1. Introduction
Patients with eating disorders require indepth diagnostics and multispecialty treatment. In the case of many occurring symptoms, a somatic background has to be ruled out, and both concomitant ailments and the effects of mental disorders need to be diagnosed and treated [
1].
Eating disorders are a group of mental illnesses manifested by a distorted perception of oneself, food, unusual eating and hygiene habits, and regurgitating digestive tract contents. The major diseases in this group include anorexia (anorexia nervosa), bulimia (bulimia nervosa), and other unclassified eating disorders. The symptoms of eating disorders may also occur in the course of other mental illnesses. These symptoms can be found in the case of mood disorders, neuroses, and anxiety disorders, as well as in patients suffering from addiction to alcohol or drugs [
1,
2,
3,
4].
The role of the dentist in recognizing these clinical problems is important since through a properly conducted interview and a detailed extraoral and intraoral examination, he may be the first physician to recognize the symptoms of an ongoing systemic disease. On the other hand, knowledge of the possible effects of eating disorders in the mouth as the first section of the gastrointestinal and respiratory tracts, and their impact on the local and general condition of the patient, should be part of the education of psychiatrists and psychologists, prompting them to include dental consultation in the management of patients [
5].
The social importance of eating disorders increases with the growing role of appearance and changes in beauty patterns in society and the increasing incidence of mental illnesses with eating disorder symptoms in industrialized countries [
6,
7]. The majority of patients are women (approx. 90%), and the overall incidence of these diseases is 0.5 to 1%. For bulimia alone, these values are 1–5%, and also 40 to 50% of patients with anorexia suffer from bulimia [
2,
8,
9,
10].
The causes of eating disorders are not fully understood. Genetic, cultural, and psychological factors appear to affect their presence. The origin is also seen in the early childhood of the patient—problems arise both from eating habits used by caregivers and unpleasant experiences from this period. Some nonspecific risk factors that increase susceptibility to mental problems leading to eating disorders include mental and sexual abuse and personality disorders in the family. People with low self esteem, those with difficulty expressing negative emotions, perfectionists, athletes, and dancers are also prone to these diseases [
11]. The genetic factor is also significant in the development of this type of disorder [
10].
Anorexia nervosa is characterized by a disturbed body image and a fatal fear of increasing its mass, regardless of the actual weight, which is often far too low (body weight is at least 15% below the expected normal weight for certain ages and heights). Anorexia is more common in women than men. This disorder consists of undertaking numerous, purposeful actions leading to weight loss and maintaining low body weight in the patient. Somatic, metabolic, or neuroendocrine disorders are added with time over the course of the disease [
8,
12].
Bulimia nervosa is unrestrained attacks of hunger, followed by compensatory behavior, aimed at getting rid of consumed food and avoiding weight gain by taking laxatives, inducing vomiting, using appetite suppressants, and many more [
8,
12].
Bulimia or anorexia may be the only diagnosis in a patient, however, more often they coexist with other mental disorders such as depression or obsessions, or are part of their symptoms, e.g., when symptoms of eating disorders occur in the course of personality disorders [
2,
3,
8].
Symptoms of nutrition-related abnormalities occur in various forms, e.g., eating disorders, aversion, eating disgust, habitual food regurgitation, digestive disorders with abdominal ailments, and stomachache, flatulence, constipation, or diarrhea [
1,
6]
Eating disorders can lead to serious metabolic and morphological problems, as well as changes in numerous organs and systems, and sometimes even to a situation that is a threat to a patient’s life [
13].
Abnormal eating habits and vomiting can also lead to bradycardia, hypothermia, or hypotension. Anorectic patients with low body weight may have an abnormal or complete loss of menstruation and changes in the ovaries, and both sexes may experience growth retardation and alopecia. In bulimia, gastric contents can be aspirated into the respiratory tract with vomiting, gastric or esophageal ruptures, hypokalemia, arrhythmia, pancreatitis, or drug-induced myopathy, and cardiomyopathy may develop. Patients causing vomiting using fingers may present Russell’s sign, which consists of thickening and redness of the skin of the fingers or hands at the place of contact with the upper incisors [
9,
14,
15,
16].
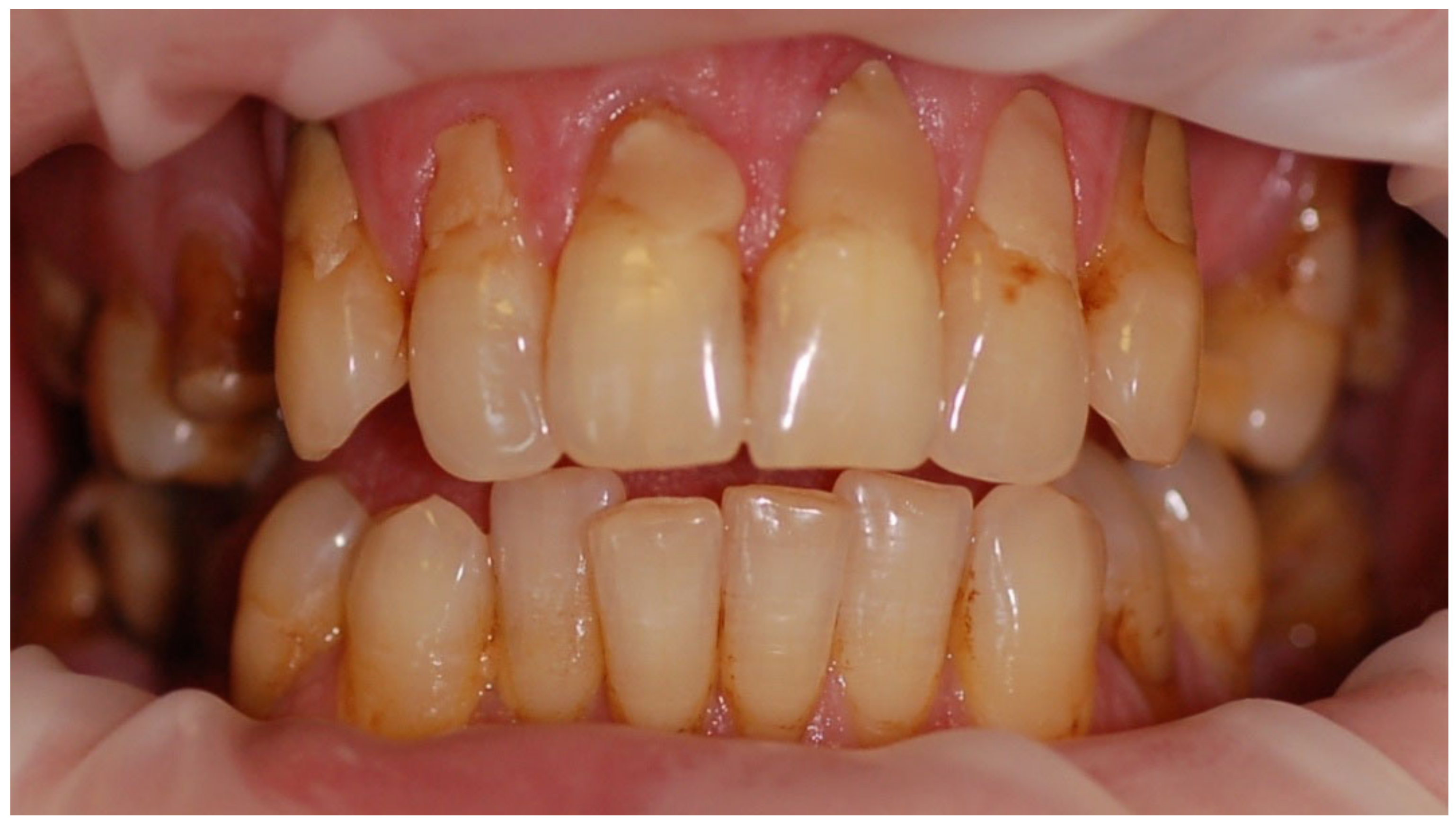
Symptoms of eating disorders in the oral cavity may occur at any stage of the disease and constitute an important indicator in assessing its course, prognosis, and treatment.
The impact of eating disorders on the soft and hard tissues of the mouth depends on both the diet and the duration and intensity of the disease. The occurring symptoms are mostly caused by the irritating effect of vomiting, nutritional deficiencies resulting in metabolic disorders, unusual dietary and hygienic habits, and improper oral hygiene [
15,
16].
The erosion of enamel and dentin is one of the most frequently observed symptoms within the oral hard tissues [
15,
17,
18]. We observe a phenomenon described as perimylolysis, which is caused by vomiting, gastroesophageal reflux, or belching, the characteristic site for the chemical loss of tooth tissues. The presence of acid of internal origin causes the formation of hard dental tissue defects covering the palatal surfaces of the upper incisors and chewing surfaces of molars and premolars [
19]. If the hygiene is not correct and the patient often brushes the teeth immediately after vomiting, the spread of acid and erosion also occurs in other areas of the oral cavity. The supply of external acids by patients in the form of drugs and specifics designed to reduce appetite (e.g., drinking vinegar or sucking a lemon), consumption of large quantities of carbonated drinks and alcohol, or large quantities of energy and isotonic drinks at increased physical activity, are additional factors intensifying this process [
20,
21,
22,
23]. The presence of acids of external and internal origin, apart from erosion, results in tooth loss [
24]. As a result of significant progress in the process, the anterior teeth may become thinner and shorter and the crowns of the posterior teeth may be shortened until the complete loss of proper anatomical characteristics. The loss of tooth tissue may result in progressive hypersensitivity and, in extreme cases, pulp inflammation, with further consequences in the form of periapical tissue inflammation [
19].
A number of scientific studies have confirmed a higher incidence of tooth wear and erosion losses in patients with symptoms of eating disorders, however, there is no consensus on the relationship between the frequency of vomiting and the intensity of observed changes [
14].
Increased dental caries in patients with eating disorders is a broad clinical problem. The authors find no correlation between the occurrence of vomiting and the severity of caries. However, there is numerous evidence of more frequent caries-related defects in patients with eating disorder symptoms. This is associated with both a diet rich in simple carbohydrates, a decrease in saliva production, and the avoidance of visits to the dentist for fear of detecting the patient’s mental illness [
3,
25].
The literature also includes assessments of the impact of eating disorders on the incidence of periodontal disease [
25]. The described patients present poor oral hygiene leading to gingivitis and further predisposing to periodontal disease [
26]. Vitamin C deficiencies resulting from malnutrition may cause changes in the marginal periodontium and promote gingivitis. Oral cavity dryness associated with deteriorated salivary gland function may affect the condition of the periodontium and oral mucosa [
15,
27,
28]. Nutritional deficiencies can lead to inflammation of the corners of the mouth, tongue mucositis, a burning sensation in the oral cavity, and candidiasis or sores in the mouth. Reduced vitamin supply and iron deficiency anemia can cause mucosal atrophy and an associated burning sensation in the mouth, especially the tongue. As a result of vomiting, there may also be chemical or mechanical (caused by the object used to induce vomiting) mucosal damage [
14,
27,
29].
The occurrence of salivary gland enlargement preceding the belching and vomiting phase is described in the literature. Initially, the swelling of the glands is transient, and it passes into a persistent form with time. This noninflammatory enlargement of the salivary glands, called sialodenosis, is associated with peripheral autonomic neuropathy leading to abnormal secretory activity and enlargement of the salivary glands. These changes may affect small and large salivary glands. In the case of large salivary glands, they are most often bilateral [
28].
Initially, both in bulimia and anorexia, there is no quantitative change in saliva production, though there are a number of biochemical deviations in its composition and a decrease in pH [
16]. Advanced cases of these disorders lead to a decrease in the flow and amount of saliva, which may be associated with increasing structural changes in the glandular tissue. In addition, xerostomy is a common adverse effect of drugs used in the therapy of individual mental illnesses [
3,
29].
In patients using dehydrating and laxative agents, there is a variable extent of dehydration and reduced saliva secretion, and the pH decrease associated with the presence of gastric acid may cause pathological changes in the hard palate within the small salivary glands located in this area [
14].
Reducing salivation may also increase the susceptibility to both bacterial and fungal infections [
28].
Other possible symptoms in the oral cavity include a burning sensation in the mucous membrane, abnormal taste sensation, difficulties in speaking, chewing, and swallowing food, and oral cavity pain of unknown origin. In patients with advanced forms of anorexia, osteopenia, and later osteoporosis may also occur, which may also include the bones of the maxilla and the mandible [
3,
9,
14,
29,
30].
Aesthetic defects are no less important, but rarely mentioned complications of eating disorders [
25].
The main aim of the study was to determine the clinical and microbiological condition of the oral cavity,
The specific objectives of the study included:
assessment of the clinical condition of the oral cavity based on the oral hygiene API index and the DMF index;
assessment of the presence of dental erosion and gingival recession;
check the correlation of eating disorder symptoms in patients with the presence of erosion, recession, and the clinical condition of the oral cavity.
The level of oral hygiene in patients with eating disorders was assessed as sufficient or bad and it did not differ from the control group.
General symptoms of mental-related eating disorders were conducive to tooth erosion development.
2. Materials and Methods
2.1. General Methodology
The study was carried out in two stages.
In the first stage, among patients of the Day Hospital for Neurotic and Behavioral Disorders of the Psychotherapy Department in the University Hospital in Krakow, the study included patients with diagnoses from categories F4.xx, F5x.x, F6x.x of International Classification of Diseases, Tenth Revision (ICD-10), accompanied by the symptoms of eating disorders. Patients were qualified for the study based on the answers provided in the KO “O” symptom questionnaire, the so-called symptom checklists “O” [
31,
32].
From among 138 symptoms present in this questionnaire, 12 potential symptoms of various types of eating disorders were selected. Patients who reported the occurrence of at least one of the 12 analyzed symptoms, regardless of their severity, were analyzed. The questionnaires were evaluated regardless of the patient’s primary diagnosis.
The study included patients who had the following symptoms listed in the symptom checklists “O” [
31,
32]:
No. 3: Choking in the throat, a sensation of having a “ball in the throat”;
No. 9: Vomiting in situations of nervousness;
No. 49: Oral dryness;
No. 54: Lack of appetite;
No. 57: Constant attention to body functions—heart rate, pulse, digestion, etc.;
No. 59: Attacks of hunger—e.g., a necessity to eat at night;
No. 69: Diarrheas;
No. 74: Constipations;
No. 94: Excessive salivation in the mouth;
No. 98: Excessive thirst;
No. 131: Burning sensation in the esophagus, heartburn;
No. 136: Nausea, queasiness.
The study included 60 patients of the Psychotherapy Department in the University Hospital, a control group with the same number was selected similar in terms of gender and age. It consisted of patients of the University Dental Clinic, who, during a medical interview denied the presence of any mental illness presently and in the past. In the second stage, patients who qualified for the study group were admitted to the University Dental Clinic, where oral clinical examination and additional examinations were carried out.
2.2. Clinical Examination Methodology
Each patient underwent a dental examination according to generally accepted principles. Next, a detailed extraoral and intraoral examination was performed. In addition, periodontal and oral hygiene examinations were carried out using a WHO periodontometer. The oral examination was assessed based on the following indicators:
Lange’s API (aproximal plaque index) assesses the presence of bacterial plaque in interdental spaces [
32]. It is calculated as the percentage ratio of the space with the current bacterial plate to all the spaces examined. The presence of the plaque was checked using a WHO periodontal probe. The index value is determined as a percentage, and the results are interpreted as follows:
API 70–100%—insufficient oral hygiene;
API 40–69%—sufficient hygiene, but improvement is recommended;
API 25–39%—quite good oral hygiene;
API < 25%—optimal oral hygiene;
DMF—decayed missing filled indicator is an index of the intensity of the dental caries process, at the same time determining the incidence of this disease [
33]. It consists of the assessment of the following components:
D—teeth with one or several primary or secondary caries defects and with temporary dressing; M—teeth lost or removed due to caries; F—teeth with one or more fillings.
The DMF index is the sum of the individual components: DMF = D + M + F. A number of DMF greater than 0 indicates that the person is or has been affected by caries. This is indicated by the presence of only one filling (D = 0, M = 0, F = 1 so DMF = 1). On the other hand, the DMF number expressing the sum of these three values does not provide clear information about the tooth condition. In extreme cases, both toothless people, as well as those who have all the teeth, but each one of them filled, will have a DMF score of 32. Due to that, both the DMF sum and the values of individual components of the index and their impact on other parameters describing the condition of the oral cavity were analyzed. For every patient and every component, the DMF index can score from 1 to 32, giving too numerous options. So for the purpose of statistical analysis, the DMF index values were grouped into ranges (
Table 1).
During the clinical examination of the patients, attention was also paid to the presence of erosive defects and gingival recessions in the 0–1 system (a single defect or recession, regardless of the number of defects/recessions and their severity, meant the patient was assigned to the group affected by this problem).
The clinical examination was supplemented by a patient’s orthopantomographic scan, allowing the assessment of alveolar processes of the jaws, and the alveolar part of the mandible, along with teeth.
Each patient had photographic documentation prepared, including photos of a smile, closed teeth with retraction, and upper and lower arch with retraction.
2.3. Statistical Test Methodology
The results of clinical tests were systematized in a Microsoft Excel table.
The collected data was first developed using descriptive statistics tools and variable distributions (Chi-square test, F = Fisher’s exact test, Mann–Whitney test depending on the situation). When testing hypotheses, the significance level was adopted at α = 0.05.
Software R, version 3.4.3, was used for the analysis.
(R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Jagiellonian University Bioethics Committee approval and code:
KBET/65/B/2012 from 22 March 2012