Global Distribution of Culex tritaeniorhynchus and Impact Factors
Abstract
:1. Background
2. Materials and Methods
2.1. Occurrence Data
2.2. Impact Factors
2.3. Model Development and Assessment
3. Results
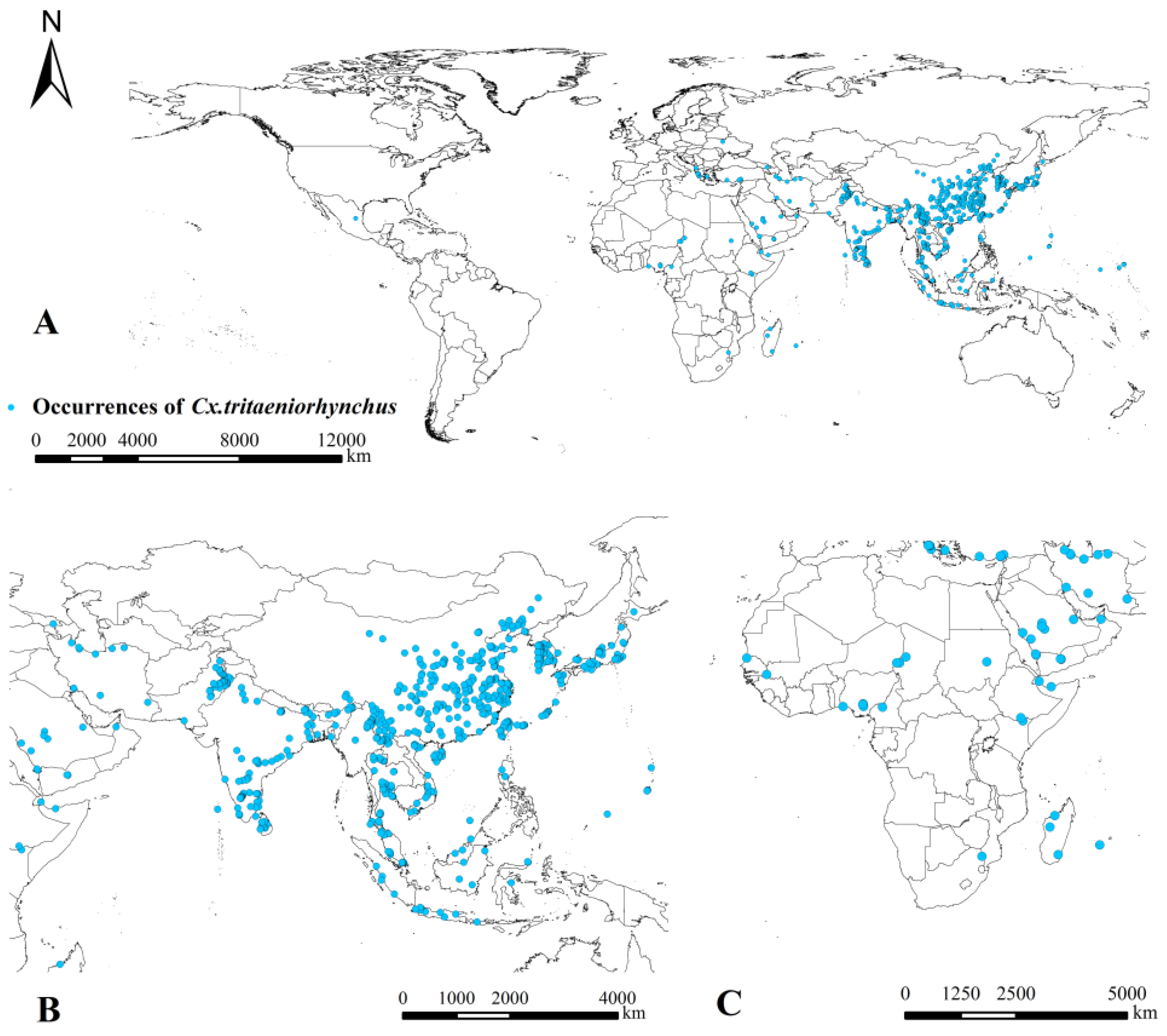
3.1. Overall Occurrences
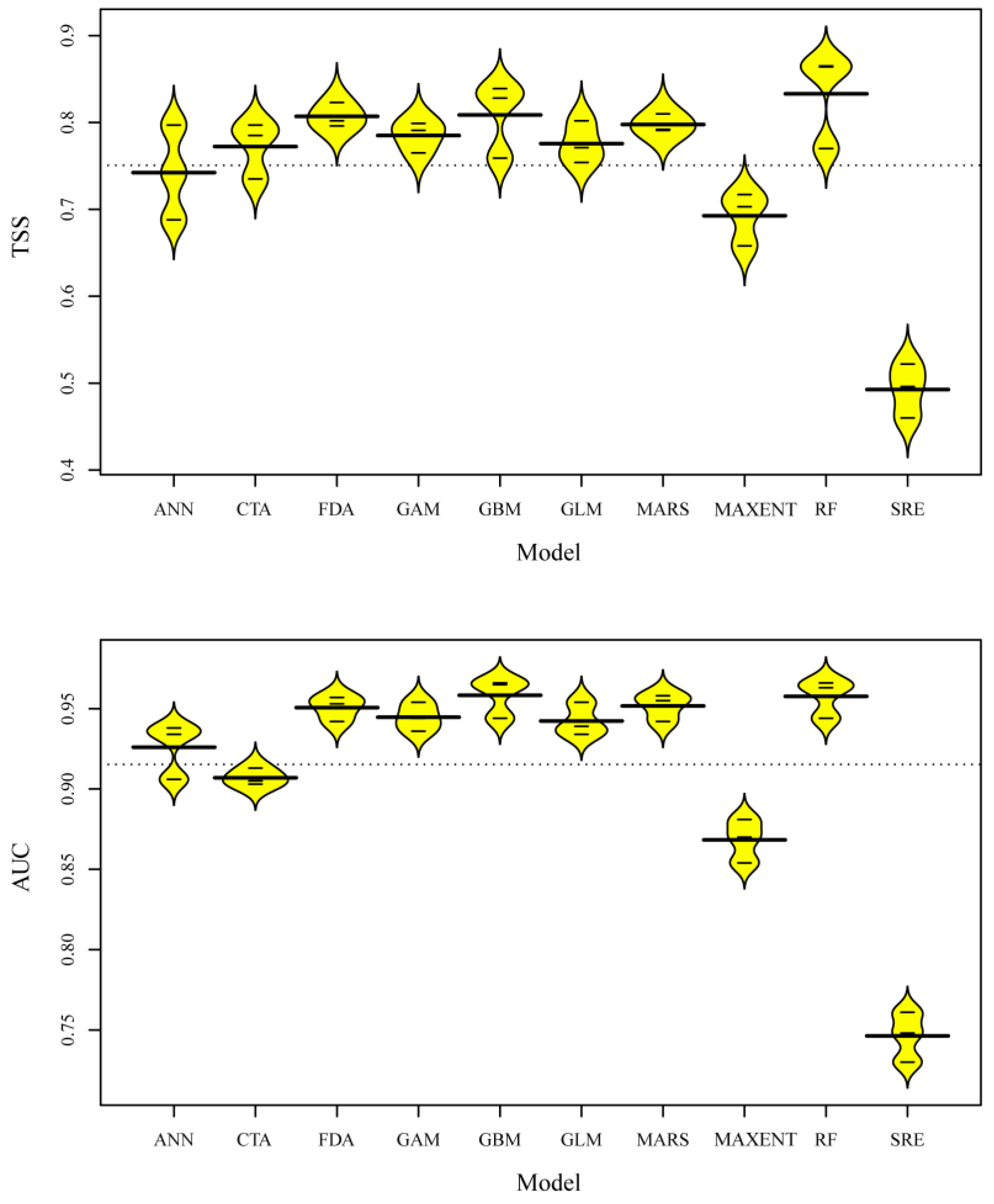
3.2. Model Evaluation
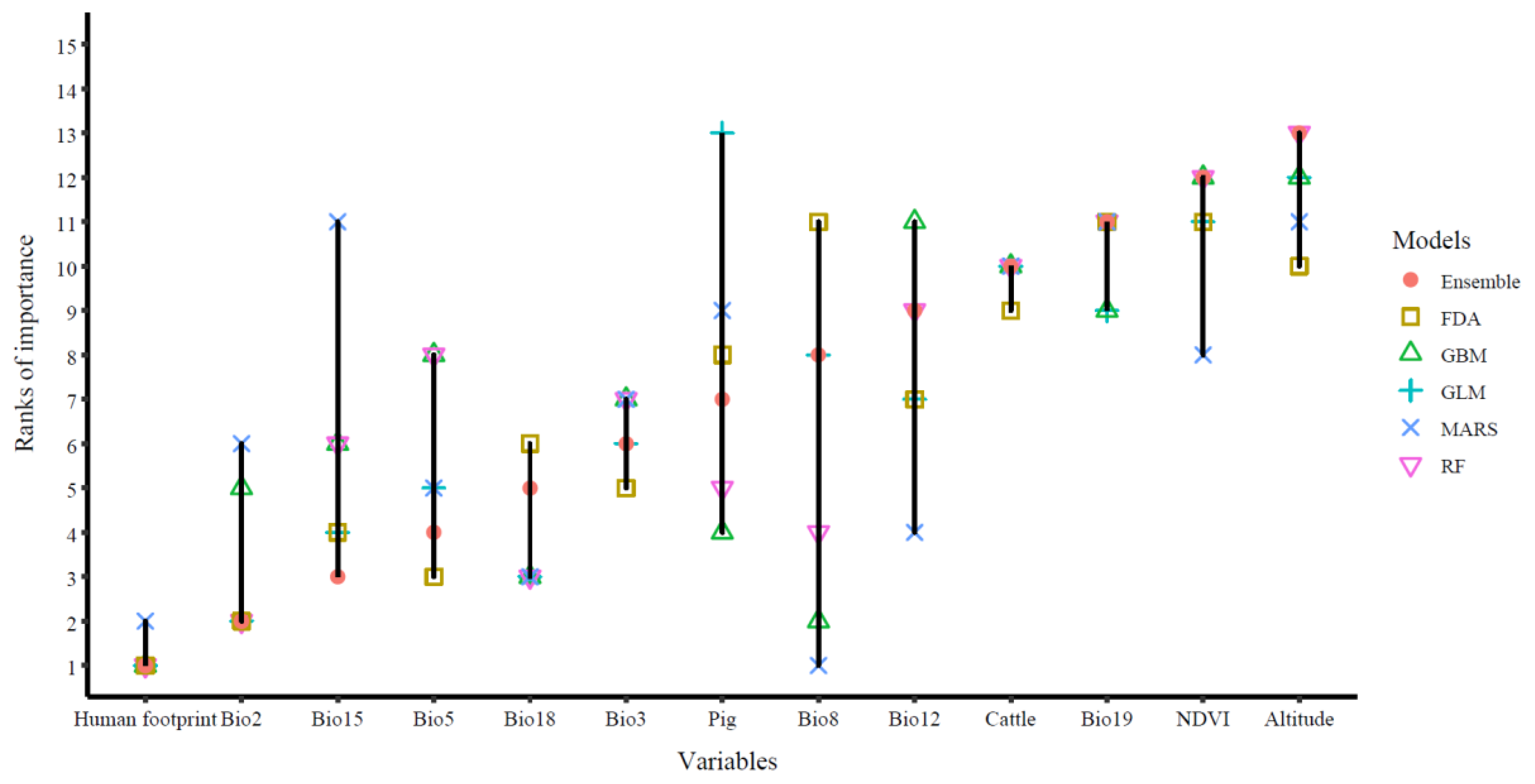
3.3. Variable Importance
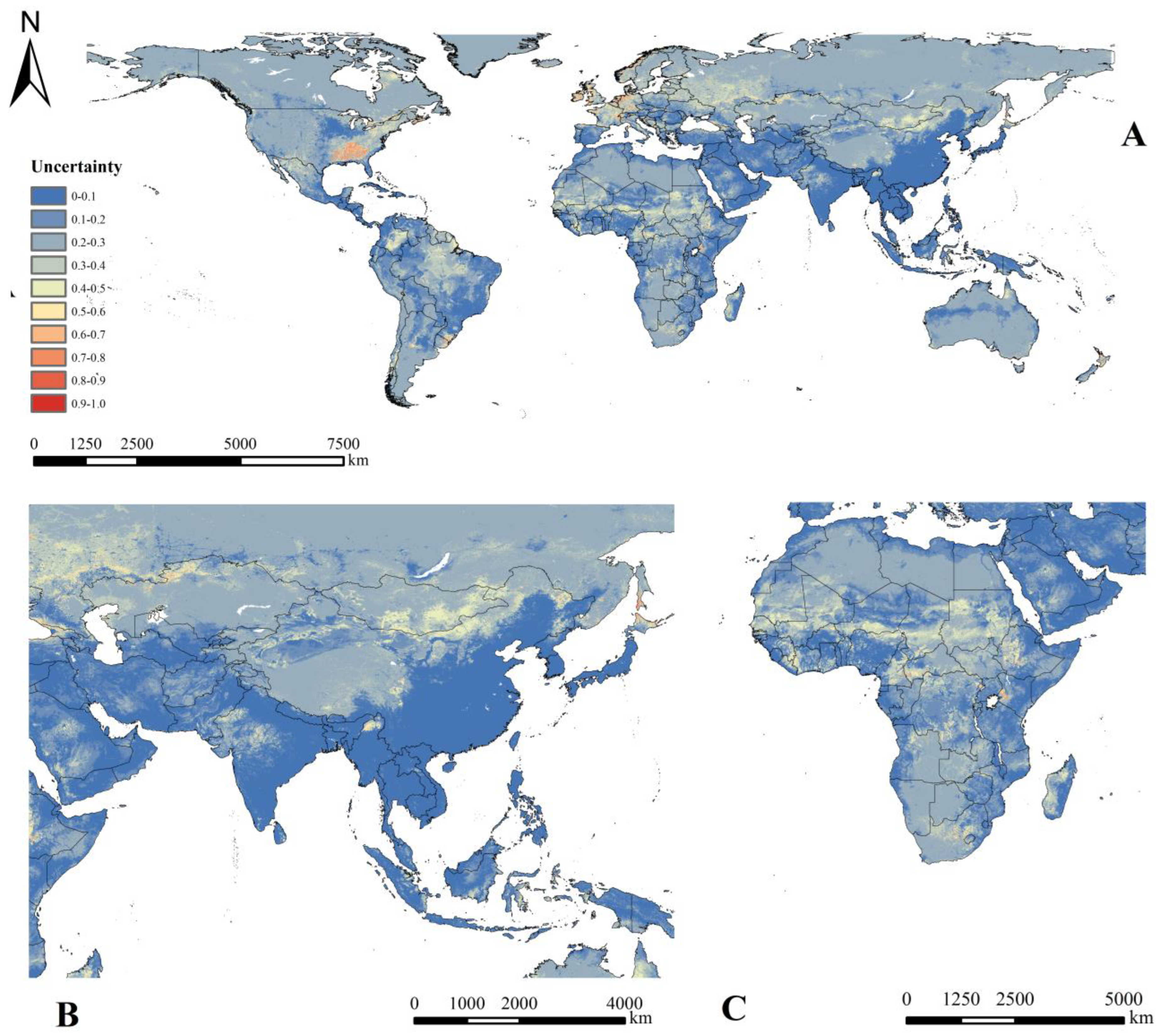
3.4. Predicted Current Geographical Distribution of Cx. tritaeniorhynchus
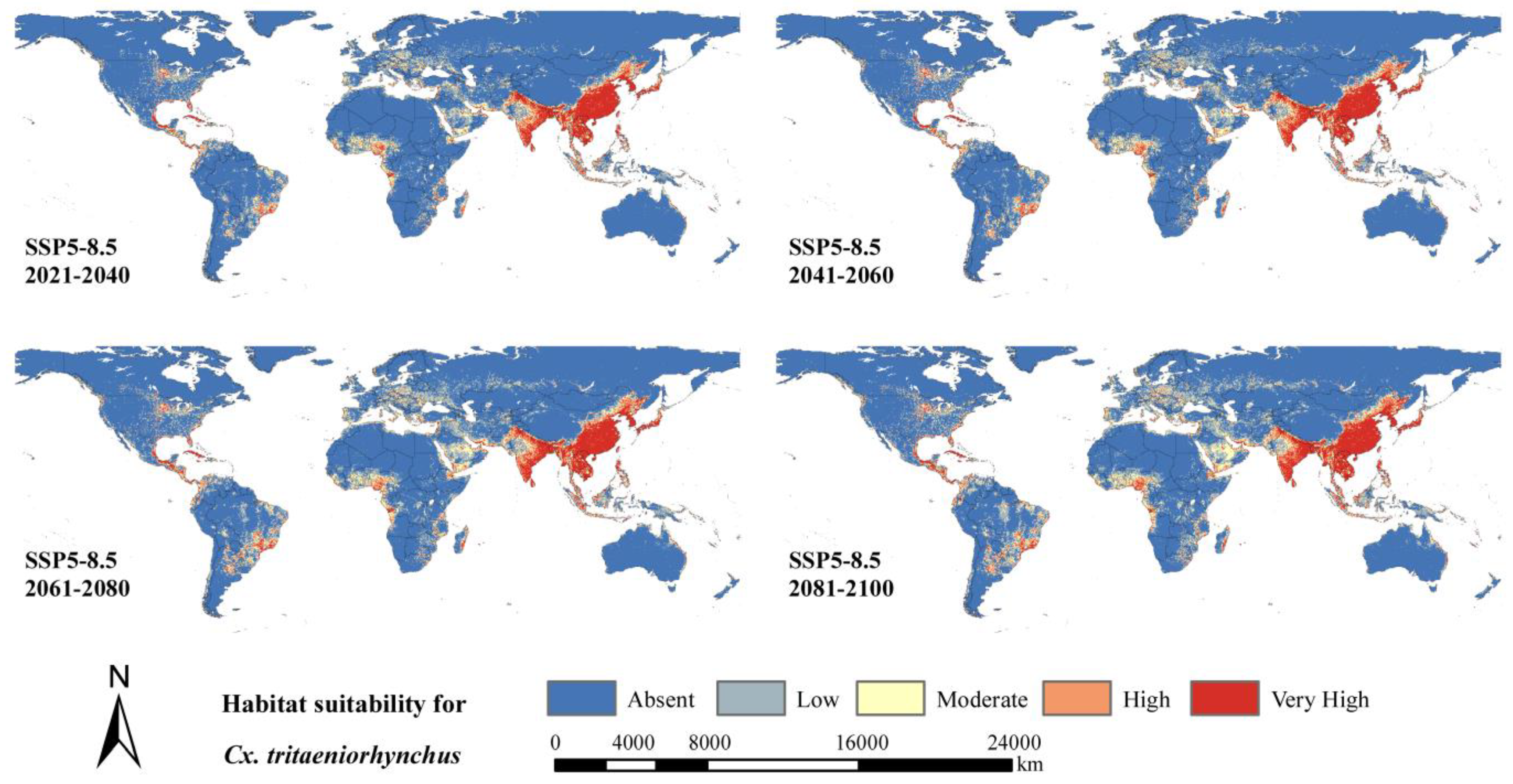
3.5. Predicted Future Geographical Distribution of Cx. tritaeniorhynchus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- World Health Organisation. The World Health Report 2004: Changing History; World Health Organisation: Geneva, Switzerland, 2004. [Google Scholar]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F. New initiatives for the control of Japanese encephalitis by vaccination: Minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 2000, 18 (Suppl. S2), 1–25. [Google Scholar] [CrossRef]
- Fischer, M.; Hills, S.; Staples, E.; Johnson, B.; Yaich, M.; Solomon, T.; Scheld, W.M.; Hammer, S.M.; Hughes, J.M. Japanese encephalitis prevention and control: Advances, challenges, and new initiatives. Emerg. Infect. 2008, 8, 93–124. [Google Scholar]
- Shi, Z.; Wei, J.; Deng, X.; Li, S.; Qiu, Y.; Shao, D.; Li, B.; Zhang, K.; Xue, F.; Wang, X.; et al. Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol. J. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Longbottom, J.; Browne, A.J.; Pigott, D.M.; Sinka, M.E.; Golding, N.; Hay, S.I.; Moyes, C.L.; Shearer, F.M. Mapping the spatial distribution of the Japanese encephalitis vector, Culex tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within areas of Japanese encephalitis risk. Parasites Vectors 2017, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeem, M.; Al-Ahmed, A.; Kheir, S.M.; Sallam, M. Spatial distribution modeling of Stegomyia aegypti and Culex tritaeniorhynchus (Diptera: Culicidae) in Al-Bahah Province, Kingdom of Saudi Arabia. Trop. Biomed. 2016, 33, 295–310. [Google Scholar]
- Kanojia, P.C. Ecological study on mosquito vectors of Japanese encephalitis virus in Bellary district, Karnataka. Indian J. Med. Res. 2007, 126, 152–157. [Google Scholar]
- Sanisuriwong, J.; Yurayart, N.; Thontiravong, A.; Tiawsirisup, S. Vector competence of Culex tritaeniorhynchus and Culex quinquefasciatus (Diptera: Culicidae) for duck Tembusu virus transmission. Acta Trop. 2021, 214, 105785. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Shin, E.H.; Lee, D.-K.; Ahn, Y.J.; Chang, K.-S.; Kim, H.-K.; Kim, S.-Y.; Park, C. Insecticide susceptibility of field-collected populations of Culex tritaeniorhynchus in the Republic of Korea. J. Insect Sci. 2013, 13, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Jiang, F.; Lu, H.; Kang, X.; Cui, F. Mosquito Diversity and Population Genetic Structure of Six Mosquito Species From Hainan Island. Front. Genet. 2020, 11, 602863. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Maltese, M.F.; Erlanger, T.E.; Bos, R.; Tanner, M.; Singer, B.H.; Utzinger, J. Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop. 2005, 95, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Lytra, I.; Emmanouel, N. Study of Culex tritaeniorhynchus and species composition of mosquitoes in a rice field in Greece. Acta Trop. 2014, 134, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hall-Mendelin, S.; Jansen, C.C.; Cheah, W.Y.; Montgomery, B.L.; Hall, R.A.; Ritchie, S.A.; Van Den Hurk, A.F. Culex annulirostris (Diptera: Culicidae) Host Feeding Patterns and Japanese Encephalitis Virus Ecology in Northern Australia. J. Med. Entomol. 2012, 49, 371–377. [Google Scholar] [CrossRef]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of Climate, Landscape, and Viral Genetics as Drivers of the Japanese Encephalitis Virus Ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef] [Green Version]
- Ilkal, M.A.; Dhanda, V.; Rao, B.U.; George, S.; Mishra, A.C.; Prasanna, Y.; Gopalkrishna, S.; Pavri, K.M. Absence of viraemia in cattle after experimental infection with Japanese encephalitis virus. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 628–631. [Google Scholar] [CrossRef]
- Gajanana, A.; Rajendran, R.; Samuel, P.P.; Thenmozhi, V.; Tsai, T.F.; Kimura-Kuroda, J.; Reuben, R. Japanese encephalitis in south Arcot district, Tamil Nadu, India: A three-year longitudinal study of vector abundance and infection frequency. J. Med. Entomol. 1997, 34, 651–659. [Google Scholar] [CrossRef]
- Lord, J.S.; Alamin, H.M.; Chakma, S.; Alam, M.S.; Gurley, E.S.; Pulliam, J. Sampling Design Influences the Observed Dominance of Culex tritaeniorhynchus: Considerations for Future Studies of Japanese Encephalitis Virus Transmission. PLoS Negl. Trop. Dis. 2016, 10, e0004249. [Google Scholar] [CrossRef] [Green Version]
- Echeverry-Cárdenas, E.; López-Castaeda, C.; Carvajal-Castro, J.D.; Obando, O. Potential geographic distribution of the Tiger Mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) in current and future conditions for Colombia. PLoS Negl. Trop. Dis. 2020, 15, e0008212. [Google Scholar] [CrossRef]
- Asigau, S.; Parker, P.G. The influence of ecological factors on mosquito abundance and occurrence in Galápagos. J. Vector Ecol. 2018, 43, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.H.; Masuoka, P.; Klein, T.A.; Kim, H.C.; Somer, T.; Grieco, J. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl. Trop. Dis. 2012, 6, e1678. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, E.; Jaiteh, M.; Levy, M.; Redford, K.; Wannebo, A.; Woolmer, G. The human footprint and the last of the wild: The human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Wang, H. Influence of Host and Environmental Factors on the Distribution of the Japanese Encephalitis Vector Culex tritaeniorhynchus in China. Int J. Environ. Res. Public Health 2018, 15, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongoh, V.; Hoen, A.G.; Aenishaenslin, C.; Waaub, J.P.; Michel, P. Spatially explicit multi-criteria decision analysis for managing vector-borne diseases. Int. J. Health Geogr. 2011, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leta, S.; Fetene, E.; Mulatu, T.; Amenu, K.; Jaleta, M.B.; Beyene, T.J.; Negussie, H.; Revie, C.W. Modeling the global distribution of Culicoides imicola: An Ensemble approach. Sci. Rep. 2019, 9, 14187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obenauer, J.F.; Andrew Joyner, T.; Harris, J.B. The importance of human population characteristics in modeling Aedes aegypti distributions and assessing risk of mosquito-borne infectious diseases. Trop. Med. Health 2017, 45, 38. [Google Scholar] [CrossRef] [Green Version]
- Dagtekin, D.; Şahan, E.A.; Denk, T.; Köse, N.; Dalfes, H.N. Past, present and future distributions of Oriental beech (Fagus orientalis) under climate change projections. PLoS ONE 2020, 15, e0242280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, W.; Wu, X.; Huang, S.; Deng, Z.; Zeng, X.; Yuan, D.; Yang, Y.; Wu, Z.; Chen, Y.; et al. Prediction of the potential global distribution for Biomphalaria straminea, an intermediate host for Schistosoma mansoni. PLoS Negl. Trop. Dis. 2018, 12, e0006548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, T.V.; Kim, W.; Nguyen-Tien, T.; Lindahl, J.; Nguyen-Viet, H.; Thi, N.Q.; Nguyen, H.V.; Unger, F.; Lee, H.S. Spatial distribution of Culex mosquito abundance and associated risk factors in Hanoi, Vietnam. PLoS Negl. Trop. Dis. 2021, 15, e0009497. [Google Scholar] [CrossRef] [PubMed]
- Shoraka, H.R.; Sofizadeh, A.; Mehravaran, A. Larval habitat characteristics and predicting the distribution of Culex tritaeniorhynchus using maximum entropy (MaxEnt) modelin Golestan Province (north of Iran). J. Vector Borne Dis. 2020, 57, 259–267. [Google Scholar]
- Masuoka, P.; Klein, T.A.; Kim, H.C.; Claborn, D.M.; Achee, N.; Andre, R.; Chamberlin, J.; Small, J.; Anyamba, A.; Lee, D.K. Modeling the distribution of Culex tritaeniorhynchus to predict Japanese encephalitis distribution in the Republic of Korea. Geospat. Health 2010, 5, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preziuso, S.; Mari, S.; Mariotti, F.; Rossi, G. Detection of Japanese Encephalitis Virus in bone marrow of healthy young wild birds collected in 1997–2000 in Central Italy. Zoonoses Public Health 2018, 65, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Patsoula, E.; Beleri, S.; Vakali, A.; Pervanidou, D.; Tegos, N.; Nearchou, A.; Daskalakis, D.; Mourelatos, S.; Hadjichristodoulou, C. Records of Aedes albopictus (Skuse, 1894) (Diptera; Culicidae) and Culex tritaeniorhynchus (Diptera; Culicidae) Expansion in Areas in Mainland Greece and Islands. Vector-Borne Zoonotic Dis. 2017, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.; Miranda, L.; Borges, R.; Saraiva, A.; Giannini, T. The Need of Species Distribution Models Metadata: Using Species Distribution Model to Address Decision Making on Climate Change. Biodivers. Inf. Sci. Stand. 2018, 2, e25478. [Google Scholar] [CrossRef]
- Angel, S.; Gonzalez, S.C.; Reed, D.L. Population distribution models: Species distributions are better modeled using biologically relevant data partitions. BMC Ecol. 2011, 11, 20. [Google Scholar]
- Tsoar, A.; Allouche, O.; Steinitz, O.; Rotem, D.; Kadmon, R. A comparative evaluation of presence-only methods for modeling species distribution. Divers. Distrib. 2007, 13, 397–405. [Google Scholar] [CrossRef]
- Machado, G.; Korennoy, F.; Alvarez, J.; Picasso-Risso, C.; Perez, A.; VanderWaal, K. Mapping changes in the spatiotemporal distribution of lumpy skin disease virus: Fitted RR spacextime. Transbound. Emerg. Dis. 2019, 66, 2045–2057. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Xia, C.; Hu, Y.; Ward, M.P.; Lynn, H.; Li, S.; Zhang, J.; Hu, J.; Xiao, S.; Lu, C.; Li, S.; et al. Identification of high-risk habitats of Oncomelania hupensis, the intermediate host of schistosoma japonium in the Poyang Lake region, China: A spatial and ecological analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007386. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2010, 32, 369–373. [Google Scholar] [CrossRef]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Hay, S.I. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife 2014, 3, e04395. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [Green Version]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2010, 36, 2290–2299. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2010, 25, 1965–1978. [Google Scholar] [CrossRef]
- Sallam, M.F.; Xue, R.D.; Pereira, R.M.; Koehler, P.G. Ecological niche modeling of mosquito vectors of West Nile virus in St. John’s County, Florida, USA. Parasites Vectors 2016, 9, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, P.M.; Sousa, C.A.; Seixas, J.; Lopes, P.; Novo, M.T.; Almeida, A. Anopheles atroparvus Density Modeling using MODIS NDVI in a Former Malarious Area in Portugal. J. Vector Ecol. 2011, 36, 279–291. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.M.R.; Gu, X.; Huang, Q.; Zhang, J.; Bai, W. Suitable habitat prediction of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) and its implications for conservation in Baihe Nature Reserve, Sichuan, China. Environ. Sci. Pollut. Res. 2019, 26, 32374–32384. [Google Scholar] [CrossRef]
- Hiroaki, T.; Tomoo, O.; Tomoko, N.; Yoshiki, K.; Koji, O.; Toshihiko, T.; Kengo, S.; Miho, S.; Manabu, A.; Fuyuki, S. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. Discuss. 2019, 12, 2727–2765. [Google Scholar]
- Mishra, V.; Kumar, D.; Ganguly, A.R.; Sanjay, J.; Mujumdar, M.; Krishnan, R.; Shah, R.D. Reliability of regional and global climate models to simulate precipitation extremes over India. J. Geophys. Res. Atmos. 2015, 119, 9301–9323. [Google Scholar] [CrossRef]
- Ayugi, B.; Shilenje, Z.W.; Babaousmail, H.; Lim Kam Sian, K.T.C.; Mumo, R.; Dike, V.N.; Iyakaremye, V.; Chehbouni, A.; Ongoma, V. Projected changes in meteorological drought over East Africa inferred from bias-adjusted CMIP6 models. Nat. Hazards 2022, 113, 1151–1176. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Kadmon, T.R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2010, 15, 59–69. [Google Scholar] [CrossRef]
- Early, R.; Rwomushana, I.; Chipabika, G.; Day, R. Comparing, evaluating and combining statistical species distribution models and CLIMEX to forecast the distributions of emerging crop pests. Pest Manag. Sci. 2022, 78, 671–683. [Google Scholar] [CrossRef]
- Lucas-Picher, P.; Laprise, R.; Winger, K. Evidence of added value in North American regional climate model hindcast simulations using ever-increasing horizontal resolutions. Clim. Dyn. 2017, 48, 2611–2633. [Google Scholar] [CrossRef] [Green Version]
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance 2012, 17, 20221. [Google Scholar] [CrossRef] [Green Version]
- van den Hurk, A.F.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses 2022, 14, 1208. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casademont, I.; Fall, G.; Fernandez-Garcia, M.D.; Diagne, M.M.; Kipela, J.M.; Fall, I.S.; Holmes, E.C.; et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N. Engl. J. Med. 2017, 376, 1483–1485. [Google Scholar] [CrossRef]
- Khan, S.U.; Ogden, N.H.; Fazil, A.A.; Gachon, P.H.; Dueymes, G.U.; Greer, A.L.; Ng, V. Current and Projected Distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S. Environ. Health Perspect. 2020, 128, 57007. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000-2015. eLife 2020, 9, e51027. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; Hassan, H.K.; Eubanks, M.; Cupp, E.W.; Unnasch, T.R. Winter severity predicts the timing of host shifts in the mosquito Culex erraticus. Biol. Lett. 2012, 8, 567. [Google Scholar] [CrossRef] [Green Version]
- Proestos, Y.; Christophides, G.K.; Ergüler, K.; Tanarhte, M.; Waldock, J.; Lelieveld, J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. Lond. 2015, 370, 219–222. [Google Scholar]
- Roche, B.; Rohani, P.; Dobson, A.P.; Guégan, J.F. The impact of community organization on vector-borne pathogens. Am. Nat. 2013, 181, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M. The effects of environmental and physiological conditions of Culex tritaeniorhynchus on the pattern of transmission of Japanese encephalitis virus. J. Med. Entomol. 1976, 13, 275–284. [Google Scholar] [CrossRef]
- Tuno, N.; Tsuda, Y.; Takagi, M. How Zoophilic Japanese Encephalitis Vector Mosquitoes Feed on Humans. J. Med. Entomol. 2017, 54, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murty, U.S.; Rao, M.S.; Arunachalam, N. The effects of climatic factors on the distribution and abundance of Japanese encephalitis vectors in Kurnool district of Andhra Pradesh, India. J. Vector Borne Dis. 2010, 47, 26–32. [Google Scholar]
- Ciota, A.T.; Chin, P.A.; Ehrbar, D.J.; Micieli, M.V.; Fonseca, D.M.; Kramer, L.D. Differential Effects of Temperature and Mosquito Genetics Determine Transmissibility of Arboviruses by Aedes aegypti in Argentina. Am. J. Trop. Med. Hyg. 2018, 99, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.C.; Kanamori, S.; Kannady, K.; Mkude, S.; Killeen, G.F.; Fillinger, U. The importance of drains for the larval development of lymphatic filariasis and malaria vectors in Dar es Salaam, United Republic of Tanzania. PLoS Negl. Trop. Dis. 2010, 4, e693. [Google Scholar] [CrossRef]
- Bashar, K.; Rahman, M.S.; Nodi, I.J.; Howlader, A.J. Species composition and habitat characterization of mosquito (Diptera: Culicidae) larvae in semi-urban areas of Dhaka, Bangladesh. Pathog. Glob. Health 2016, 110, 48–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynès, C.; Sabatier, R.; Molinari, N. Choice of B-splines with free parameters in the flexible discriminant analysis context. Comput. Stat. Data Anal. 2006, 51, 1765–1778. [Google Scholar] [CrossRef]
- Menon, R. Multivariate adaptive regression splines analysis to predict biomarkers of spontaneous preterm birth. Acta Obs. Gynecol. Scand. 2013, 208, S210. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, Q. Tolerance niche expansion and potential distribution prediction during Asian openbill bird range expansion. Ecol. Evol. 2021, 11, 5562–5574. [Google Scholar] [CrossRef]
- Lei, J.; Chen, L.; Li, H. Using ensemble forecasting to examine how climate change promotes worldwide invasion of the golden apple snail (Pomacea canaliculata). Environ. Monit. Assess. 2017, 189, 404. [Google Scholar] [CrossRef]
- Deka, M.A.; Morshed, N. Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia. Trop. Med. Infect. Dis. 2018, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W. Editorial commentary on ‘BIOMOD—Optimizing predictions of species distributions and projecting potential future shifts under global change’. Glob. Chang. Biol. 2014, 20, 3591–3592. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.; Thuiller, W.; Williams, P.; Reginster, I. Downscaling European species atlas distributions to a finer resolution. Glob. Ecol. Biogeogr. 2005, 14, 17–30. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Liu, Y. ModEco: An integrated software package for ecological niche modeling. Ecography 2010, 33, 637–642. [Google Scholar] [CrossRef]


| Variables | Description | Sources |
|---|---|---|
| Bio2 | Mean diurnal temperature range | WorldClim database (http://www.worldclim.org/ (accessed on 31 August 2021) |
| Bio3 | Isothermality | |
| Bio5 | Max temperature of warmest month | |
| Bio8 | Mean temperature of wettest quarter | |
| Bio12 | Annual precipitation | |
| Bio15 | Precipitation seasonality | |
| Bio18 | Precipitation of warmest quarter | |
| Bio19 | Precipitation of coldest quarter | |
| Altitude | Altitude from DEM | Global Land One-km Base Elevation Project (GLOBE) (https://www.ngdc.noaa.gov/mgg/topo/globe.html (accessed on 31 August 2021) |
| NDVI | SPOT VEGETATION Images from spectral bands (SWIR, NIR, RED, BLUE). | https://www.vito-eodata.be/ (accessed on 31 August 2021) |
| Human footprint | Anthropogenic impacts on the environment | Wildlife Conservation Society (WCS) and Center for International Earth Science Information Network (CIESIN) (http://sedac.ciesin.columbia.edu/data/collection/wildareas-v2 (accessed on 31 August 2021) |
| Pig | Global livestock distribution | Global pig density grid data (http://www.geo-wiki.org (accessed on 31 August 2021) |
| Cattle | Global livestock distribution | Global cattle density grid data (http://www.geo-wiki.org (accessed on 31 August 2021) |
| Scenario | Period | Area of Different Habitat Suitability/Km2 (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Low | Moderate | High | Very High | ||
| Current | 11,041 (81.8) | 983 (7.3) | 513 (3.8) | 377 (2.8) | 586 (4.3) | |
| SSP1-2.6 | 2021–2040 | 10,711 (79.3) | 1129 (8.4) | 584 (4.3) | 436 (3.2) | 640 (4.7) |
| 2041–2060 | 10,662 (79.0) | 1150 (8.5) | 595 (4.4) | 444 (3.3) | 650 (4.8) | |
| 2061–2080 | 10,694 (79.2) | 1134 (8.4) | 587 (4.3) | 446 (3.3) | 639 (4.7) | |
| 2081–2100 | 10,733 (79.5) | 1129 (8.4) | 573 (4.2) | 435 (3.2) | 630 (4.7) | |
| SSP5-8.5 | 2021–2040 | 10,692 (79.2) | 1135 (8.4) | 594 (4.4) | 439 (3.3) | 640 (4.7) |
| 2041–2060 | 10,507 (77.8) | 1218 (9.0) | 625 (4.6) | 476 (3.5) | 674 (5.0) | |
| 2061–2080 | 10,279 (76.1) | 1321 (9.8) | 684 (5.1) | 512 (3.8) | 703 (5.2) | |
| 2081–2100 | 9995 (74.0) | 1451 (10.7) | 752 (5.6) | 553 (4.1) | 749 (5.5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Jiang, H.; Xu, N.; Wang, Z.; Xiong, Y.; Yin, J.; Huang, J.; Chen, Y.; Jiang, Q.; Zhou, Y. Global Distribution of Culex tritaeniorhynchus and Impact Factors. Int. J. Environ. Res. Public Health 2023, 20, 4701. https://doi.org/10.3390/ijerph20064701
Tong Y, Jiang H, Xu N, Wang Z, Xiong Y, Yin J, Huang J, Chen Y, Jiang Q, Zhou Y. Global Distribution of Culex tritaeniorhynchus and Impact Factors. International Journal of Environmental Research and Public Health. 2023; 20(6):4701. https://doi.org/10.3390/ijerph20064701
Chicago/Turabian StyleTong, Yixin, Honglin Jiang, Ning Xu, Zhengzhong Wang, Ying Xiong, Jiangfan Yin, Junhui Huang, Yue Chen, Qingwu Jiang, and Yibiao Zhou. 2023. "Global Distribution of Culex tritaeniorhynchus and Impact Factors" International Journal of Environmental Research and Public Health 20, no. 6: 4701. https://doi.org/10.3390/ijerph20064701





