationships between Sleep Duration, Quality, Architecture and Chemosensory Function in Non-Obese Females. Chem. Senses 2018, 43, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Garneau, N.L.; Nuessle, T.M.; Mendelsberg, B.J.; Shepard, S.; Tucker, R.M. Sweet Liker Status in Children and Adults: Consequences for Beverage Intake in Adults. Food Qual. Prefer. 2018, 65, 175–180. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Tepper, B.J.; Rietzschel, J.; Prescott, J. Human Hedonic Responses to Sweetness: Role of Taste Genetics and Anatomy. Physiol. Behav. 2007, 91, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Iatridi, V.; Hayes, J.E.; Yeomans, M.R. Quantifying Sweet Taste Liker Phenotypes: Time for Some Consistency in the Classification Criteria. Nutrients 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Prescott, J.; Kim, K.-O. Patterns of Sweet Liking in Sucrose Solutions and Beverages. Food Qual. Prefer. 2014, 36, 96–103. [Google Scholar] [CrossRef]
- Mennella, J.A.; Lukasewycz, L.D.; Griffith, J.W.; Beauchamp, G.K. Evaluation of the Monell Forced-Choice, Paired-Comparison Tracking Procedure for Determining Sweet Taste Preferences across the Lifespan. Chem. Senses 2011, 36, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-Hour Dietary Recall (ASA24): A Resource for Researchers, Clinicians, and Educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowski, T.; Baranowski, J.C.; Watson, K.B.; Martin, S.; Beltran, A.; Islam, N.; Dadabhoy, H.; Adame, S.; Cullen, K.; Thompson, D.; et al. Children’s Accuracy of Portion Size Estimation Using Digital Food Images: Effects of Interface Design and Size of Image on Computer Screen. Public Health Nutr. 2011, 14, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Frankenfeld, C.L.; Poudrier, J.K.; Waters, N.M.; Gillevet, P.M.; Xu, Y. Dietary Intake Measured from a Self-Administered, Online 24-Hour Recall System Compared with 4-Day Diet Records in an Adult US Population. J. Acad. Nutr. Diet. 2012, 112, 1642–1647. [Google Scholar] [CrossRef]
- Subar, A.F.; Crafts, J.; Zimmerman, T.P.; Wilson, M.; Mittl, B.; Islam, N.G.; McNutt, S.; Potischman, N.; Buday, R.; Hull, S.G.; et al. Assessment of the Accuracy of Portion Size Reports Using Computer-Based Food Photographs Aids in the Development of an Automated Self-Administered 24-Hour Recall. J. Am. Diet. Assoc. 2010, 110, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Loparo, K.A.; Kelly, M.R.; Kaplan, R.F. Evaluation of an Automated Single-Channel Sleep Staging Algorithm. Nat. Sci. Sleep 2015, 7, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, E.R.; Troxel, W.M.; Shih, R.A.; Pinder, E.; Lee, L.D.; Geyer, C.L. Increasing Resilience through Promotion of Healthy Sleep among Service Members. Mil. Med. 2015, 180, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Stein, L.J.; Cowart, B.J.; Beauchamp, G.K. The Development of Salty Taste Acceptance Is Related to Dietary Experience in Human Infants: A Prospective Study123. Am. J. Clin. Nutr. 2012, 95, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.-S.; Loh, W.-Y. A Comparison of Tests of Equality of Variances. Comput. Stat. Data Anal. 1996, 22, 287–301. [Google Scholar] [CrossRef]
- Zimmerman, D.W. A Note on Preliminary Tests of Equality of Variances. Br. J. Math. Stat. Psychol. 2004, 57, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Riddell, L.; Liem, G.; Whitelock, S.; Keast, R. The Influence of Sodium on Liking and Consumption of Salty Food. J. Food Sci. 2011, 76, S72–S76. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Scaling. In Sensory Evaluation of Food: Principles and Practices; Lawless, H.T., Heymann, H., Eds.; Springer: New York, NY, USA, 2010; pp. 149–177. ISBN 978-1-4419-6488-5. [Google Scholar]
- Bishop, P.A.; Herron, R.L. Use and Misuse of the Likert Item Responses and Other Ordinal Measures. Int. J. Exerc. Sci. 2015, 8, 297–302. [Google Scholar] [PubMed]
- Li, Q.; Cui, Y.; Jin, R.; Lang, H.; Yu, H.; Sun, F.; He, C.; Ma, T.; Li, Y.; Zhou, X.; et al. Enjoyment of Spicy Flavor Enhances Central Salty-Taste Perception and Reduces Salt Intake and Blood Pressure. Hypertension 2017, 70, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T.; Heymann, H. Measurement of Sensory Thresholds. In Sensory Evaluation of Food: Principles and Practices; Lawless, H.T., Heymann, H., Eds.; Food Science Text Series; Springer: New York, NY, USA, 2010; pp. 125–147. ISBN 978-1-4419-6488-5. [Google Scholar]
- Holt, S.H.A.; Cobiac, L.; Beaumont-Smith, N.E.; Easton, K.; Best, D.J. Dietary Habits and the Perception and Liking of Sweetness among Australian and Malaysian Students: A Cross-Cultural Study. Food Qual. Prefer. 2000, 11, 299–312. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [Green Version]
- Mattes, R.D. Gustation as a Determinant of Ingestion: Methodological Issues. Am. J. Clin. Nutr. 1985, 41, 672–683. [Google Scholar] [CrossRef]
- Sartor, F.; Donaldson, L.F.; Markland, D.A.; Loveday, H.; Jackson, M.J.; Kubis, H.-P. Taste Perception and Implicit Attitude toward Sweet Related to Body Mass Index and Soft Drink Supplementation. Appetite 2011, 57, 237–246. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Boakes, R.A.; Oaten, M.J.; Yeomans, M.R.; Mahmut, M.; Francis, H.M. Chemosensory Abilities in Consumers of a Western-Style Diet. Chem. Senses 2016, 41, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.; Bolhuis, D.P.; Cicerale, S.; Hayes, J.E.; Keast, R. The Relationships Between Common Measurements of Taste Function. Chemosens. Percept. 2015, 8, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Spetter, M.S.; Smeets, P.A.M.; de Graaf, C.; Viergever, M.A. Representation of Sweet and Salty Taste Intensity in the Brain. Chem. Senses 2010, 35, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Gujar, N.; Yoo, S.-S.; Hu, P.; Walker, M.P. Sleep Deprivation Amplifies Reactivity of Brain Reward Networks, Biasing the Appraisal of Positive Emotional Experiences. J. Neurosci. 2011, 31, 4466–4474. [Google Scholar] [CrossRef] [Green Version]
- Duraccio, K.M.; Krietsch, K.N.; Zhang, N.; Whitacre, C.; Howarth, T.; Pfeiffer, M.; Beebe, D.W. The Impact of Short Sleep on Food Reward Processes in Adolescents. J. Sleep Res. 2021, 30, e13054. [Google Scholar] [CrossRef]
- Pritchard, T.C.; Hamilton, R.B.; Morse, J.R.; Norgren, R. Projections of Thalamic Gustatory and Lingual Areas in the Monkey, Macaca Fascicularis. J. Comp. Neurol. 1986, 244, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Gregory, M.D.; Mak, Y.E.; Gitelman, D.; Mesulam, M.M.; Parrish, T. Dissociation of Neural Representation of Intensity and Affective Valuation in Human Gustation. Neuron 2003, 39, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The Sleep-Deprived Human Brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Mullin, B.C.; Phillips, M.L.; Siegle, G.J.; Buysse, D.J.; Forbes, E.E.; Franzen, P.L. Sleep Deprivation Amplifies Striatal Activation to Monetary Reward. Psychol. Med. 2013, 43, 2215–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, V.; Chuah, Y.M.L.; Huettel, S.A.; Chee, M.W.L. Sleep Deprivation Elevates Expectation of Gains and Attenuates Response to Losses Following Risky Decisions. Sleep 2007, 30, 603–609. [Google Scholar] [CrossRef] [Green Version]
- FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 5 September 2022).
- Wimmer, F.; Hoffmann, R.F.; Bonato, R.A.; Moffitt, A.R. The Effects of Sleep Deprivation on Divergent Thinking and Attention Processes. J. Sleep Res. 1992, 1, 223–230. [Google Scholar] [CrossRef]
- Linde, L.; Bergström, M. The Effect of One Night without Sleep on Problem-Solving and Immediate Recall. Psychol. Res. 1992, 54, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J.; Appleton, S.L.; Taylor, A.W.; Gill, T.K.; Lang, C.; McEvoy, R.D.; Antic, N.A. Sleep Health of Australian Adults in 2016: Results of the 2016 Sleep Health Foundation National Survey. Sleep Health 2017, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Lin, C.-Y.; Wang, P.-Y.; Yang, S.-Y. Association between Sleeping Duration and Health-Related Behaviors in College Student. Soc. Health Behav. 2018, 1, 31. [Google Scholar] [CrossRef]
- Stranges, S.; Tigbe, W.; Gómez-Olivé, F.X.; Thorogood, M.; Kandala, N.-B. Sleep Problems: An Emerging Global Epidemic? Findings From the INDEPTH WHO-SAGE Study Among More Than 40,000 Older Adults From 8 Countries Across Africa and Asia. Sleep 2012, 35, 1173–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philips Global Sleep Survey Shows We Want Better Sleep, but Only If It Comes Easily. Available online: https://www.philips.com/a-w/about/news/archive/standard/news/press/2019/20190307-philips-global-sleep-survey-shows-we-want-better-sleep-but-only-if-it-comes-easily.html (accessed on 5 September 2022).
- Kim, S.; DeRoo, L.A.; Sandler, D.P. Eating Patterns and Nutritional Characteristics Associated with Sleep Duration. Public Health Nutr. 2011, 14, 889–895. [Google Scholar] [CrossRef]
- Driver, H.S.; Dijk, D.J.; Werth, E.; Biedermann, K.; Borbély, A.A. Sleep and the Sleep Electroencephalogram across the Menstrual Cycle in Young Healthy Women. J. Clin. Endocrinol. Metab. 1996, 81, 728–735. [Google Scholar] [CrossRef]
- Romans, S.E.; Kreindler, D.; Einstein, G.; Laredo, S.; Petrovic, M.J.; Stanley, J. Sleep Quality and the Menstrual Cycle. Sleep Med. 2015, 16, 489–495. [Google Scholar] [CrossRef]
- Barbosa, D.E.C.; de Souza, V.R.; dos Santos, L.A.S.; de Jesus Chiappini, C.C.; de Sa, S.A.; de Azeredo, V.B. Changes in Taste and Food Intake during the Menstrual Cycle. J. Nutr. Food Sci. 2015, 5, 1. [Google Scholar]



| Sex | N | % |
|---|---|---|
| Male | 18 | 30.5 |
| Female | 41 | 69.5 |
| Race | ||
| Asian | 23 | 39.0 |
| Black | 5 | 8.5 |
| White | 27 | 45.8 |
| Other | 3 | 5.1 |
| Ethnicity | ||
| Hispanic | 2 | 3.4 |
| Non-Hispanic | 51 | 86.4 |
| Anthropometrics | Mean ± SD | Range |
| Body mass index (kg/m2) | 23.3 ± 4.4 | 15.5–36.6 |
| Body fat (%) | 24.7 ± 10.8 | 3.0–49.5 |
| Age (y) | 26.2 ± 6.0 | 19–41 |
| Sleep measures | Mean ± SD | Range |
| Pittsburg Sleep Quality Index (score) | 4.0 ± 1.7 | 0–8 |
| Sleep duration (h, self-reported) | 8.1 ± 0.6 | 7.1–9.1 |
| Geometric Mean of the Preferred Salt Concentration for Those Who Experienced the Habitual Sleep Night First (n = 27) (Mean ± SD) | Geometric Mean of the Preferred Salt Concentration for Those Who Experienced the Habitual Sleep Night Second (n = 32) (Mean ± SD) | Geometric Mean of the Preferred Salt Concentration after the Habitual Sleep Night for the Overall Sample (n = 59) (Mean ± SD) | p Value for the Comparison of the Habitual Sleep Night Experienced as the First Night vs. the Second Night | |
|---|---|---|---|---|
| Habitual sleep night | 0.71 ± 0.38 mg/dL | 0.70 ± 0.36 mg/dL | 0.70 ± 0.37 mg/dL | 0.920 |
| Habitual | Curtailed | % Reduction | p-Value | ||
|---|---|---|---|---|---|
| Objective sleep measures (h) | Time in bed | 8.6 ± 0.8 | 5.5 ± 0.7 | 36.0% | < 0.001 |
| Total sleep time | 7.2 ± 0.6 | 4.6 ± 0.7 | 36.1% | < 0.001 | |
| SWS sleep | 1.5 ± 0.4 | 1.3 ± 0.5 | 13.3% | 0.002 | |
| REM sleep | 1.8 ± 0.6 | 1.1 ± 0.4 | 38.9% | <0.001 | |
| Subjective sleep measures (0–100 scale) | Sleep quality satisfaction | 64.1± 17.5 | 41.4 ± 20.4 | 35.4% | <0.001 |
| Sleep duration satisfaction | 67.9 ± 17.6 | 27.3 ± 14.1 | 59.8% | <0.001 |
| Habitual | Curtailed | p-Value | |
|---|---|---|---|
| Na (mg/d) | 3251.9 ± 1866.6 | 3156.9 ± 1400.0 | 0.621 |
| Energy-corrected Na (mg/Kcal) | 1.7 ± 0.5 | 1.7 ± 0.6 | 0.986 |
| Energy (Kcal/d) | 1984.3 ± 1101.6 | 1948.6 ± 818.0 | 0.757 |
| Carbohydrate (g/d) | 249.0 ± 156.7 | 235.6 ± 120.3 | 0.454 |
| Protein (g/d) | 73.5 ± 54.0 | 70.8 ± 37.7 | 0.657 |
| Fat (g/d) | 76.7 ± 47.5 | 77.6 ± 36.9 | 0.864 |
| Measures | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| (1) Age (years) | - | 0.213 | 0.295 † | −0.087 | −0.138 | −0.129 | 0.161 | −0.078 | −0.239 |
| (2) BMI (Kg/m2) | - | 0.798 ** | 0.012 | −0.029 | −0.004 | 0.098 | −0.001 | 0.021 | |
| (3) BF% | - | −0.046 | 0.046 | −0.110 | 0.121 | −0.030 | −0.119 | ||
| (4) PSQI | - | 0.020 | 0.072 | −0.174 | 0.177 | −0.154 | |||
| (5) Total sleep time (h) | - | −0.104 | −0.248 | 0.075 | 0.024 | ||||
| (6) Liking slope | − | 0.009 | 0.593 ** | 0.338 ** | |||||
| (7) Intensity slope | - | −0.199 | 0.005 | ||||||
| (8) Preferred salt concentration (M) | - | 0.165 | |||||||
| (9) Energy-corrected Na intake (mg/Kcal) | - |
| Measures | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| (1) Age (years) | - | 0.213 | 0.295 † | −0.087 | −0.084 | −0.315 † | 0.158 | −0.021 | 0.037 |
| (2) BMI (Kg/m2) | - | 0.798 ** | 0.012 | −0.214 | 0.074 | 0.294 † | −0.014 | 0.217 | |
| (3) BF% | - | −0.046 | −0.195 | −0.119 | 0.259 † | −0.170 | 0.077 | ||
| (4) PSQI | - | −0.061 | 0.136 | 0.162 | 0.060 | 0.203 | |||
| (5) Total sleep time (h) | - | −0.171 | −0.259 † | −0.032 | −0.255 | ||||
| (6) Liking slope | − | −0.029 | 0.671 ** | 0.132 | |||||
| (7) Intensity slope | - | −0.099 | 0.299 † | ||||||
| (8) Preferred salt concentration (M) | - | −0.016 | |||||||
| (9) Energy-corrected Na intake (mg/Kcal) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Keast, R.; Tan, S.-Y.; Tucker, R.M. The Effects of Acute Sleep Curtailment on Salt Taste Measures and Relationships with Energy-Corrected Sodium Intake: A Randomized Cross-Over Trial with Methodology Validation. Int. J. Environ. Res. Public Health 2023, 20, 4140. https://doi.org/10.3390/ijerph20054140
Du C, Keast R, Tan S-Y, Tucker RM. The Effects of Acute Sleep Curtailment on Salt Taste Measures and Relationships with Energy-Corrected Sodium Intake: A Randomized Cross-Over Trial with Methodology Validation. International Journal of Environmental Research and Public Health. 2023; 20(5):4140. https://doi.org/10.3390/ijerph20054140
Chicago/Turabian StyleDu, Chen, Russell Keast, Sze-Yen Tan, and Robin M. Tucker. 2023. "The Effects of Acute Sleep Curtailment on Salt Taste Measures and Relationships with Energy-Corrected Sodium Intake: A Randomized Cross-Over Trial with Methodology Validation" International Journal of Environmental Research and Public Health 20, no. 5: 4140. https://doi.org/10.3390/ijerph20054140
APA StyleDu, C., Keast, R., Tan, S.-Y., & Tucker, R. M. (2023). The Effects of Acute Sleep Curtailment on Salt Taste Measures and Relationships with Energy-Corrected Sodium Intake: A Randomized Cross-Over Trial with Methodology Validation. International Journal of Environmental Research and Public Health, 20(5), 4140. https://doi.org/10.3390/ijerph20054140







