Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. PECO Question
2.2. Sources of Information and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Screening and Data Charting
3. Results
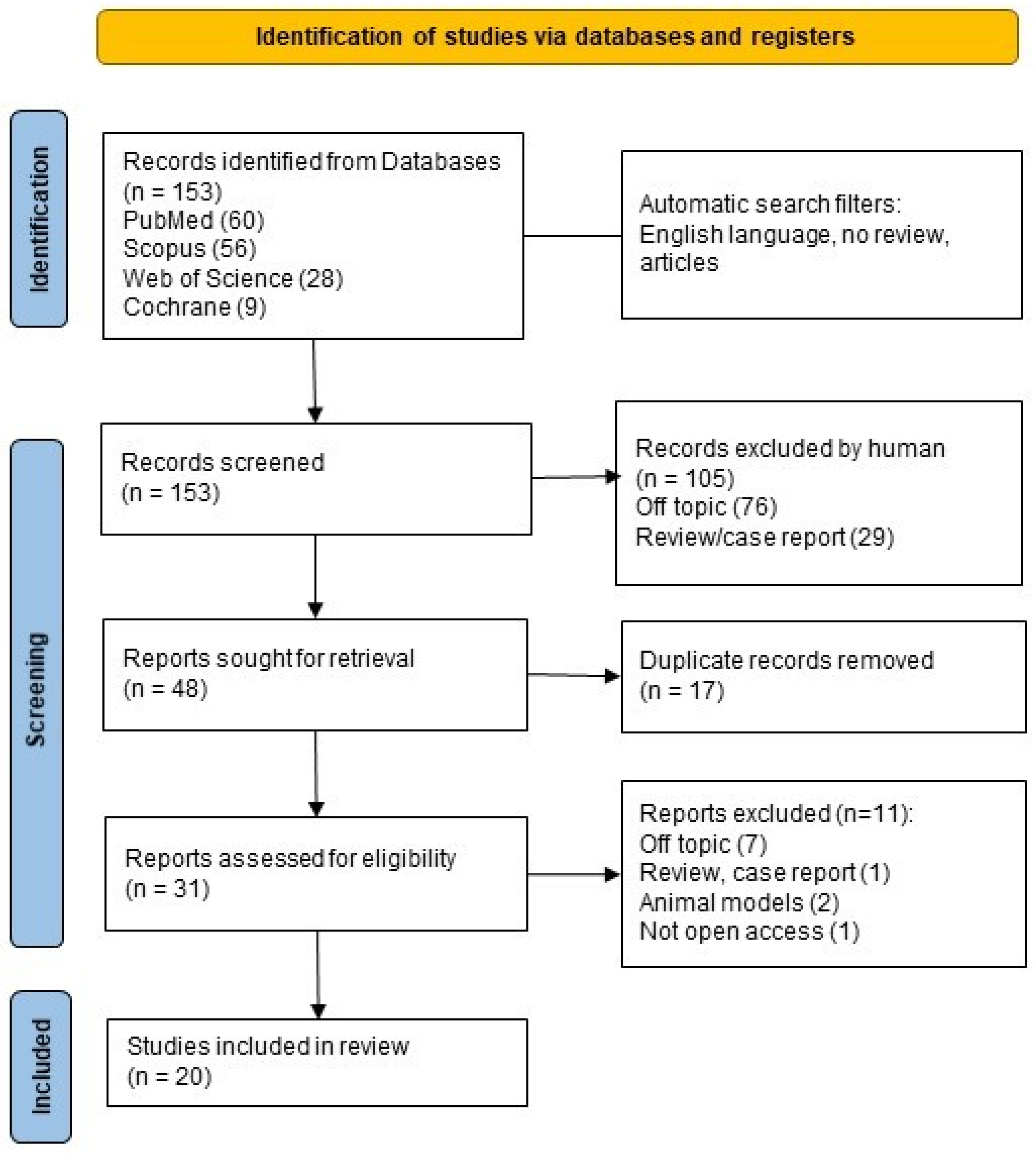
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Critical Appraisal within Sources of Evidence
3.4. Results of Individual Sources of Evidence
3.5. Synthesis of Results
4. Discussion
4.1. Summary of Evidence
4.1.1. Epidemiology of the Association between Thyroid Disease and Periodontitis
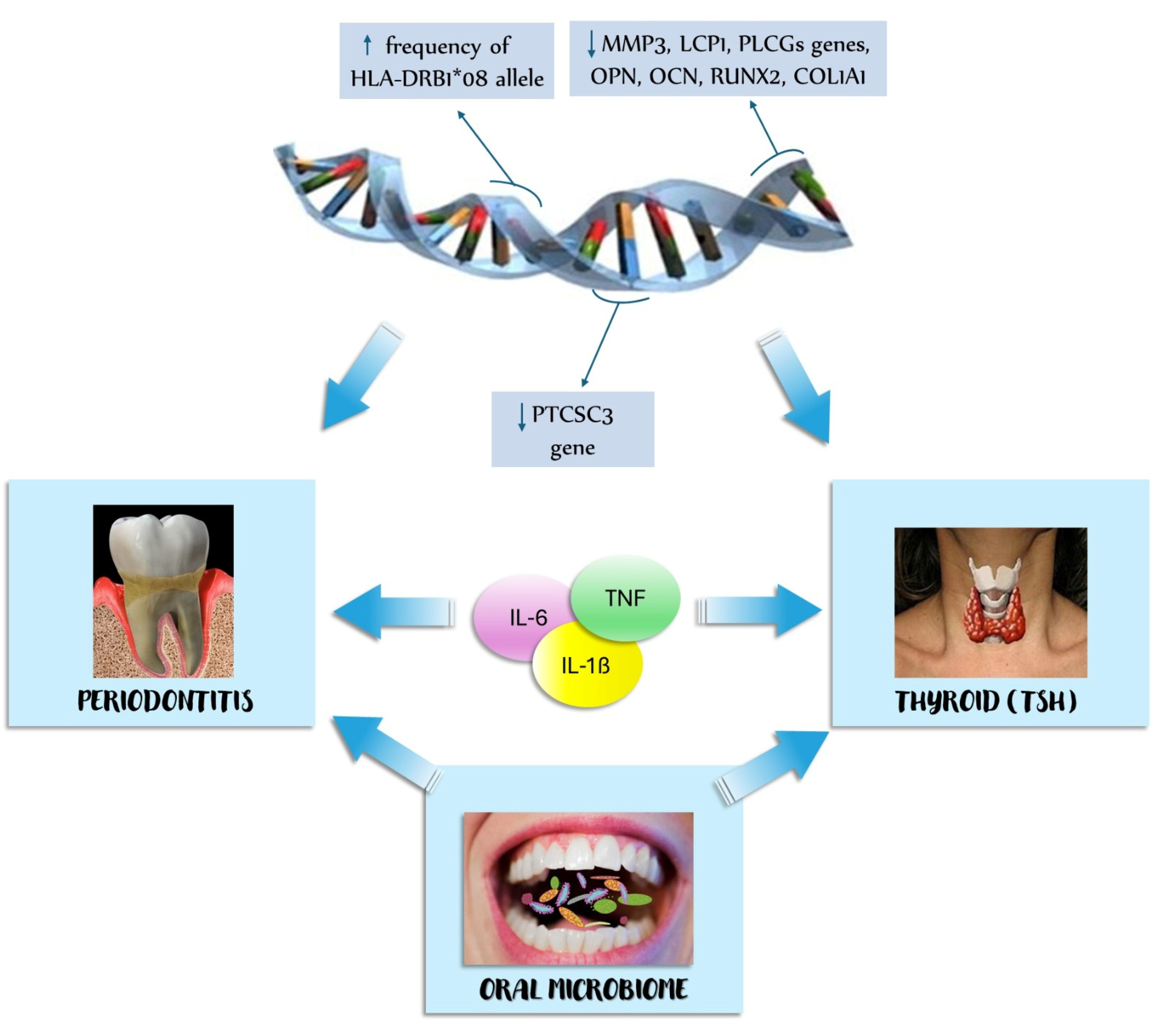
4.1.2. Pathogenesis of the Association between Thyroid Disease and Periodontitis
4.1.3. Genetic Factors
4.1.4. Oral Microbiome
4.1.5. Pro-Inflammatory Cytokines
4.1.6. The Effect of Periodontal Treatment on Thyroid Disease and the Effect of Thyroxine Replacement on Periodontitis
4.2. Limitations of the Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aHR | adjusted hazard ratio |
| COL1A1 | collagen type I alpha 1 chain |
| BOP | bleeding of probing |
| CAL | clinical attachment loss |
| GI | gingival index |
| HLA | human leukocyte antigen |
| IL | interleukine |
| MDA | malondialdehyde |
| NSPT | non-surgical periodontal treatment |
| OCN | osteocalcin |
| OPN | osteopontin |
| OR | odds ratio |
| PI | plaque index, |
| PPD | probing pocket depth |
| PDLSCs | periodontal ligament stem cells |
| PTCSC3 | candidate papillary thyroid carcinoma susceptibility gene 3 |
| RCT | randomized controlled trials |
| RUNX2 | RUNX family transcription factor 2 |
| SOD | superoxide dismutase |
| SRP | scaling and root planning |
| TLR4 | Toll-like receptor 4 |
| TNF | tumor necrosis factor |
| TSH | thyroid-stimulating hormone |
References
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, Fallacies and the Future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Glickman, I. Periodontal Disease. N. Engl. J. Med. 1971, 284, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Serra, P.; Francesco, I.; Di Carmine, M.; Tari, S.R.; Leo, L.; Lorusso, F. Current Status of Peri-Implant Diseases: A Clinical Review for Evidence-Based Decision Making. J. Funct. Biomater. 2023, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Songhee Song, K.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral. Biol. 2016, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Highfield, J. Diagnosis and Classification of Periodontal Disease. Aust Dent J. 2009, 54 (Suppl. 1), S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal Diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, B.K. Periodontal Ligament: Structural and Clinical Correlates. Dent. Update 2004, 31, 46–50, 52, 54. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The Intricate Anatomy of the Periodontal Ligament and Its Development: Lessons for Periodontal Regeneration. J. Periodontal. Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef]
- Page, R.C. Gingivitis. J. Clin. Periodontol 1986, 13, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F. Causation and Pathogenesis of Periodontal Disease. Periodontol. 2000 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Lovegrove, J.M. Dental Plaque Revisited: Bacteria Associated with Periodontal Disease. J. N. Z. Soc. Periodontol. 2004, 87, 7–21. [Google Scholar]
- Marsh, P.D.; Bradshaw, D.J. Dental Plaque as a Biofilm. J. Ind. Microbiol. 1995, 15, 169–175. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary Prevention of Periodontitis: Managing Gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S71–S76. [Google Scholar] [CrossRef]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Evidence-Based Update on Diagnosis and Management of Gingivitis and Periodontitis. Dent. Clin. North Am. 2019, 63, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Ray, R.R. Periodontitis: An Oral Disease with Severe Consequences. Appl. Biochem. Biotechnol. 2023, 195, 17–32. [Google Scholar] [CrossRef]
- Lamster, I.B.; Karabin, S.D. Periodontal Disease Activity. Curr. Opin. Dent. 1992, 2, 39–52. [Google Scholar]
- Elemek, E. Periodontal Disease Severity, Tooth Loss, and Periodontal Stability in Private Practice. Niger J. Clin. Pr. 2022, 25, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Scaravilli, M.S.; Ingenito, A. Dental and Periodontal Health Status in Campanian Children and Relation between Caries Experience and Socio-Economic Behavioural Factors. Eur. J. Paediatr. Dent. 2006, 7, 174–178. [Google Scholar] [PubMed]
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.d.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal Disease and Its Impact on General Health in Latin America. Section V: Treatment of Periodontitis. Braz Oral. Res. 2020, 34, e026. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, A.M.; Cutler, C.W. Pathophysiological Relationships Between Periodontitis and Systemic Disease: Recent Concepts Involving Serum Lipids. J. Periodontol. 2000, 71, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- John, V.; Alqallaf, H.; De Bedout, T. Periodontal Disease and Systemic Diseases: An Update for the Clinician. J. Indiana Dent. Assoc. 2016, 95, 16–23. [Google Scholar] [PubMed]
- Kalhan, A.C.; Wong, M.L.; Allen, F.; Gao, X. Periodontal Disease and Systemic Health: An Update for Medical Practitioners. Ann. Acad Med. Singap. 2022, 51, 567–574. [Google Scholar] [CrossRef]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mainas, G.; Ide, M.; Rizzo, M.; Magan-Fernandez, A.; Mesa, F.; Nibali, L. Managing the Systemic Impact of Periodontitis. Medicina 2022, 58, 621. [Google Scholar] [CrossRef]
- Shangase, S.L.; Mohangi, G.U.; Hassam-Essa, S.; Wood, N.H. The Association between Periodontitis and Systemic Health: An Overview. SADJ 2013, 68, 10–12. [Google Scholar]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Uzunçıbuk, H.; Marrapodi, M.M.; Meto, A.; Ronsivalle, V.; Cicciù, M.; Minervini, G. Prevalence of Temporomandibular Disorders in Clear Aligner Patients Using Orthodontic Intermaxillary Elastics Assessed with Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) Axis II Evaluation: A Cross-Sectional Study. J. Oral. Rehabil. 2024, 51, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Isola, G.; Cicciù, M. Conservative Treatment of Temporomandibular Joint Condylar Fractures: A Systematic Review Conducted According to PRISMA Guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J. Oral. Rehabil. 2023, 50, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Post-traumatic Stress, Prevalence of Temporomandibular Disorders in War Veterans: Systematic Review with Meta-analysis. J. Oral. Rehabil. 2023, 50, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G. The Association between Parent Education Level, Oral Health, and Oral-Related Sleep Disturbance. An Observational Crosssectional Study. Eur. J. Paediatr. Dent. 2023, 24, 218–223. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Ronsivalle, V.; Cicciù, M. Children Oral Health and Parents Education Status: A Cross Sectional Study. BMC Oral. Health 2023, 23, 787. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Marrapodi, M.M.; Cicciù, M. Online Bruxism-related Information: Can People Understand What They Read? A Cross-Sectional Study. J. Oral. Rehabil. 2023, 50, 1211–1216. [Google Scholar] [CrossRef]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 152–169. [Google Scholar] [CrossRef]
- Serón, C.; Olivero, P.; Flores, N.; Cruzat, B.; Ahumada, F.; Gueyffier, F.; Marchant, I. Diabetes, Periodontitis, and Cardiovascular Disease: Towards Equity in Diabetes Care. Front. Public Health 2023, 11, 1270557. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.C.; Clarkson, J.E.; Worthington, H.V.; MacDonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of Periodontitis for Glycaemic Control in People with Diabetes Mellitus. Cochrane Database Syst. Rev. 2022, 4, CD004714. [Google Scholar] [CrossRef] [PubMed]
- Gualtero, D.F.; Lafaurie, G.I.; Buitrago, D.M.; Castillo, Y.; Vargas-Sanchez, P.K.; Castillo, D.M. Oral Microbiome Mediated Inflammation, a Potential Inductor of Vascular Diseases: A Comprehensive Review. Front. Cardiovasc. Med. 2023, 10, 1250263. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.W.; DalBó, S.; Striechen, T.M.; Farias, J.M.; Olchanheski, L.R.; Mendes, R.T.; Vellosa, J.C.R.; Fávero, G.M.; Sordi, R.; Assreuy, J.; et al. Experimental Periodontitis Promotes Transient Vascular Inflammation and Endothelial Dysfunction. Arch. Oral. Biol. 2013, 58, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Severino, M.; Campanella, V.; Barlattani, A.; Quinzi, V.; Marchetti, E. Periodontal Disease in Subjects Suffering from Coronary Heart Disease. J. Biol. Regul. Homeost Agents 2019, 33, 73–82. [Google Scholar] [PubMed]
- Giorgini, E.; Sabbatini, S.; Conti, C.; Rubini, C.; Rocchetti, R.; Fioroni, M.; Memè, L.; Orilisi, G. Fourier Transform Infrared Imaging Analysis of Dental Pulp Inflammatory Diseases. Oral. Dis. 2017, 23, 484–491. [Google Scholar] [CrossRef]
- de Molon, R.S.; Rossa, C.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef] [PubMed]
- Nesse, W.; Dijkstra, P.U.; Abbas, F.; Spijkervet, F.K.L.; Stijger, A.; Tromp, J.A.H.; van Dijk, J.L.; Vissink, A. Increased Prevalence of Cardiovascular and Autoimmune Diseases in Periodontitis Patients: A Cross-Sectional Study. J. Periodontol. 2010, 81, 1622–1628. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial Colonization of the Periodontal Pocket and Its Significance for Periodontal Therapy. Periodontol. 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Ogrendik, M. Rheumatoid Arthritis Is an Autoimmune Disease Caused by Periodontal Pathogens. Int. J. Gen. Med. 2013, 6, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Faizuddin, M.; Dharmapalan, J. Role of Autoimmune Responses in Periodontal Disease. Autoimmune Dis. 2014, 2014, 596824. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Papapanou, P.N. Diabetes Mellitus and Periodontitis: A Tale of Two Common Interrelated Diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Graziani, F.; Hasturk, H. Effects of Periodontal Disease on Glycemic Control, Complications, and Incidence of Diabetes Mellitus. Periodontol. 2000 2020, 83, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional Association between Periodontal Disease and Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Isacco, C.G.; Nguyen, K.C.D.; Pham, V.H.; Di Palma, G.; Aityan, S.K.; Tomassone, D.; Distratis, P.; Lazzaro, R.; Balzanelli, M.G.; Inchingolo, F. Searching for a Link between Bone Decay and Diabetes Type 2. Endocr. Metab Immune. Disord. Drug Targets 2022, 22, 904–910. [Google Scholar] [CrossRef]
- Dias Lopes, N.M.; Mendonça Lens, H.H.; Armani, A.; Marinello, P.C.; Cecchini, A.L. Thyroid Cancer and Thyroid Autoimmune Disease: A Review of Molecular Aspects and Clinical Outcomes. Pathol. Res. Pr. 2020, 216, 153098. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Uricoechea, H. Autoimmune Thyroid Disease and Differentiated Thyroid Carcinoma: A Review of the Mechanisms That Explain an Intriguing and Exciting Relationship. World J. Oncol. 2024, 15, 14–27. [Google Scholar] [CrossRef]
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. N. Engl. J. Med. 2003, 348, 2646–2655. [Google Scholar] [CrossRef]
- McIver, B.; Morris, J.C. The Pathogenesis of Graves’ Disease. Endocrinol. Metab Clin. North Am. 1998, 27, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L. Diagnosis and Management of Graves Disease: A Global Overview. Nat. Rev. Endocrinol. 2013, 9, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Walfish, P.G.; Chan, J.Y. Post-Partum Hyperthyroidism. Clin. Endocrinol. Metab 1985, 14, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Schubert, L.; Bricaire, L.; Groussin, L. Amiodarone-Induced Thyrotoxicosis. Ann. Endocrinol. 2021, 82, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Martinez Quintero, B.; Yazbeck, C.; Sweeney, L.B. Thyroiditis: Evaluation and Treatment. Am. Fam. Physician. 2021, 104, 609–617. [Google Scholar] [PubMed]
- Hoang, T.D.; Stocker, D.J.; Chou, E.L.; Burch, H.B. 2022 Update on Clinical Management of Graves Disease and Thyroid Eye Disease. Endocrinol. Metab Clin. North Am. 2022, 51, 287–304. [Google Scholar] [CrossRef]
- Subekti, I.; Pramono, L.A. Current Diagnosis and Management of Graves’ Disease. Acta Med. Indones 2018, 50, 177–182. [Google Scholar]
- Sherman, S.I. Thyroid Carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab Clin. North Am. 2019, 48, 23–35. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer-Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid Cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid Cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Chandna, S.; Bathla, M. Oral Manifestations of Thyroid Disorders and Its Management. Indian J. Endocrinol. Metab 2011, 15, S113–S116. [Google Scholar] [CrossRef]
- Zahid, T.M.; Wang, B.-Y.; Cohen, R.E. The Effects of Thyroid Hormone Abnormalities on Periodontal Disease Status. J. Int. Acad Periodontol. 2011, 13, 80–85. [Google Scholar]
- De Angelis, F.; Brauner, E.; Pranno, N.; Visca, A.; Stefanelli, L.V.; Di Carlo, S. Correlazione Tra Ormoni Tiroidei e Peri-Implantiti: Stato Dell’arte. Dent. Cadmos 2020, 88, 650. [Google Scholar] [CrossRef]
- Souza, A.C.O.D.; Castro, J.Z.M.; Meira, G.D.F.; Rego, J.T.M. Influence of hyperthyroidism and hypothyroidism on the oral cavity: Literature review. Rev. Científica Multidiscip. Núcleo Do Conhecimento 2023, 3, 82–106. [Google Scholar] [CrossRef]
- Egido-Moreno, S.; Valls-Roca-Umbert, J.; Perez-Sayans, M.; Blanco-Carrión, A.; Jane-Salas, E.; López-López, J. Role of Thyroid Hormones in Burning Mouth Syndrome. Systematic Review. Med. Oral. Patol. Oral. Cir. Bucal. 2023, 28, e81–e86. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-P.; Wu, Y.-C.; Wu, Y.-H.; Chang, J.Y.-F.; Wang, Y.-P.; Sun, A. Gastric Parietal Cell and Thyroid Autoantibodies in Patients with Burning Mouth Syndrome. J. Formos Med. Assoc. 2020, 119, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Aldulaijan, H.A.; Cohen, R.E.; Stellrecht, E.M.; Levine, M.J.; Yerke, L.M. Relationship between Hypothyroidism and Periodontitis: A Scoping Review. Clin. Exp. Dent. Res. 2020, 6, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Goel, K.; Solanki, J.; Gupta, S. Oral Manifestations of Hypothyroidism: A Case Report. J. Clin. Diagn Res. 2014, 8, ZD20–ZD22. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Tartaglia, G.; Inchingolo, F.; Scarano, A. Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial. Dent. J. 2022, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.C. Periodontal Disease in Hypothyroid Adult Rats. Arch. Oral. Biol. 1969, 14, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Shcherba, V.; Krynytska, I.; Marushchak, M.; Korda, M. Does Thyroid Dysfunction Influence Inflammatory Mediators in Experimental Periodontitis? Endocr. Regul. 2021, 55, 131–141. [Google Scholar] [CrossRef]
- Shcherba, V.; Havrylenko, Y.; Krynytska, I.; Marushchak, M.; Korda, M. A Comparative Study of Oral Microbiocenosis Structure in Experimental Comorbidity-Free Periodontitis and in Periodontitis Combined with Thyroid Dysfunction. Pol. Merkur Lek. 2020, 48, 32–38. [Google Scholar]
- Poumpros, E.; Loberg, E.; Engström, C. Thyroid Function and Root Resorption. Angle Orthod 1994, 64, 389–393, discussion 394. [Google Scholar] [CrossRef]
- Shcherba, V.; Demkovych, A.; Hasiuk, P.; Lebid, O.; Duda, K.; Stoikevych, H. Morphological Changes of Periodontal Components Under Experimental Lipopolysaccharide Periodontitis Combined with Hyperthyroidism. Wiad Lek. 2022, 75, 1960–1964. [Google Scholar] [CrossRef]
- Rahangdale, S.; Galgali, S. Periodontal Status of Hypothyroid Patients on Thyroxine Replacement Therapy: A Comparative Cross-Sectional Study. J. Indian Soc. Periodontol. 2018, 22, 535. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Ursomanno, B.L.; Cohen, R.E.; Levine, M.J.; Yerke, L.M. The Effect of Hypothyroidism on Bone Loss at Dental Implants. J. Oral. Implant. 2021, 47, 131–134. [Google Scholar] [CrossRef]
- Dhanwal, D.K. Thyroid Disorders and Bone Mineral Metabolism. Indian J. Endocrinol. Metab 2011, 15, S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Almeida, L.E.; Ronsivalle, V.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Obesity Patients: A Systematic Review and Meta-Analysis. J. Oral. Rehabil. 2023, 50, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Werneck, F.Z.; Coelho, E.F.; de Lima, J.R.P.; Laterza, M.C.; Barral, M.M.; Teixeira, P.d.F.D.S.; Vaisman, M. Pulmonary Oxygen Uptake Kinetics during Exercise in Subclinical Hypothyroidism. Thyroid 2014, 24, 931–938. [Google Scholar] [CrossRef]
- Vargas, F.; Moreno, J.M.; Rodríguez-Gómez, I.; Wangensteen, R.; Osuna, A.; Alvarez-Guerra, M.; García-Estañ, J. Vascular and Renal Function in Experimental Thyroid Disorders. Eur J. Endocrinol. 2006, 154, 197–212. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Mohammed, A.A. Effects of Thyroid Dysfunction on Hematological Parameters: Case Controlled Study. Ann. Med. Surg. 2020, 57, 52–55. [Google Scholar] [CrossRef]
- Shcherba, V.; Machogan, V.; Luchynskyi, V.; Korda, M.; Delibashvili, D.; Svanishvili, N. Correlation between Connective Tissue Metabolism and Thyroid Dysfunction in Rats with Periodontitis. Georgian. Med. News 2019, 145–149. [Google Scholar]
- Silver, S.; Crohn, E.B.; Porto, P. Hypermetabolism without Hyperthyroidism. Bull N. Y. Acad. Med. 1949, 25, 441. [Google Scholar] [PubMed]
- Roth, R.N.; McAuliffe, M.J. Hyperthyroidism and Thyroid Storm. Emerg. Med. Clin. North Am. 1989, 7, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.S.; Patil, S.; Gururaj, T.R. Probable Autoimmune Causal Relationship between Periodontitis and Hashimotos Thyroidits: A Systemic Review. Niger J. Clin. Pr. 2011, 14, 253–261. [Google Scholar] [CrossRef]
- Morais, A.; Resende, M.; Pereira, J. [Hashimoto Thyroiditis and Periodontal Disease: A Narrative Review]. Acta Med. Port 2016, 29, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Liu, X.; Shi, C.; Sun, H.C. Roles of Immune Cells and Mechanisms of Immune Responses in Periodontitis. Chin. J. Dent. Res. 2021, 24, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hammerstad, S.S.; Jahnsen, F.L.; Tauriainen, S.; Hyöty, H.; Paulsen, T.; Norheim, I.; Dahl-Jørgensen, K. Inflammation and Increased Myxovirus Resistance Protein A Expression in Thyroid Tissue in the Early Stages of Hashimoto’s Thyroiditis. Thyroid 2013, 23, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Vural, S.; Muhtaroğlu, A.; Güngör, M. Systemic Immune-Inflammation Index: A New Marker in Differentiation of Different Thyroid Diseases. Medicine 2023, 102, e34596. [Google Scholar] [CrossRef] [PubMed]
- Shcherba, V.; Kyryliv, M.; Bekus, I.; Krynytska, I.; Marushchak, M.; Korda, M. A Comparative Study of Connective Tissue Metabolism Indices in Experimental Comorbidity-Free Periodontitis and Periodontitis Combined with Thyroid Dysfunction. J. Med. Life 2020, 13, 219–224. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Liang, Y.-C.; Zhang, H.-H.; Li, H.-L.; Liu, D.-L. Association between the Systemic Immune-Inflammation Index and Thyroid Function in U.S. Adults. Mediat. Inflamm 2023, 2023, 5831858. [Google Scholar] [CrossRef]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef] [PubMed]
- Pink, C.; Holtfreter, B.; Völzke, H.; Nauck, M.; Dörr, M.; Kocher, T. Periodontitis and Systemic Inflammation as Independent and Interacting Risk Factors for Mortality: Evidence from a Prospective Cohort Study. BMC Med. 2023, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.; Miśkiewicz, J.; Strzelec, K.; Wcisło-Dziadecka, D.; Strzalka-Mrozik, B. Apoptosis in Autoimmunological Diseases, with Particular Consideration of Molecular Aspects of Psoriasis. Med. Sci. Monit. 2020, 26, e922035. [Google Scholar] [CrossRef] [PubMed]
- Arscott, P.L.; Baker, J.R. Apoptosis and Thyroiditis. Clin. Immunol. Immunopathol. 1998, 87, 207–217. [Google Scholar] [CrossRef]
- Salmaso, C.; Bagnasco, M.; Pesce, G.; Montagna, P.; Brizzolara, R.; Altrinetti, V.; Richiusa, P.; Galluzzo, A.; Giordano, C. Regulation of Apoptosis in Endocrine Autoimmunity: Insights from Hashimoto’s Thyroiditis and Graves’ Disease. Ann. N. Y. Acad. Sci. 2002, 966, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune Disorders in Hashimoto’s Thyroiditis: What Do We Know so Far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Pham, V.H.; Del Prete, R.; Mosca, A.; Inchingolo, F.; Aityan, S.K.; Santacroce, L.; Nguyen, K.C.D.; et al. From Pathogens to Cancer: Are Cancer Cells Evolved Mitochondrial Super Cells? Diagnostics 2023, 13, 813. [Google Scholar] [CrossRef]
- Ruan, Y.; Heng, X.-P.; Yang, L.-Q.; He, W.-D.; Li, L.; Wang, Z.-T.; Huang, S.-P.; Chen, Q.-W.; Han, Z. Relationship between Autoimmune Thyroid Antibodies and Anti-Nuclear Antibodies in General Patients. Front. Endocrinol. 2024, 15, 1368088. [Google Scholar] [CrossRef]
- Orgiazzi, J. Thyroid Autoimmunity. Presse Med. 2012, 41, e611–e625. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Weetman, A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in Our Understanding. Horm. Metab Res. 2015, 47, 702–710. [Google Scholar] [CrossRef]
- Weetman, A.P. An Update on the Pathogenesis of Hashimoto’s Thyroiditis. J. Endocrinol. Investig. 2021, 44, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Sapio, S.; Falco, A.; Vittorini, O.L.; Quinzi, V. Management of Pedodontic Patients in Moderate Sedation in Clinical Dentistry: Evaluation of Behaviour before and after Treatment. J. Biol. Regul. Homeost Agents 2020, 34, 55–62. [Google Scholar] [PubMed]
- Fleischer, B. Superantigens. APMIS 1994, 102, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.A.; Kominsky, S.; Perrin, G.Q.; Hobeika, A.C.; Johnson, H.M. Superantigens: The Good, the Bad, and the Ugly. Exp. Biol. Med. 2001, 226, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Tomer, Y.; Davies, T.F. Infection, Thyroid Disease, and Autoimmunity. Endocr. Rev. 1993, 14, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Kita, M.; Flynn, J.C.; Panos, J.C.; Motte, R.W.; Davies, T.F.; Giraldo, A.A.; David, C.S.; Kong, Y.C. Participation of Vbeta13(+) and Vbeta1(+) T Cells in Transfer Thyroiditis after Activation of Mouse Thyroglobulin-Primed T Cells by Superantigen Staphylococcal Enterotoxin A. Cell Immunol. 2001, 213, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.J.; Garzón, H.; Arboleda, S.; Rodríguez, A. Oral Dysbiosis and Autoimmunity: From Local Periodontal Responses to an Imbalanced Systemic Immunity. A Review. Front. Immunol. 2020, 11, 591255. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated Methodological Guidance for the Conduct of Scoping Reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Env. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, M.M.; Dodamani, A.S.; Karibasappa, G.N.; Vishwakarma, P.Y.; Vathar, J.B.; Sonawane, K.R. Assessment of Oral Health Status and Treatment Needs among Individuals with Thyroid Dysfunction in Nashik City (Maharashtra): A Cross-Sectional Study. Contemp Clin. Dent. 2018, 9, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthakopoulos, N.A.; Chrysanthakopoulos, P.A. Association between Indices of Clinically-defined Periodontitis and Self-reported History of Systemic Medical Conditions. J. Invest. Clin. Dent. 2016, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Jeong, Y.; Kwak, J.; Jung, K.; Baek, S. Association between Oral Health and Thyroid Disorders: A Population-based Cross-sectional Study. Oral. Dis. 2022, 28, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chen, J.-F.; Hung, Y.-T.; Hsu, T.-J.; Chiu, C.-C.; Kuo, S.-J. Exploring the Relationship between Periodontitis, Anti-Periodontitis Therapy, and Extra-Oral Cancer Risk: Findings from a Nationwide Population-Based Study. Biomedicines 2023, 11, 1949. [Google Scholar] [CrossRef]
- Venkatesh Babu, N.; Patel, P. Oral Health Status of Children Suffering from Thyroid Disorders. J. Indian Soc. Pedod Prev. Dent. 2016, 34, 139. [Google Scholar] [CrossRef]
- Zeigler, C.C.; Wondimu, B.; Marcus, C.; Modéer, T. Pathological Periodontal Pockets Are Associated with Raised Diastolic Blood Pressure in Obese Adolescents. BMC Oral. Health 2015, 15, 41. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Jiang, Z.; Marka, N.; Wolff, L.F. Association between Periodontitis Extent, Severity, and Progression Rate with Systemic Diseases and Smoking: A Retrospective Study. JPM 2023, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, D.; Liu, Y.; Qiu, Y.; Lu, S. Periodontitis and Thyroid Function: A Bidirectional Mendelian Randomization Study. J. Periodontal. Res. 2024, 59, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Nam, S.; Park, C.H.; Kim, Y.; Lee, M.; Ahn, J.B.; Shin, S.J.; Park, Y.R.; Jung, H.I.; Kim, B.-I.; et al. Periodontal Disease and Cancer Risk: A Nationwide Population-Based Cohort Study. Front. Oncol. 2022, 12, 901098. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Park, M.J.; Kim, J.A.; Roh, E.; Yu, J.H.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; et al. Implication of Thyroid Function in Periodontitis: A Nationwide Population-Based Study. Sci. Rep. 2021, 11, 22127. [Google Scholar] [CrossRef] [PubMed]
- Al-Hindawi, S.; Al-Ghurabi, B.; Luaibi, N. The Role of HLA-DRB1 Allele in Hypothyroid Patients with and without Periodontitis. Pak. J. Biotechnol. 2017, 14, 629–634. Available online: https://api.semanticscholar.org/CorpusID:96438029 (accessed on 23 April 2024).
- Liu, W.; Zheng, Y.; Chen, B.; Ke, T.; Shi, Z. LncRNA Papillary Thyroid Carcinoma Susceptibility Candidate 3 (PTCSC3) Regulates the Proliferation of Human Periodontal Ligament Stem Cells and Toll-like Receptor 4 (TLR4) Expression to Improve Periodontitis. BMC Oral. Health 2019, 19, 108. [Google Scholar] [CrossRef]
- Zeng, Y.; Deng, J.; Jiang, Q.; Wang, C.; Zhang, L.; Li, T.; Jiang, J. Thyrotropin Inhibits Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. J. Periodontal. Res. 2023, 58, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, R.; Li, R.; Chen, W.; Zhang, J.; Lv, W.; Lin, B.; Luo, J. Exploring the Association Between Thyroid Function and Oral Microbiome Diversity: An NHANES Analysis. J. Endocr. Soc. 2023, 7, bvad125. [Google Scholar] [CrossRef]
- Al-Hindawi, S.H.; Al-Hindawi, B.H. Interleukin-1β, Interleukin-6 and Tumour Necrosis Factor-Alpha Levels in Blood and Saliva in Hypothyroidism Accompanied with Periodontitis. ijrps 2019, 10, 1361–1366. [Google Scholar] [CrossRef]
- Vallabhan, C.G.; Koshi, E.; Sadasivan, A.; Saraswathi, I.R.; Vijayakumar, S.; Vrinda, S.M. Effect of Nonsurgical Periodontal Therapy on Serum Level of Interleukin-6 and Tumor Necrosis Factor-α in Chronic Periodontitis Patients with and without Hypothyroidism. J. Contemp. Dent. Pract. 2020, 21, 410–415. [Google Scholar] [CrossRef]
- Bhankhar, R.; Hungund, S.; Kambalyal, P.; Singh, V.; Jain, K. Effect of Nonsurgical Periodontal Therapy on Thyroid Stimulating Hormone in Hypothyroid Patients with Periodontal Diseases. Indian J. Dent. Res. 2017, 28, 16. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-M.; Sun, L.-M.; Lin, C.-L.; Lee, C.-F.; Kao, C.-H. Periodontal Disease with Treatment Reduces Subsequent Cancer Risks. QJM 2014, 107, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Yusubova, S.R.; Mamedov, F.Y. Increasing the Efficiency of Treatment of Inflammatory Periodontal Diseases in Patients with Thyroid Diseases. Womab 2021, 17, 184. [Google Scholar] [CrossRef]
- Duda-Sobczak, A.; Zozulinska-Ziolkiewicz, D.; Wyganowska, M. Better Gingival Status in Patients with Comorbidity of Type 1 Diabetes and Thyroiditis in Comparison with Patients with Type 1 Diabetes and No Thyroid Disease—A Preliminary Study. Int. J. Environ. Res. Public Health 2023, 20, 3008. [Google Scholar] [CrossRef] [PubMed]
- Klewin-Steinböck, S.; Wyganowska, M. Reduction in Gingival Bleeding after Atelocollagen Injection in Patients with Hashimoto’s Disease—A Pilot Study. IJERPH 2023, 20, 2954. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchingolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; Dipalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I.A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health 2020, 17, 3168. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and Growth Factors in Oral Squamous Cell Carcinoma: Useful Source of Dental-Derived Stem Cells to Develop a Steroidogenic Model in New Clinical Strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [CrossRef]
- Dizdar, O.; Hayran, M.; Guven, D.C.; Yılmaz, T.B.; Taheri, S.; Akman, A.C.; Bilgin, E.; Hüseyin, B.; Berker, E. Increased Cancer Risk in Patients with Periodontitis. Curr. Med. Res. Opin. 2017, 33, 2195–2200. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Z.; Chen, Z.; Song, J.; Wang, B.; Jin, F. Association between Periodontitis and Breast Cancer: Two-Sample Mendelian Randomization Study. Clin. Oral. Investig. 2023, 27, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Amiri Moghaddam, M.; Etajuri, E.A.; Badkoobeh, A.; Tavakol, O.; Rafinejad, M.; Forutan Mirhosseini, A.; Fathi, A. Periodontitis and Progression of Gastrointestinal Cancer: Current Knowledge and Future Perspective. Clin. Transl. Oncol. 2023, 25, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-E.; Lin, F.-G.; Huang, R.-Y.; Fang, W.-H.; Cheng, W.-C.; Tsai, Y.-W.C.; Chen, W.-L. Periodontitis, Helicobacter Pylori Infection, and Gastrointestinal Tract Cancer Mortality. J. Clin. Periodontol. 2022, 49, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Prevention and Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Fusco, A.; Stiuso, P.; Lama, S.; Gravina, A.G.; Itro, A.; Federico, A.; Itro, A.; Dipalma, G.; Inchingolo, F.; et al. Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 2021, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ji, R.; Zhan, W. Long Noncoding RNA Papillary Thyroid Carcinoma Susceptibility Candidate 3 (PTCSC3) Inhibits Proliferation and Invasion of Glioma Cells by Suppressing the Wnt/β-Catenin Signaling Pathway. BMC Neurol. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine Gene Polymorphisms Associate with Microbiogical Agents in Periodontal Disease: Our Experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Evans, S.; Barry, B.; Barratt, D.; Wang, Y.; Lin, C.; Wang, X.; Hutchinson, M.R. Toll-Like Receptor 4 in Pain: Bridging Molecules-to-Cells-to-Systems. Handb Exp. Pharmacol. 2022, 276, 239–273. [Google Scholar] [CrossRef]
- Wangzhou, K.; Gong, L.; Liu, C.; Tan, Y.; Chen, J.; Li, C.; Lai, Z.; Hao, C. LncRNA MAFG-AS1 Regulates Human Periodontal Ligament Stem Cell Proliferation and Toll-like Receptor 4 Expression. Oral. Dis. 2020, 26, 1302–1307. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The Microbiome in Autoimmune Diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Chimenos-Küstner, E.; Giovannoni, M.L.; Schemel-Suárez, M. Dysbiosis as a Determinant Factor of Systemic and Oral Pathology: Importance of Microbiome. Med. Clin. 2017, 149, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Corriero, A.; Giglio, M.; Inchingolo, F.; Moschetta, A.; Varrassi, G.; Puntillo, F. Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review. Pain Ther. 2024, 13, 33–51. [Google Scholar] [CrossRef]
- Güngör Borsöken, A.; Gursel Surmelıoglu, D. The Effect of Saliva and Dental Caries of the Patients with Hashimoto Thyroiditis on Cytokine Levels. Niger J. Clin. Pr. 2024, 27, 8–15. [Google Scholar] [CrossRef]
- Lumachi, F.; Basso, S.M.M.; Orlando, R. Cytokines, Thyroid Diseases and Thyroid Cancer. Cytokine 2010, 50, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S.; Gruwell, S.F.; Powell, C.A. Scaling and Root Planing vs. Conservative Surgery in the Treatment of Chronic Periodontitis. Periodontol. 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Sottosanti, J.S. A Re-Evaluation of Scaling and Root Planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Bakdash, B. Oral Hygiene and Compliance as Risk Factors in Periodontitis. J. Periodontol. 1994, 65, 539–544. [Google Scholar] [CrossRef]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New Tendencies in Non-Surgical Periodontal Therapy. Braz Oral. Res. 2021, 35, e095. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Comparison between Traditional Surgery, CO2 and Nd:Yag Laser Treatment for Generalized Gingival Hyperplasia in Sturge-Weber Syndrome: A Retrospective Study. J. Investig Clin. Dent. 2010, 1, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sato, K.; Miyazaki, T.; Aizaki, Y.; Tanaka, S.; Sekikawa, M.; Kozu, N.; Kadono, Y.; Oda, H.; Mimura, T. Characterization and Function of Tumor Necrosis Factor and Interleukin-6-Induced Osteoclasts in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Stanton, H.; Cowell, S.; Butler, G.; Knäuper, V.; Atkinson, S.; Gavrilovic, J. Mechanisms for pro Matrix Metalloproteinase Activation. APMIS 1999, 107, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.H.; Naji, N.S.; Al-Saadi, A.H. Study of the Relationship between FT3, FT4, and TSH with Bone Resorption Indices in Men with Hypothyroidism. J. Popul. Ther. Clin. Pharmacol. 2022, 29, e33–e39. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, N.M.; Tulgar Kınık, S.; Özdemir, B.; Muratoğlu Şahin, N.; Tekindal, M.A.; Haberal, A. Congenital Hypothyroidism and Bone Remodeling Cycle. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Servili, A.; Inchingolo, A.M.; Dipalma, G. A Hypothetical Correlation between Hyaluronic Acid Gel and Development of Cutaneous Metaplastic Synovial Cyst. Head Face Med. 2010, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Bhati, A.; Fageeh, H.; Ibraheem, W.; Fageeh, H.; Chopra, H.; Panda, S. Role of Hyaluronic Acid in Periodontal Therapy (Review). Biomed. Rep. 2022, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic Acid: Perspectives in Dentistry. A Systematic Review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef] [PubMed]
| Articles Screening strategy | KEYWORDS: A: “periodontitis”; B: “thyroid” |
| Boolean Indicators: “A” AND “B” | |
| Timespan: from 1 January 2014, to January 2024 | |
| Electronic Databases: Pubmed, Scopus, Web of Science, and Cochrane |
| Authors | Study Design | Study Sample (n. Patients) | Parameters Evaluated | Results |
|---|---|---|---|---|
| Kshirsagar et al., 2018 [135] | Cross-sectional | 200 participants Study group (thyroid disease and periodontitis): 100 Control group (healthy): 100 | Gingival bleeding, loss of attachment, and caries | Greater severity of dental caries status and periodontal destruction in patients with thyroid disease and periodontitis, especially in women |
| Chrysanthakopoulos et al., 2016 [136] | Retrospective | 3.360 outpatients | Systemic diseases as independent variables and the relative frequency of periodontal pockets measuring ≥5 mm and CAL measuring ≥6 mm as dependent variables | Male gender, vascular illness, hypertension, stroke, heart attack, diabetes mellitus, endocrine disorders, thyroid disease, respiratory allergies, and rheumatoid arthritis are associated with periodontal pocket depth. CAL was linked to the illnesses, as well as infective endocarditis and chronic obstructive pulmonary disease, but not to other endocrine or thyroid disorders |
| Kwon et al., 2022 [137] | Cross-sectional | 18.034 adults | Oral health-related parameters and the prevalence of thyroid diseases; oral health status, and TFTs | Thyroid disease histories are more prevalent in those who regularly clean their teeth or use mouthwash. CPI and a history of periodontitis do not significantly correlate with thyroid illness. In individuals without a prior history of thyroid diseases, the association of higher CPI with abnormal TFTs |
| Chen et al., 2023 [138] | Comparative cohort | 683.854 participants | aHR for cancer aHR for specific types of cancer regardless of gender, age, Charlson comorbidity index, and the use of nonsteroidal anti-inflammatory drugs | Lower aHR for cancer in periodontitis patients Increased aHR for prostate cancer in men and thyroid cancer (aHR = 1.20 for women and aHR = 1.51 for men) in periodontitis patients Periodontal treatment reduced the risk of cancer |
| Venkatesh Babu et al., 2016 [139] | Comparative cohort | 200 children Study group: 100 with thyroid dysfunction Control group: 100 healthy | GI, PI, DMFT, Dmft, and modified DDE index | Higher GI, PI, and DDE indexes in the thyroid group Higher DMFT and dmft index in the thyroid group, but this difference was not statistically significant Children with thyroid disease have additional oral symptoms (macroglossia, open bites, and altered eruption patterns) |
| Zeigler et al., 2015 [140] | Cross-sectional pilot | 75 obese adolescents with PD < 4 mm: 61 PD ≥ 4 mm: 14 | VPI, BOP, and PD ≥ 4 mm Systolic and diastolic blood pressures | Adolescents with PD ≥ 4 mm had significantly higher BOP, higher diastolic blood pressure, and higher serum levels of IL-6, Leptin, MCP-1, and TSH |
| Chatzopoulos et al., 2023 [141] | Retrospective | 2.069 records | Grade of periodontitis | Men have widespread periodontitis, and older adults have grade B and IV periodontitis. Those with generalized disease and stage IV had greater tooth loss rates. Multiple sclerosis and smoking have been associated with grade C PD. No association between stage and extension of periodontitis and thyroid disorders (p value > 0.99). |
| Gao et al., 2024 [142] | Bidirectional, univariable MR framework | 17.353 cases 28.210 controls | OR for periodontitis, FT4, TSH levels, hypothyroidism, hyperthyroidism, and AT | Increased OR (1.24) for hypothyroidism in individuals with a genetic predisposition to periodontitis |
| Kim et al., 2022 [143] | Retrospective cohort | 713.201 individuals Periodontitis group: 53.075 Control group: 660.126 | Cumulative incidence of cancer, aHR for specific types of cancer | Increased cumulative incidence of cancer (2.2) for cancer in the periodontitis group; higher aHR (1.307) for cancer in the periodontitis group adjusted for age, sex, comorbidities, BMI, and smoking history Increased aHR for leukemia, stomach cancer, colon cancer, lung cancer, bladder cancer, and thyroid cancer in the periodontitis group No significant association between the development of secondary malignancy and periodontitis in cancer survivors at 5 years |
| Song et al., 2021 [144] | Comparative cohort | 5468 participants, 1423 with periodontitis First terzile: TSH < 1.76 mIU/L Second terzile: TSH 1.76–2.83 mIU/L Third terzile: TSH > 2.83 mIU/L) | CPI | OR for periodontitis is 1.34 in the first tertile vs. the third tertile, adjusted OR for risk factors is 1.39, and adjusted OR for log-transformed urine iodine levels and thyroid peroxidase antibodies is 1.36 |
| Authors | Study Design | Study Sample | Parameters Evaluated | Results |
|---|---|---|---|---|
| Al-Hindawi S., 2017 [145] | Cross-sectional | 30 hypothyroid subjects with periodontitis 30 hypothyroid subjects without periodontitis 30 healthy controls | Frequency of HLA | Increased frequency of HLA-DRB1*03 and *04 alleles in hypothyroid patients with or without periodontitis The increased frequency of the HLA-DRB1*08 allele in a healthy group Increased frequency of the HLA DRB1*03 allele was in patients with hypothyroidism and periodontitis |
| Liu W., 2019 [146] | Experimental laboratory-based | Periodontal ligament tissues from 34 subjects with periodontitis and 34 non-periodontitis subjects | Expression levels of PTCSC3 and TLR4 mRNA in periodontitis-affected PDLSCs. Effects of PTCSC3 and TLR4 Proliferation ability of periodontitis-affected PDLSCs | PTCSC3 was downregulated, TLR4 was upregulated in PD-affected PDLSCs, and PTCSC3 overexpression inhibited proliferation |
| Zeng Y., 2023 [147] | Observational cross-sectional | Human periodontal ligament tissue was obtained from extracted bicuspid teeth before orthodontic treatment. | Osteogenic differentiation parameters in human PDLSCs exposed to various concentrations of TSH: alkaline phosphatase activity, alizarin red staining for calcium nodules, and expression levels of osteogenic genes, namely, OPN, RUNX2, COL1A1, and OCN | TSH hindered osteogenic differentiation in periodontal ligament stem cells, as evidenced by reduced alkaline phosphatase activity, calcium nodule formation, and expression of OPN, RUNX2, COL1A1, and OCN |
| Zheng L., 2023 [148] | Observational cross-sectional | 2.943 subjects after applying appropriate NHANES sample weights. | Thyroid function markers TSH, fT3, and fT4 levels, alongside oral microbiome diversity metrics such as species richness and evenness | Both clinical and subclinical hyperthyroidism are associated with reduced diversity of the oral microbiome. Anti-thyroid oxidase antibodies and hypothyroidism are associated with increased oral biodiversity |
| Sahar H. Al-Hindawi, 2019 [149] | Analytical cross-sectional | 30 hypothyroid subjects with periodontitis 30 hypothyroid subjects without periodontitis 30 healthy subjects | Serum and salivary levels of IL-1β, IL-6, and TNF-α | Increased serum and salivary IL-1β levels in hypothyroid patients with and without periodontitis. Increased salivary IL-6 levels in hypothyroid patients with periodontitis |
| Authors | Study Design | Study Sample (n.) | Treatment | Follow-Up | Parameter Evaluated | Outcomes |
|---|---|---|---|---|---|---|
| Vallabhan C.G., 2020 [150] | Comparative interventional | Study group: 20 hypothyroid subjects with periodontitis Control group: 20 patients with periodontitis | SRP | 4 weeks | PI, GI, CAL, PPD, IL-6, and TNF-α | Reduction of PI, GI, CAL, PPD, IL-6, and TNF-α in both group |
| Bhankhar R., 2017 [151] | Prospective interventional | Study group: 15 hypothyroid subjects under medication Control group: 15 healthy subjects | NSPT | 3 months | OHI-S, PBI, PSR, CAL, and serum TSH levels | Improvements in OHI-S, PBI, PSR, and CAL Reduction in serum TSH levels after NSPT |
| Hwang I.M., 2014 [152] | Retrospective cohort | 38.902 subjects with periodontitis and 77.804 comparison subjects | SRP and periodontal flap surgery | 13 years | Cancer incidence, demographics, comorbidities, sex- and age-specific cancer risks, aHR, cancer site-specific incidence rates, and aHR | Reduced overall cancer (aHR = 0.77) but increased aHR for prostate and thyroid cancers (aHR = 1.54) |
| Yusubova, Sh.R., 2021 [153] | Prospective cohort | Study group: 13 subjects with thyroid dysfunction and periodontitis Comparison group: 11 healthy subjects Control group: 12 subjects undergoing various treatment methods | Hyaluronic acid in the study group, chlorhexidine in the comparison group, and curettage in the control group | Before and after treatment | Periodontal indices Biochemical parameters in oral fluid: MDA levels, SOD activity, catalase activity Concentration of IgA, IgG, and IgA in saliva | Improved periodontal health, decreased MDA, increased SOD, catalase, and enhanced salivary immunoglobulin level |
| Rahangdal S.I., Galgali S.R., 2018 [92] | Comparative cross-sectional | 52 hypothyroid subjects on thyroxine therapy and 50 healthy controls | Thyroxine therapy | 12 months | PI, BI, PPD CAL, radiographic mandibular cortical thickness, and panoramic mandibular index parameters | Higher PPD and CAL in hypothyroid patients on thyroxine therapy indicate an increased risk for periodontal destruction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, F.; Inchingolo, A.M.; Inchingolo, A.D.; Fatone, M.C.; Ferrante, L.; Avantario, P.; Fiore, A.; Palermo, A.; Amenduni, T.; Galante, F.; et al. Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 860. https://doi.org/10.3390/ijerph21070860
Inchingolo F, Inchingolo AM, Inchingolo AD, Fatone MC, Ferrante L, Avantario P, Fiore A, Palermo A, Amenduni T, Galante F, et al. Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review. International Journal of Environmental Research and Public Health. 2024; 21(7):860. https://doi.org/10.3390/ijerph21070860
Chicago/Turabian StyleInchingolo, Francesco, Angelo Michele Inchingolo, Alessio Danilo Inchingolo, Maria Celeste Fatone, Laura Ferrante, Pasquale Avantario, Arianna Fiore, Andrea Palermo, Tommaso Amenduni, Francesco Galante, and et al. 2024. "Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review" International Journal of Environmental Research and Public Health 21, no. 7: 860. https://doi.org/10.3390/ijerph21070860
APA StyleInchingolo, F., Inchingolo, A. M., Inchingolo, A. D., Fatone, M. C., Ferrante, L., Avantario, P., Fiore, A., Palermo, A., Amenduni, T., Galante, F., & Dipalma, G. (2024). Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review. International Journal of Environmental Research and Public Health, 21(7), 860. https://doi.org/10.3390/ijerph21070860











