Polypharmacy and the Change of Self-Rated Health in Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measures
2.2.1. Polypharmacy Status
2.2.2. Self-Rated Health (SRH)
2.2.3. Determination of SRH-Change Categories
2.2.4. Covariable Assessment
2.3. Statistical Analyses
3. Results
3.1. Main Characteristics of the Study Population
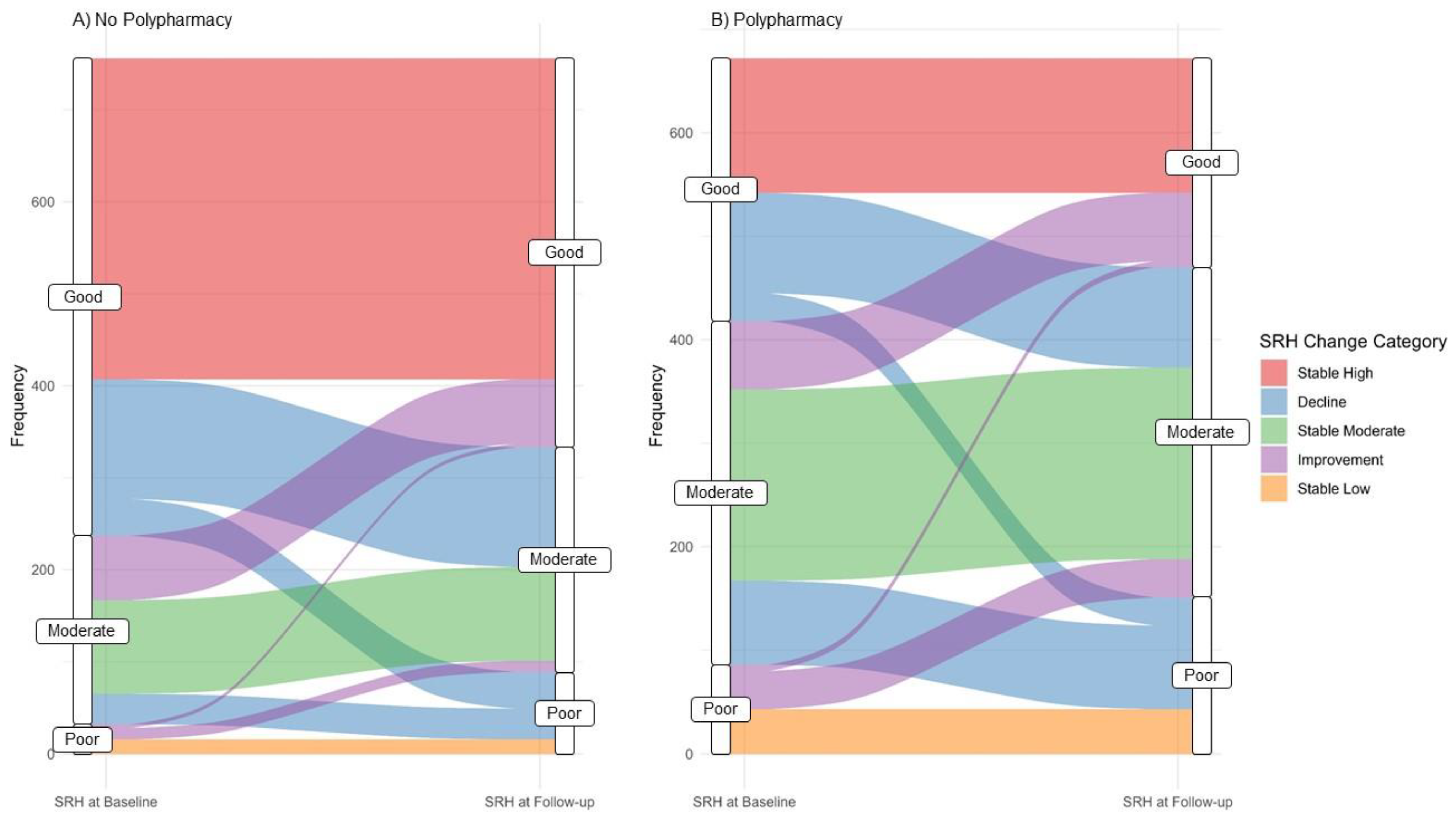
3.2. SRH Transition by Polypharmacy Status
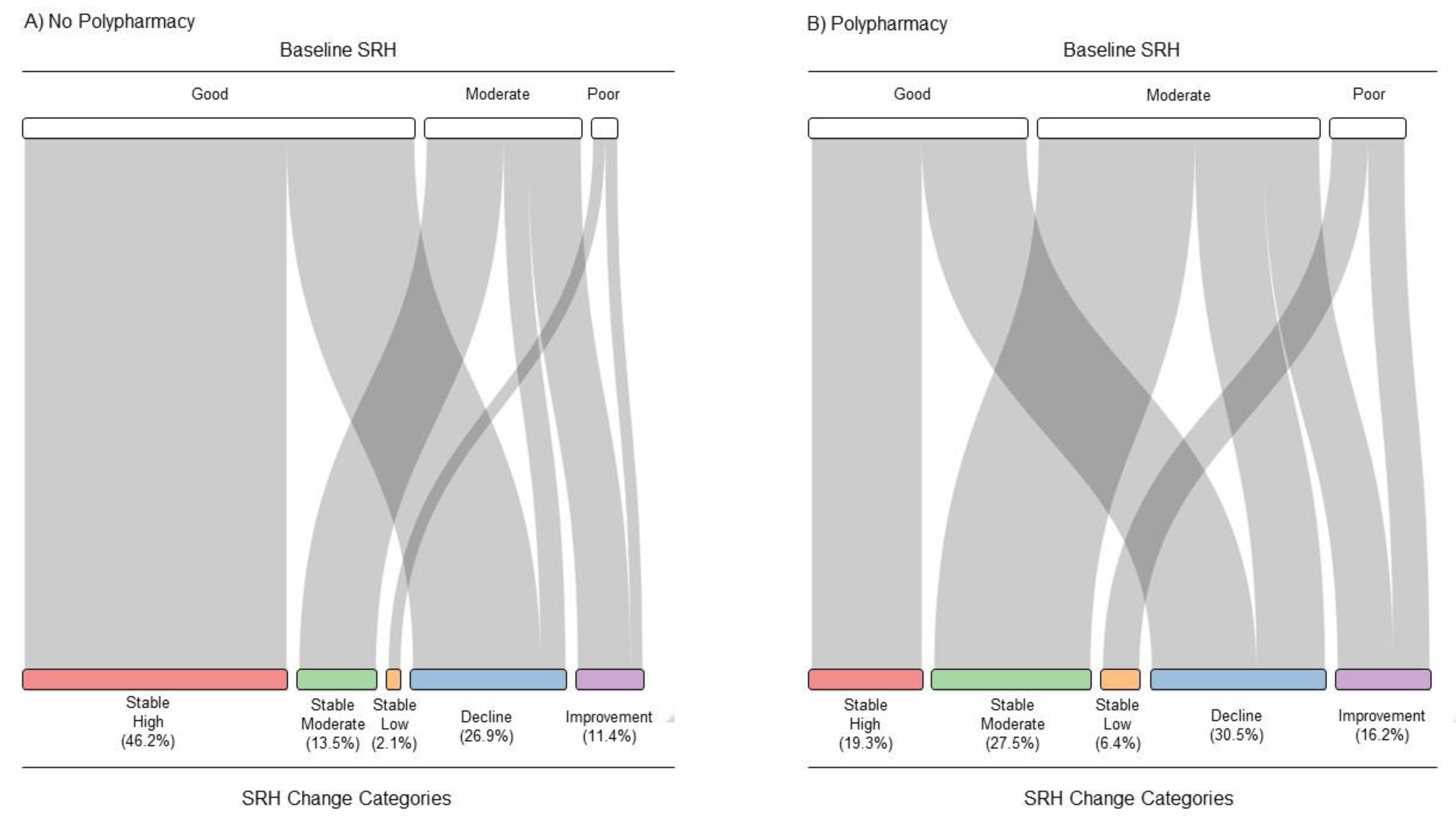
3.3. SRH-Change Categories by Polypharmacy
3.4. Polypharmacy and SRH-Change Categories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistisches Bundesamt. Anteil der Bevölkerung ab 65 Jahren an der Gesamtbevölkerung in Deutschland von 1991 bis 2021. Available online: https://de.statista.com/statistik/daten/studie/548267/umfrage/anteil-der-bevoelkerung-ab-65-jahren-und-aelter-in-deutschland/ (accessed on 28 June 2022).
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puth, M.-T.; Weckbecker, K.; Schmid, M.; Münster, E. Prevalence of multimorbidity in Germany: Impact of age and educational level in a cross-sectional study on 19,294 adults. BMC Public Health 2017, 17, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilici, E.; Despotou, G.; Arvanitis, T.N. The use of computer-interpretable clinical guidelines to manage care complexities of patients with multimorbid conditions: A review. Digital Health 2018, 4, 2055207618804927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, J. Polypharmacy: A necessary evil. BMJ Br. Med. J. 2013, 347, f7033. [Google Scholar] [CrossRef] [Green Version]
- Wastesson, J.W.; Morin, L.; Tan, E.C.K.; Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Fernández, A.; Gómez, F.; Curcio, C.L.; Pineda, E.; Fernandes de Souza, J. Prevalence and impact of potentially inappropriate medication on community-dwelling older adults. Biomed. Rev. Inst. Nac. Salud 2021, 41, 111–122. [Google Scholar] [CrossRef]
- Chang, T.I.; Park, H.; Kim, D.W.; Jeon, E.K.; Rhee, C.M.; Kalantar-Zadeh, K.; Kang, E.W.; Kang, S.W.; Han, S.H. Polypharmacy, hospitalization, and mortality risk: A nationwide cohort study. Sci. Rep. 2020, 10, 18964. [Google Scholar] [CrossRef]
- Hsu, H.F.; Chen, K.M.; Belcastro, F.; Chen, Y.F. Polypharmacy and pattern of medication use in community-dwelling older adults: A systematic review. J. Clin. Nurs 2021, 30, 918–928. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [Green Version]
- Bjälkefur, K.; Nasic, S.; Bertholds, E.; Jood, K.; Rejnö, Å. Self-rated health over the first five years after stroke. BMC Neurol. 2020, 20, 389. [Google Scholar] [CrossRef]
- Hamplová, D.; Klusáček, J.; Mráček, T. Assessment of self-rated health: The relative importance of physiological, mental, and socioeconomic factors. PLoS ONE 2022, 17, e0267115. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Mir, N.; Al-Obaidi, M.; Clark, D.; Kenzik, K.M.; McDonald, A.; Young-Smith, C.; Paluri, R.; Nandagopal, L.; Gbolahan, O.; et al. Use of Single-Item Self-Rated Health Measure to Identify Frailty and Geriatric Assessment-Identified Impairments Among Older Adults with Cancer. Oncologist 2022, 27, e45–e52. [Google Scholar] [CrossRef]
- Isaac, V.; McLachlan, C.S.; Baune, B.T.; Huang, C.-T.; Wu, C.-Y. Poor Self-Rated Health Influences Hospital Service Use in Hospitalized Inpatients with Chronic Conditions in Taiwan. Medicine 2015, 94, e1477. [Google Scholar] [CrossRef] [PubMed]
- Wuorela, M.; Lavonius, S.; Salminen, M.; Vahlberg, T.; Viitanen, M.; Viikari, L. Self-rated health and objective health status as predictors of all-cause mortality among older people: A prospective study with a 5-, 10-, and 27-year follow-up. BMC Geriatr. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Krantz, E.; Wide, U.; Trimpou, P.; Bryman, I.; Landin-Wilhelmsen, K. Comparison between different instruments for measuring health-related quality of life in a population sample, the WHO MONICA Project, Gothenburg, Sweden: An observational, cross-sectional study. BMJ Open 2019, 9, e024454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilleron, S.; Le Goff, M.; Ajana, S.; Helmer, C.; Pérès, K.; Dartigues, J.F.; Tabue-Teguo, M.; Féart, C. Self-Rated Health and Frailty in Older Adults from the Population-Based Three-City Bordeaux Cohort. Gerontology 2022, 68, 755–762. [Google Scholar] [CrossRef]
- Machón, M.; Vergara, I.; Dorronsoro, M.; Vrotsou, K.; Larrañaga, I. Self-perceived health in functionally independent older people: Associated factors. BMC Geriatr. 2016, 16, 66. [Google Scholar] [CrossRef] [Green Version]
- Colombijn, J.M.T.; Bonenkamp, A.A.; van Eck van der Sluijs, A.; Bijlsma, J.A.; Boonstra, A.H.; Özyilmaz, A.; Abrahams, A.C.; van Jaarsveld, B.C. Impact of Polypharmacy on Health-Related Quality of Life in Dialysis Patients. Am. J. Nephrol. 2021, 52, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Kivimäki, M.; Jylhä, M.; Kawachi, I.; Westerlund, H.; Pentti, J.; Goldberg, M.; Zins, M.; Vahtera, J. Trajectories of self-rated health in the last 15 years of life by cause of death. Eur. J. Epidemiol. 2016, 31, 177–185. [Google Scholar] [CrossRef]
- Aljeaidi, M.S.; Haaksma, M.L.; Tan, E.C.K. Polypharmacy and trajectories of health-related quality of life in older adults: An Australian cohort study. Qual. Life Res. 2022, 31, 2663–2671. [Google Scholar] [CrossRef]
- Schaeffner, E.S.; van der Giet, M.; Gaedeke, J.; Tölle, M.; Ebert, N.; Kuhlmann, M.K.; Martus, P. The Berlin initiative study: The methodology of exploring kidney function in the elderly by combining a longitudinal and cross-sectional approach. Eur. J. Epidemiol. 2010, 25, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, H. Social participation and the health and well-being of Canadian seniors. Health Rep. 2012, 23, 23–32. [Google Scholar]
- Pfarr, C.; Schmid, A.; Schneider, U. Reporting heterogeneity in self-assessed health among elderly Europeans. Health Econ. Rev. 2012, 2, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauns, H.; Scherer, S.; Steinmann, S. The CASMIN Educational Classification in International Comparative Research. In Advances in Cross-National Comparison: A European Working Book for Demographic and Socio-Economic Variables; Hoffmeyer-Zlotnik, J.H.P., Wolf, C., Eds.; Springer: New York, NY, USA, 2003; pp. 221–244. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar]
- Saum, K.U.; Schöttker, B.; Meid, A.D.; Holleczek, B.; Haefeli, W.E.; Hauer, K.; Brenner, H. Is Polypharmacy Associated with Frailty in Older People? Results From the ESTHER Cohort Study. J. Am. Geriatr. Soc. 2017, 65, e27–e32. [Google Scholar] [CrossRef] [PubMed]
- Junius-Walker, U.; Theile, G.; Hummers-Pradier, E. Prevalence and predictors of polypharmacy among older primary care patients in Germany. Fam Pract. 2007, 24, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borim, F.S.A.; de Assumpção, D.; Neri, A.L.; Batistoni, S.S.T.; Francisco, P.M.S.B.; Yassuda, M.S. Impact of functional capacity on change in self-rated health among older adults in a nine-year longitudinal study. BMC Geriatr. 2021, 21, 627. [Google Scholar] [CrossRef]
- Verropoulou, G. Determinants of change in self-rated health among older adults in Europe: A longitudinal perspective based on SHARE data. Eur. J. Ageing 2012, 9, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Tur-Sinai, A.; Soskolne, V. Socioeconomic status and health behaviors as predictors of changes in self-rated health among older persons in Israel. Health Soc. Care Community 2021, 29, 1461–1472. [Google Scholar] [CrossRef]
- Rowe, J.W.; Kahn, R.L. Successful aging. Gerontologist 1997, 37, 433–440. [Google Scholar] [CrossRef]
- Leinonen, R.; Heikkinen, E.; Jylhä, M. Changes in health, functional performance and activity predict changes in self-rated health: A 10-year follow-up study in older people. Arch. Gerontol. Geriatr. 2002, 35, 79–92. [Google Scholar] [CrossRef]
- Fayers, P.M.; Sprangers, M.A. Understanding self-rated health. Lancet 2002, 359, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; McGarrigle, C.A.; Kenny, R.A. More than health: Quality of life trajectories among older adults-findings from The Irish Longitudinal Study of Ageing (TILDA). Qual. Life Res. 2019, 28, 429–439. [Google Scholar] [CrossRef]
- Beyer, A.K.; Wolff, J.K.; Warner, L.M.; Schüz, B.; Wurm, S. The role of physical activity in the relationship between self-perceptions of ageing and self-rated health in older adults. Psychol Health 2015, 30, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Faß, E.; Schlesinger, T. The Relation of Physical Activity and Self-Rated Health in Older Age—Cross Country Analysis Results from SHARE. J. Popul. Ageing 2020, 13, 347–364. [Google Scholar] [CrossRef]
- Hellgren, M.I.; Kitsche, E.; Groot-Zevert, M.; Lindblad, U.; Daka, B. Association between body mass index and self-rated health: A Swedish population-based longitudinal study. Scand. J. Public Health 2021, 49, 369–376. [Google Scholar] [CrossRef]
- Lee, H.L.; Huang, H.C.; Lee, M.D.; Chen, J.H.; Lin, K.C. Factors affecting trajectory patterns of self-rated health (SRH) in an older population--a community-based longitudinal study. Arch. Gerontol Geriatr. 2012, 54, e334–e341. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shaw, B.A.; Krause, N.; Bennett, J.M.; Kobayashi, E.; Fukaya, T.; Sugihara, Y. How does self-assessed health change with age? A study of older adults in Japan. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005, 60, S224–S232. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.T.; Fung, H.; Chan, A. Maintaining self-rated health through social comparison in old age. J. Gerontol. B Psychol. Sci. Soc. Sci. 2007, 62, P277–P285. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.L.; Arnold, A.M.; Hirsch, C.H.; Thielke, S.M.; Kim, D.; Mukamal, K.J.; Kizer, J.R.; Ix, J.H.; Kaplan, R.C.; Kritchevsky, S.B.; et al. Effects of Disease Burden and Functional Adaptation on Morbidity and Mortality on Older Adults. J. Am. Geriatr. Soc. 2016, 64, 1242–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, D.; Pascuzzi, K. Polypharmacy in the elderly: Focus on drug interactions and adherence in hypertension. Clin. Geriatr. Med. 2009, 25, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Luque, A.; Núñez-Montenegro, A.J.; Martín-Aurioles, E.; Canca-Sánchez, J.C.; Toro-Toro, M.C.; González-Correa, J.A. Medication-related factors associated with health-related quality of life in patients older than 65 years with polypharmacy. PLoS ONE 2017, 12, e0171320. [Google Scholar] [CrossRef] [Green Version]
- Benyamini, Y.; Leventhal, E.A.; Leventhal, H. Gender Differences in Processing Information for Making Self-Assessments of Health. Psychosom. Med. 2000, 62, 354–364. [Google Scholar] [CrossRef]
- Venturini, C.D.; Engroff, P.; Ely, L.S.; Zago, L.F.; Schroeter, G.; Gomes, I.; De Carli, G.A.; Morrone, F.B. Gender differences, polypharmacy, and potential pharmacological interactions in the elderly. Clinics 2011, 66, 1867–1872. [Google Scholar] [CrossRef]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Dalastra, L.; Pagotto, V. Polypharmacy, chronic diseases and nutritional markers in community-dwelling older. Rev. Bras. Epidemiol. 2014, 17, 818–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, D.; Garvey, J.; McKee, G. Factors associated with ADL/IADL disability in community dwelling older adults in the Irish longitudinal study on ageing (TILDA). Disabil. Rehabil. 2017, 39, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, J.; Pivetta, N.; Batistoni, S.; Neri, A.; Borim, F. The association between self-rated health and functional capacity indicators. Geriatr. Gerontol. Aging 2017, 11, 61–67. [Google Scholar] [CrossRef] [Green Version]
- García-Lara, J.M.; Navarrete-Reyes, A.P.; Medina-Méndez, R.; Aguilar-Navarro, S.G.; Avila-Funes, J.A. Successful Aging, a New Challenge for Developing Countries: The Coyoacán Cohort. J. Nutr. Health Aging 2017, 21, 215–219. [Google Scholar] [CrossRef]
- Cross, A.J.; Elliott, R.A.; Petrie, K.; Kuruvilla, L.; George, J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst. Rev. 2020, CD012419. [Google Scholar] [CrossRef]
- Tavares, N.U.; Bertoldi, A.D.; Mengue, S.S.; Arrais, P.S.; Luiza, V.L.; Oliveira, M.A.; Ramos, L.R.; Farias, M.R.; Pizzol, T.D. Factors associated with low adherence to medicine treatment for chronic diseases in Brazil. Rev. Saude Publica 2016, 50 (Suppl. 2), 10s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochon, P.A.; Gurwitz, J.H. Optimising drug treatment for elderly people: The prescribing cascade. BMJ 1997, 315, 1096–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzer, S.; Blüher, S.; Teti, A.; Schaeffner, E.; Ebert, N.; Martus, P.; Suhr, R.; Kuhlmey, A. Risk Profiles for Care Dependency: Cross-Sectional Findings of a Population-Based Cohort Study in Germany. J. Aging Health 2020, 32, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Category | No Polypharmacy | Polypharmacy | Total (N = 1428) |
|---|---|---|---|---|
| 756 (52.9%) | 672 (47.1%) | |||
| Sociodemographic Factors | ||||
| Age Mean (SD) | 78.3 (6.1) | 79.9 (5.9) | 79.1 (6.1) | |
| Gender N (%) | Female | 426 (56.3%) | 345 (51.3%) | 771 (54.0%) |
| SRH Level at baseline N (%) | Good | 519 (68.7%) | 254 (37.8%) | 773 (54.1%) |
| Moderate | 205 (27.1%) | 332 (49.4%) | 537 (37.6%) | |
| Poor | 32 (4.2%) | 86 (12.8%) | 118 (8.3%) | |
| Income (in EUR) N (%) | <1000 | 215 (28.4%) | 194 (28.9%) | 409 (28.6%) |
| 1000–1999 | 388 (51.3%) | 366 (54.5%) | 754 (52.8%) | |
| ≥2000 | 49 (6.5%) | 31 (4.6%) | 80 (5.6%) | |
| Missing | 104 (13.8%) | 81 (12.1%) | 185 (13%) | |
| CASMIN N (%) | Low | 435 (57.5%) | 412 (61.3%) | 847 (59.3%) |
| Intermediate | 155 (20.5%) | 133 (19.8%) | 288 (20.2%) | |
| High | 162 (21.4%) | 124 (18.5%) | 286 (20%) | |
| Missing | 4 (0.5%) | 3 (0.4%) | 7 (0.5%) | |
| Having a Partner N (%) | 457 (60.4%) | 403 (60.0%) | 860 (60.2%) | |
| Lifestyle Factors | ||||
| Frequency of Alcohol Consumption N (%) | Less than once a month | 290 (38.4%) | 317 (47.2%) | 607 (42.5%) |
| ≤2 times per week | 300 (39.7%) | 221 (32.9%) | 521 (36.5%) | |
| Regularly | 166 (22.0%) | 130 (19.3%) | 296 (20.7%) | |
| Missing | 0 | 4 (0.6%) | 4 (0.3%) | |
| Physical Activity N (%) | Less than once a week | 104 (13.8%) | 208 (31%) | 312 (21.8%) |
| 1–5 times per week | 376 (49.7%) | 305 (45.4%) | 681 (47.7%) | |
| More than 5 times per week | 275 (36.4%) | 158 (23.5%) | 433 (30.3%) | |
| Missing | 1 (0.1%) | 1 (0.1%) | 2 (0.1%) | |
| BMI (in kg/m2) N (%) | <25 | 228 (30.2%) | 127 (18.9%) | 355 (24.9%) |
| 25–<30 | 367 (48.5%) | 321 (47.8%) | 688 (48.2%) | |
| ≥30 | 161 (21.3%) | 223 (33.2%) | 384 (26.9%) | |
| Missing | 0 | 1 (0.1%) | 1 (0.1%) | |
| Medical Status | ||||
| CCI Category N (%) | 0 | 119 (15.7%) | 19 (2.8%) | 138 (9.7%) |
| 1–2 | 295 (39%) | 115 (17.1%) | 410 (28.7%) | |
| 3–4 | 196 (25.9%) | 176 (26.2%) | 372 (26.1%) | |
| ≥5 | 136 (18.0%) | 360 (53.6%) | 496 (34.7%) | |
| Missing | 2 (0.3%) | 10 (1.3%) | 12 (0.8%) | |
| (a) No Polypharmacy Group | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | SRH-Change Category | Total (N = 756) | ||||
| Stable High | Stable Moderate | Stable Low | Decline | Improvement | |||
| 349 (46.2%) | 102 (13.5%) | 16 (2.1%) | 203 (26.9%) | 86 (11.4%) | |||
| Sociodemographic Factors | |||||||
| Age Mean (SD) | 77.5 (5.5) | 78.2 (6.4) | 79.0 (6.5) | 79.7 (6.6) | 78.4 (6.1) | 78.3 (6.1) | |
| Gender N (%) | Female | 185 (53.0%) | 74 (72.5%) | 13 (81.2%) | 106 (52.2%) | 48 (55.8%) | 426 (56.3%) |
| Income (in EUR) N (%) | <1000 | 86 (24.6%) | 34 (33.3%) | 8 (50.0%) | 60 (29.6%) | 27 (31.4%) | 215 (28.4%) |
| 1000–1999 | 185 (53.0%) | 43 (42.2%) | 6 (37.5%) | 110 (54.2%) | 44 (51.2%) | 388 (51.3%) | |
| ≥2000 | 26 (7.4%) | 8 (7.8%) | 1 (6.2%) | 10 (4.9%) | 4 (4.7%) | 49 (6.5%) | |
| Missing | 52 (14.9%) | 17 (16.7%) | 1 (6.2%) | 23 (11.3%) | 11 (12.8%) | 104 (13.8%) | |
| CASMIN N (%) | Low | 198 (56.7%) | 64 (62.7%) | 10 (62.5%) | 111 (54.7%) | 52 (60.5%) | 435 (57.5%) |
| Intermediate | 73 (20.9%) | 17 (16.7%) | 5 (31.2%) | 47 (23.2%) | 5 (31.2%) | 155 (20.5%) | |
| High | 78 (22.3%) | 21 (20.6%) | 1 (6.2%) | 42 (20.7%) | 20 (23.3%) | 162 (21.4%) | |
| Missing | 0 | 0 | 0 | 3 (1.5%) | 1 (1.2%) | 4 (0.5%) | |
| Having a Partner N (%) | 229 (65.6%) | 55 (53.9%) | 8 (50.0%) | 112 (55.2%) | 53 (61.6%) | 457 (60.4%) | |
| Lifestyle Factors | |||||||
| Frequency of Alcohol Consumption N (%) | Less the once a month | 108 (30.9%) | 48 (47.1%) | 12 (75.0%) | 84 (41.4%) | 38 (44.2%) | 290 (38.4%) |
| ≤2 times per week | 143 (41.0%) | 40 (39.2%) | 2 (12.5%) | 82 (40.4%) | 33 (38.4%) | 300 (39.7%) | |
| Regularly | 98 (28.1%) | 14 (13.7%) | 2 (12.5%) | 37 (18.2%) | 15 (17.4%) | 166 (22.0%) | |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | |
| Physical Activity N (%) | Less than once a week | 35 (10.0%) | 16 (15.7%) | 5 (31.2%) | 29 (14.3%) | 19 (22.1%) | 104 (13.8%) |
| 1–5 times per week | 162 (46.4%) | 56 (54.9%) | 8 (50.0%) | 113 (55.7%) | 37 (43.0%) | 376 (49.7%) | |
| More than 5 times per week | 151 (43.3%) | 30 (29.4%) | 3 (18.8%) | 61 (30.0%) | 30 (34.9%) | 275 (36.4%) | |
| Missing | 1 (0.3%) | 0 | 0 | 0 | 0 | 1 (0.1%) | |
| BMI (in kg/m2) N (%) | <25 | 107 (30.7%) | 36 (35.3%) | 4 (25.0%) | 57 (28.1%) | 24 (27.9%) | 228 (30.2%) |
| 25–<30 | 175 (50.1%) | 45 (44.1%) | 8 (50.0%) | 97 (47.8%) | 42 (48.8%) | 367 (48.5%) | |
| ≥30 | 67 (19.2%) | 21 (20.6%) | 4 (25.0%) | 49 (24.1%) | 20 (23.3%) | 161 (21.3%) | |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | |
| Medical Status | |||||||
| CCI Category N (%) | 0 | 74 (21.2%) | 12 (11.8%) | 0 | 25 (12.3%) | 8 (9.3%) | 119 (15.7%) |
| 1–2 | 143 (41.0%) | 36 (35.3%) | 7 (43.8%) | 79 (38.9%) | 30 (34.9%) | 295 (39.0%) | |
| 3–4 | 85 (24.4%) | 30 (29.4%) | 2 (12.5%) | 51 (25.1%) | 28 (32.6%) | 196 (25.9%) | |
| ≥5 | 41 (11.7%) | 24 (23.5%) | 7 (43.8%) | 44 (21.7%) | 20 (23.3%) | 136 (18.0%) | |
| Missing | 6 (1.7%) | 0 | 0 | 4 (2.0%) | 0 | 10 (1.3%) | |
| (b) Polypharmacy Group | |||||||
| Variable | Category | SRH-Change Category | Total (N = 672) | ||||
| Stable High | Stable Moderate | Stable Low | Decline | Improvement | |||
| 130 (19.3%) | 185 (27.5%) | 43 (6.4%) | 205 (30.5%) | 109 (16.2%) | |||
| Sociodemographic Factors | |||||||
| Age Mean (SD) | 79.7 (5.9) | 79.4 (5.9) | 79.8 (6.0) | 81.0 (6.0) | 78.9 (5.7) | 79.9 (6.0) | |
| Gender N (%) | Female | 52 (40.0%) | 111 (60.0%) | 28 (65.1%) | 110 (53.7%) | 44 (40.4%) | 345 (51.3%) |
| Income (in EUR) N (%) | <1000 | 33 (25.4%) | 64 (34.6%) | 16 (37.2%) | 49 (23.9%) | 32 (29.4%) | 194 (28.9%) |
| 1000–1999 | 68 (52.3%) | 95 (51.4%) | 21 (48.8%) | 123 (60.0%) | 59 (54.1%) | 366 (54.5%) | |
| >=2000 | 9 (6.9%) | 8 (4.3%) | 2 (4.7%) | 8 (3.9%) | 4 (3.7%) | 31 (4.6%) | |
| Missing | 20 (15.4%) | 18 (9.7%) | 4 (9.3%) | 25 (12.2%) | 14 (9.7%) | 81 (12.1%) | |
| CASMIN N (%) | Low | 77 (59.2%) | 117 (63.2%) | 29 (67.4%) | 123 (60.0%) | 66 (60.6%) | 412 (61.3%) |
| Intermediate | 22 (16.9%) | 36 (19.5%) | 9 (20.9%) | 42 (20.5%) | 24 (22.0%) | 133 (19.8%) | |
| High | 31 (23.8%) | 31 (16.8%) | 5 (11.6%) | 39 (19.0%) | 18 (16.5%) | 124 (18.5%) | |
| Missing | 0 | 1 (0.5%) | 0 | 1 (0.5%) | 1 (0.9%) | 3 (0.4%) | |
| Having a Partner N (%) | 82 (63.1%) | 107 (57.8%) | 22 (51.2%) | 121 (59.0%) | 71 (65.1%) | 403 (60.0%) | |
| Lifestyle Factors | |||||||
| Frequency of Alcohol Consumption N (%) | Less the once a month | 44 (33.8%) | 107 (57.8%) | 27 (62.8%) | 83 (40.5%) | 56 (51.4%) | 317 (47.2%) |
| ≤2 times per week | 49 (37.7%) | 49 (26.5%) | 8 (18.6%) | 84 (41.0%) | 31 (28.4%) | 221 (32.9%) | |
| Regularly | 36 (27.7%) | 28 (15.1%) | 8 (18.6%) | 37 (18.0%) | 21 (19.3%) | 130 (19.3%) | |
| Missing | 1 (0.8%) | 1 (0.5%) | 0 | 1 (0.5%) | 1 (0.9%) | 4 (0.6%) | |
| Physical Activity N (%) | Less than once a week | 27 (20.8%) | 58 (31.4%) | 24 (55.8%) | 65 (31.7%) | 34 (31.2%) | 208 (31.0%) |
| 1–5 times per week | 56 (43.1%) | 87 (47.0%) | 11 (25.6%) | 98 (47.8%) | 53 (48.6%) | 305 (45.4%) | |
| More than 5 times per week | 47 (36.2%) | 39 (21.1%) | 8 (18.6%) | 42 (20.5%) | 22 (20.2%) | 158 (23.5%) | |
| Missing | 0 | 1 (0.5%) | 0 | 0 | 0 | 1 (0.1%) | |
| BMI (kg/m2) N (%) | <25 | 30 (23.1%) | 32 (17.3%) | 7 (16.3%) | 39 (19.0%) | 19 (17.4%) | 127 (18.9%) |
| 25–<30 | 68 (52.3%) | 85 (45.9%) | 19 (44.2%) | 94 (45.9%) | 55 (50.5%) | 321 (47.8%) | |
| ≥30 | 32 (24.6%) | 68 (36.8%) | 17 (39.5%) | 71 (34.6%) | 35 (32.1%) | 223 (33.2%) | |
| Missing | 0 | 0 | 0 | 1 (0.5%) | 0 | 1 (0.1%) | |
| Medical Status | |||||||
| CCI Category N (%) | 0 | 5 (3.8%) | 7 (3.8%) | 0 | 6 (2.9%) | 1 (0.9%) | 19 (2.8%) |
| 1–2 | 36 (27.7%) | 24 (13.0%) | 8 (18.6%) | 34 (16.6%) | 13 (11.9%) | 115 (17.1%) | |
| 3–4 | 30 (23.1%) | 58 (31.4%) | 9 (20.9%) | 54 (26.3%) | 25 (22.9%) | 176 (26.2%) | |
| ≥5 | 59 (45.4%) | 95 (51.4%) | 26 (60.5%) | 110 (53.7%) | 70 (64.2%) | 360 (53.6%) | |
| Missing | 0 | 1 (0.5%) | 0 | 1 (0.5%) | 0 | 2 (0.3%) | |
| SRH-Change Categories | ||||||
|---|---|---|---|---|---|---|
| Stable High | Stable Moderate | Stable Low | Decline | Improvement | ||
| N (%) | ||||||
| Polypharmacy | <0.001 | |||||
| Yes | 130 (19.3) | 185 (27.5) | 43 (6.4) | 205 (30.5) | 109 (16.2) | |
| No | 349 (46.2) | 102 (13.5) | 16 (2.1) | 203 (26.9) | 86 (11.4) | |
| Crude Model OR (95% CI) | ||||||
| Polypharmacy (Yes) | Reference | 4.87 (3.56–6.67) | 7.22 (3.93–13.26) | 2.71 (2.05–3.59) | 3.40 (2.40–4.81) | |
| Adjusted Model 1 a OR (95% CI) | ||||||
| Polypharmacy (Yes) | Reference | 3.55 (2.43–5.20) | 3.32 (1.65–6.70) | 1.87 (1.34–2.62) | 2.01 (1.33–3.05) | |
| Adjusted Model 2 b OR (95% CI) | ||||||
| Polypharmacy (Yes) | Reference | 3.54 (2.41–5.18) | 3.34 (1.65–6.76) | 1.83 (1.31–2.57) | 2.01 (1.33–3.06) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barghouth, M.H.; Schaeffner, E.; Ebert, N.; Bothe, T.; Schneider, A.; Mielke, N. Polypharmacy and the Change of Self-Rated Health in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2023, 20, 4159. https://doi.org/10.3390/ijerph20054159
Barghouth MH, Schaeffner E, Ebert N, Bothe T, Schneider A, Mielke N. Polypharmacy and the Change of Self-Rated Health in Community-Dwelling Older Adults. International Journal of Environmental Research and Public Health. 2023; 20(5):4159. https://doi.org/10.3390/ijerph20054159
Chicago/Turabian StyleBarghouth, Muhammad Helmi, Elke Schaeffner, Natalie Ebert, Tim Bothe, Alice Schneider, and Nina Mielke. 2023. "Polypharmacy and the Change of Self-Rated Health in Community-Dwelling Older Adults" International Journal of Environmental Research and Public Health 20, no. 5: 4159. https://doi.org/10.3390/ijerph20054159
APA StyleBarghouth, M. H., Schaeffner, E., Ebert, N., Bothe, T., Schneider, A., & Mielke, N. (2023). Polypharmacy and the Change of Self-Rated Health in Community-Dwelling Older Adults. International Journal of Environmental Research and Public Health, 20(5), 4159. https://doi.org/10.3390/ijerph20054159





