Effect of Combining Impact-Aerobic and Strength Exercise, and Dietary Habits on Body Composition in Breast Cancer Survivors Treated with Aromatase Inhibitors
Abstract
1. Introduction
2. Materials and Methods
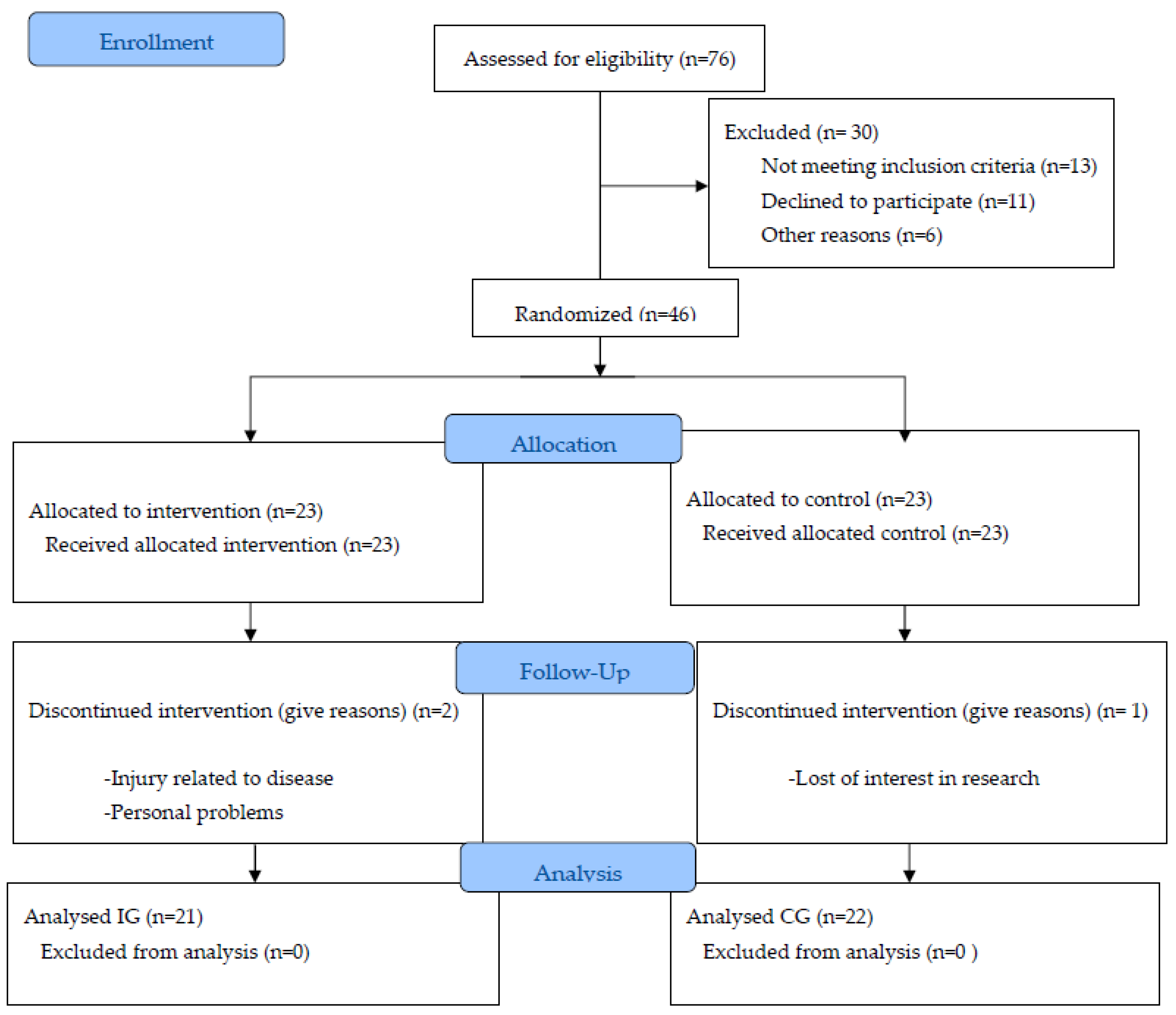
2.1. Participants
2.2. Procedure
2.3. Training Program
2.4. Body Composition and Nutritional Status
2.5. Level of Physical Activity
2.6. Dietary Habits
2.7. Statistical Analyses
3. Results
3.1. Body Composition and Nutritional Status
3.2. Physical Activity Level
3.3. Dietary Habits
4. Discussion
4.1. Body Composition and Nutritional Status
4.2. Lifestyle: Physical Activity Level and Dietary Habits
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimauro, I.; Grazioli, E.; Antinozzi, C.; Duranti, G.; Arminio, A.; Mancini, A.; Greco, E.A.; Caporossi, D.; Parisi, A.; Di Luigi, L. Estrogen-Receptor-Positive Breast Cancer in Postmenopausal Women: The Role of Body Composition and Physical Exercise. Int. J. Environ. Res. Public Health 2021, 18, 9834. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N. Advances in targeting HER2-positive breast cancer. Curr. Opin. Obstet. Gynecol. 2018, 30, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body Mass Index and Breast Cancer Risk According to Postmenopausal Estrogen-Progestin Use and Hormone Receptor Status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar]
- Fahad Ullah, M. Breast Cancer: Current Perspectives on the Disease Status. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; pp. 51–64. [Google Scholar]
- Visvanathan, K.; Fabian, C.J.; Bantug, E.; Brewster, A.M.; Davidson, N.E.; DeCensi, A.; Floyd, J.D.; Garber, J.E.; Hofstatter, E.W.; Khan, S.A.; et al. Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, 37, 3152–3165. [Google Scholar] [CrossRef]
- Force, U.P.S.T.; Owens, D.K.; Davidson, K.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; et al. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. J. Am. Med. Assoc. 2019, 322, 857–867. [Google Scholar] [CrossRef]
- Clines, G.A.; Choksi, P.; Van Poznak, C. Adjuvant Endocrine Therapy and Bone Health in Breast Cancer. Curr. Osteoporos. Rep. 2015, 13, 263–273. [Google Scholar] [CrossRef]
- Goldvaser, H.; Barnes, T.A.; Šeruga, B.; Cescon, D.W.; Ocana, A.; Ribnikar, D.; Amir, E. Toxicity of Extended Adjuvant Therapy with Aromatase Inhibitors in Early Breast Cancer: A Systematic Review and Meta-analysis. Gynecol. Oncol. 2018, 110, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Ramchand, S.K.; Milat, F.; Vincent, A.; Lim, E.; Kotowicz, M.A.; Hicks, J.; Teede, H. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: Position statement of the Endocrine Society of Australia, the Australian and New Zealand Bone & Mineral Society, the Australasian. Clin. Endocrinol. 2018, 89, 280–296. [Google Scholar] [CrossRef]
- Roberts, K.; Rickett, K.; Greer, R.; Woodward, N. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early Breast cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. 2017, 111, 66–80. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA: A Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- WHO. OMS, Serie de Informes Técnicos 916 Dieta, Nutrición Y Prevención de Enfermedades Crónicas Organización Mundial de la Salud Ginebra; WHO: Geneva, Switzerland, 2018.
- National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Patients. Survivorship Care for Healthy Living. 2020. Available online: https://www.nccn.org/patients/guidelines/content/PDF/survivorship-hl-patient.pdf. (accessed on 30 January 2023).
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; et al. Randomized Exercise Trial of Aromatase Inhibitor–Induced Arthralgia in Breast Cancer Survivors. J. Clin. Oncol. 2015, 33, 1104–1111. [Google Scholar] [CrossRef]
- Casla, S.; López-Tarruella, S.; Jerez, Y.; Marquez-Rodas, I.; Galvão, D.A.; Newton, R.U.; Cubedo, R.; Calvo, I.; Sampedro, J.; Barakat, R.; et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: A randomized controlled trial. Breast Cancer Res. Treat. 2015, 153, 371–382. [Google Scholar] [CrossRef]
- Dalla Via, J.; Daly, R.M.; Fraser, S.F. The effect of exercise on bone mineral density in adult cancer survivors: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 287–303. [Google Scholar] [CrossRef]
- Grossmann, M.; Ramchand, S.K.; Milat, F.; Vincent, A.; Lim, E.; Kotowicz, M.A.; Hicks, J.; Teede, H.J. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: Position statement summary. Med. J. Aust. 2019, 211, 224–229. [Google Scholar] [CrossRef]
- Saarto, T.; Sievänen, H.; Kellokumpu-Lehtinen, P.; Nikander, R.; Vehmanen, L.; Huovinen, R.; Kautiainen, H.; Järvenpää, S.; Penttinen, H.M.; Utriainen, M.; et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos. Int. 2012, 23, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical Usefulness of a New Equation for Estimating Body Fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; McManus, C.; Smith, J.; Stevens, V.; Nixon, D.W. Anthropometric measurement of muscle mass: Revised equations for calcu-lating bone-free arm muscle area. Am. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Markfell-Jones, M.; Olds, T.; Ridder, H. Protocolo Internacional para la valoracion antropométrica. Isak 2011, 82, 1–117. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008; pp. 1–47.
- Idoate, F.; Ibañez, J.; Gorostiaga, E.M.; García-Unciti, M.; Martínez-Labari, C.; Izquierdo, M. Weight-loss diet alone or combined with resistance training induces different regional visceral fat changes in obese women. Int. J. Obes. 2011, 35, 700–713. [Google Scholar] [CrossRef]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- Martín-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and Validation of a Food Frequency Questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sport. Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Zanuso, S.; Bergamin, M.; Jimenez, A.; Pugliese, G.; D’Errico, V.; Nicolucci, A.; Ermolao, A.; Balducci, S. Determination of metabolic equivalents during low- and high-intensity resistance exercise in healthy young subjects and patients with type 2 diabetes. Biol. Sport 2016, 33, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Métodos de evaluación de la ingesta actual: Registro o diario dietético. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Rusolillo, G.; Marques, I. Álbum Fotográfico de Porciones de Alimentos, 1st ed.; ICM: Madrid, España, 2011. [Google Scholar]
- AEND. Academia Española de Nutrición y Dietética; Programa Informático Easydiet®: Brisbane, Australia, 2008. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campà, A.; Martínez-González, M.A.; Ruiz-Canela, M. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Bartrina, J.A.; Val, V.A.; Aldalur, E.M.; de Victoria Muñoz, E.M.; Anta, R.M.O.; Pérez-Rodrigo, C.; Izquierdo, J.Q.; Martín, A.R.; Viñas, B.R.; Castell, G.S.; et al. Dietary Guidelines for the Spanish population (SENC, diciembre 2016); the new graphic icon of healthy food. Nutr. Hosp. 2016, 33, 1–48. [Google Scholar] [CrossRef]
- Aranceta-Bartrina, J.; Partearroyo, T.; López-Sobaler, A.M.; Ortega, R.M.; Varela-Moreiras, G.; Serra-Majem, L.; Pérez-Rodrigo, C. Updating the Food-Based Dietary Guidelines for the Spanish Population: The Spanish Society of Community Nutrition (SENC) Proposal. Nutrients 2019, 11, 2675. [Google Scholar] [CrossRef]
- Clèries, R.; Ameijide, A.; Buxó, M.; Vilardell, M.; Martínez, J.M.; Font, R.; Marcos-Gragera, R.; Puigdemont, M.; Viñas, G.; Carulla, M.; et al. Ten-Year Probabilities of Death Due to Cancer and Cardiovascular Disease among Breast Cancer Patients Diagnosed in North-Eastern Spain. Int. J. Env. Res. Public Health 2023, 20, 405. [Google Scholar] [CrossRef]
- Nichols, H.B.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Holmes, M.D.; Bersch, A.J.; Holick, C.N.; Hampton, J.M.; Stampfer, M.J.; Willett, W.C. Body mass index before and after breast cancer diagnosis: Associations with all-cause, breast cancer, and cardiovascular disease mortality. Canc. Epidem. Preven. Biomark. 2009, 18, 1403–1409. [Google Scholar] [CrossRef]
- Nechuta, S.; Chen, W.Y.; Cai, H.; Poole, E.M.; Kwan, M.L.; Flatt, S.W.; Patterson, R.E.; Pierce, J.P.; Caan, B.J.; Shu, X.O. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int. J. Cancer J. Int. Du Cancer 2016, 138, 2088–2097. [Google Scholar] [CrossRef]
- Battisti, S.; Guida, F.M.; Coppa, F.; Vaccaro, D.M.; Santini, D.; Tonini, G.; Zobel, B.B.; Semelka, R.C. Modification of Abdominal Fat Distribution After Aromatase Inhibitor Therapy in Breast Cancer Patients Visualized Using 3-D Computed Tomography Volumetry. Clin. Breast Cancer 2014, 14, 365–370. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Wang, Y.; Xiong, H.; Zhao, Y.; Sun, F. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 2016, 9, 2153–2168. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.A.; Cartmel, B.; Harrigan, M.; Fiellin, M.; Capozza, S.; Zhou, Y.; Ercolano, E.; Gross, C.P.; Hershman, D.; Ligibel, J.; et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity 2017, 25, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, W.D.N.; Vieira, A.; de Lira, C.; Mota, J.F.; Gentil, P.; Junior, R.D.F.; Battaglini, C.L.; Bottaro, M.; Vieira, C.A. Once a Week Resistance Training Improves Muscular Strength in Breast Cancer Survivors: A Randomized Controlled Trial. Integr. Cancer Ther. 2019, 18, 1534735419879748. [Google Scholar] [CrossRef] [PubMed]
- HHS. Physical Activity Guidelines for Americans, 2nd ed.; HHS: Washington, DC, USA, 2018.
- Crespo-Salgado, J.J.; Delgado-Martín, J.L.; Blanco-Iglesias, O.; Aldecoa-Landesa, S. Guía básica de detección del sedentarismo y recomendaciones de actividad física en atención primaria. Aten. Prim. 2015, 47, 175–183. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; LaMonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sport. Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Jones, S.M.W.; LaCroix, A.Z.; Li, W.; Zaslavsky, O.; Wassertheil-Smoller, S.; Weitlauf, J.; Brenes, G.A.; Nassir, R.; Ockene, J.K.; Caire-Juvera, G.; et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J. Cancer Surviv. 2015, 9, 620–629. [Google Scholar] [CrossRef]
- Beasley, J.M.; Kwan, M.L.; Chen, W.Y.; Weltzien, E.K.; Kroenke, C.H.; Lu, W.; Nechuta, S.J.; Cadmus-Bertram, L.; Patterson, R.E.; Sternfeld, B.; et al. Meeting the physical activity guidelines and survival after breast cancer: Findings from the after breast cancer pooling project. Breast Cancer Res. Treat. 2012, 131, 637–643. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1293–1311. [Google Scholar] [CrossRef]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control. 2011, 22, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Spei, M.-E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Stone, C.R.; Cheung, W.Y.; Hayes, S.C. Physical Activity and Mortality in Cancer Survivors: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2019, 4, pkz080. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA A Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, M.; Halmøy, A.; Faraone, S.V.; Haavik, J. Long-term efficacy and safety of treatment with stimulants and atomoxetine in adult ADHD: A review of controlled and naturalistic studies. Eur. Neuropsychopharmacol. 2013, 23, 508–527. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Mori, T.A. Dietary patterns, dietary nutrients and cardiovascular disease. Rev. Cardiovasc. Med. 2022, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.F.; Woodside, J.V.; Lunny, P.M.; Cardwell, C.R.; Cantwell, M.M. Dietary fat and breast cancer mortality: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Morimoto, Y.; Cooney, R.V.; Franke, A.A.; Shvetsov, Y.B.; Le Marchand, L.; Haiman, C.A.; Kolonel, L.N.; Goodman, M.T.; Maskarinec, G. Dietary Red and Processed Meat Intake and Markers of Adiposity and Inflammation: The Multiethnic Cohort Study. J. Am. Coll. Nutr. 2017, 36, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.I. Review: Dairy foods, red meat and processed meat in the diet: Implications for health at key life stages. Animal 2018, 12, 1709–1721. [Google Scholar] [CrossRef]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e4. [Google Scholar] [CrossRef]
- Sayon-Orea, C.; Martinez-Gonzalez, M.A.; Gea, A.; Alonso, A.; Pimenta, A.M.; Bes-Rastrollo, M. Baseline consumption and changes in sugar-sweetened beverage consumption and the incidence of hypertension: The SUN project. Clin. Nutr. 2015, 34, 1133–1140. [Google Scholar] [CrossRef]
- Brandt, P.A.V.D.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef]
- Weigl, J.; Hauner, H.; Hauner, D. Can Nutrition Lower the Risk of Recurrence in Breast Cancer? Breast Care 2018, 13, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Agudo, A.; Fonseca-Nunes, A.; Navarro, C.; Lagiou, P.; Demetriou, C.A.; Amiano, P.; Dorronsoro, M.; Chirlaque, M.-D.; et al. Olive oil intake and breast cancer risk in the Mediterranean countries of the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2012, 131, 2465–2469. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Boldo, E.; Pérez-Gómez, B.; Lope, V.; Altzibar, J.M.; Martín, V.; Castaño-Vinyals, G.; Guevara, M.; Dierssen-Sotos, T.; Tardón, A.; et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 2017, 103, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.; Wesselius, A.; Van Schooten, F.J.; Cheng, K.K.; Zeegers, M. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open 2017, 8, e014530. [Google Scholar] [CrossRef]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Baena Ruiz, R.; Salinas Hernández, P. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Couto, E.; Sandin, S.; Löf, M.; Ursin, G.; Adami, H.-O.; Weiderpass, E. Mediterranean Dietary Pattern and Risk of Breast Cancer. PLoS ONE 2013, 8, e55374. [Google Scholar] [CrossRef]
- de Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and breast cancer: A literature review on prevention, treatment and recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef]
- Savanelli, M.C.; Barrea, L.; Macchia, P.E.; Savastano, S.; Falco, A.; Renzullo, A.; Scarano, E.; Nettore, I.C.; Colao, A.; Di Somma, C. Preliminary results demonstrating the impact of Mediterranean diet on bone health. J. Transl. Med. 2017, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Sieri, S.; Chiodini, P.; Agnoli, C.; Pala, V.; Berrino, F.; Trichopoulou, A.; Benetou, V.; Vasilopoulou, E.; Sánchez, M.-J.; Chirlaque, M.-D.; et al. Dietary Fat Intake and Development of Specific Breast Cancer Subtypes. Gynecol. Oncol. 2014, 106, dju068. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Ávila, J.M.; Valero, T.; Del Pozo, S.; Rodriguez, P.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; et al. Energy Intake, Profile, and Dietary Sources in the Spanish Population: Findings of the ANIBES Study. Nutrients 2015, 7, 4739–4762. [Google Scholar] [CrossRef] [PubMed]
- Fassier, P.; Zelek, L.; Lécuyer, L.; Bachmann, P.; Touillaud, M.; Druesne-Pecollo, N.; Galan, P.; Cohen, P.; Hoarau, H.; Latino-Martel, P.; et al. Modifications in dietary and alcohol intakes between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Santé cohort. Int. J. Cancer 2017, 141, 457–470. [Google Scholar] [CrossRef]
- Song, D.; Deng, Y.; Liu, K.; Zhou, L.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; Wu, Y.; Zhai, Z.; et al. Vitamin D intake, blood vitamin D levels, and the risk of breast cancer: A dose-response meta-analysis of observational studies. Aging 2019, 11, 12708–12732. [Google Scholar] [CrossRef]
- Vance, V.; Campbell, S.; McCargar, L.; Mourtzakis, M.; Hanning, R. Dietary changes and food intake in the first year after breast cancer treatment. Appl. Physiol. Nutr. Metab. 2014, 39, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Wayne, S.J.; Lopez, S.T.; Butler, L.M.; Baumgartner, K.B.; Baumgartner, R.N.; Ballard-Barbash, R. Changes in dietary intake after diagnosis of breast cancer. J. Am. Diet. Assoc. 2004, 104, 1561–1568. [Google Scholar] [CrossRef]
- Maróti-Nagy, Á.; Paulik, E.; Thurzó, L.; Az egészségügyi személyzet életmód-tanácsadó tevékenységének szerepe daganatos betegséggel kezelt nők táplálkozási szokásainak megváltoztatásában. Magy. Onkol. 2010, 54, 41–45. [Google Scholar] [CrossRef]
- Coa, K.I.; Smith, K.C.; Klassen, A.C.; Caulfield, L.E.; Helzlsouer, K.; Peairs, K.; Shockney, L. Capitalizing on the “teachable moment” to promote healthy dietary changes among cancer survivors: The perspectives of health care providers. Support. Care Cancer 2015, 23, 679–686. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.C.C. Ingestas recomendadas de energia y nutrientes para la poblacion espanola. In Ediciones Piramide Grupo Anaya SE (ed) Tablas de Composicion de Alimentos, 17th ed.; Moreir: Madrid, Spain, 2017; pp. 258–259. [Google Scholar]
- Ruiz, E.; Ávila, J.M.; Valero, T.; Del Pozo, S.; Rodriguez, P.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; et al. Macronutrient Distribution and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2016, 8, 177. [Google Scholar] [CrossRef]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef]
- Tramm, R.; Mccarthy, A.L.; Yates, P. Dietary modification for women after breast cancer treatment: A narrative review. Eur. J. Cancer Care 2011, 20, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Essén, A.; Santaolalla, A.; Garmo, H.; Hammar, N.; Walldius, G.; Jungner, I.; Malmström, H.; Holmberg, L.; Van Hemelrijck, M. Baseline serum folate, vitamin B12 and the risk of prostate and breast cancer using data from the Swedish AMORIS cohort. Cancer Causes Control. 2019, 30, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Matejcic, M.; de Batlle, J.; Ricci, C.; Biessy, C.; Perrier, F.; Huybrechts, I.; Weiderpass, E.; Boutron-Ruault, M.; Cadeau, C.; His, M.; et al. Biomarkers of folate and vitamin B12 and breast cancer risk: Report from the EPIC cohort. Int. J. Cancer 2017, 140, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Foglietta, J.; Inno, A.; de Iuliis, F.; Sini, V.; Duranti, S.; Turazza, M.; Tarantini, L.; Gori, S. Cardiotoxicity of Aromatase Inhibitors in Breast Cancer Patients. Clin. Breast Cancer 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Freisling, H.; Noh, H.; Slimani, N.; Chajès, V.; May, A.M.; Peeters, P.H.; Weiderpass, E.; Cross, A.J.; Skeie, G.; Jenab, M.; et al. Nut intake and 5-year changes in body weight and obesity risk in adults: Results from the EPIC-PANACEA study. Eur. J. Nutr. 2018, 57, 2399–2408. [Google Scholar] [CrossRef]


| Control Group (CG) | Intervention Group (IG) | CG vs. IG | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| Age (years) | 61.54 | 5.19 | 60.48 | 4.96 | 0.494 |
| Tumor stage, n (%) | n | (%) | n | (%) | 0.495 |
| Stage I | 14 | 63.6 | 17 | 81.0 | |
| Stage II | 7 | 31.8 | 3 | 14.3 | |
| Stage III | 1 | 4.5 | 1 | 4.8 | |
| Treatment, n (%) | n | (%) | n | (%) | 0.251 |
| Surgery | 3 | 13.6 | 0 | 0.0 | |
| Radiotherapy | 9 | 40.9 | 13 | 61.9 | |
| Chemotherapy | 3 | 13.6 | 1 | 4.8 | |
| Radiotherapy and Chemotherapy | 7 | 31.8 | 7 | 33.3 | |
| Score PG-SGA (SD) | 4 | 2.0 | 5 | 3.0 | 0.417 |
| Nutritional status according to BMI, n (%) | n | (%) | n | (%) | 1.000 |
| Underweight | 0 | 0.0 | 0 | 0.0 | |
| Normal weight | 8 | 36.4 | 8 | 38.1 | |
| Overweight | 9 | 40.9 | 9 | 42.9 | |
| Obesity Class I | 4 | 18.2 | 4 | 19 | |
| Obesity Class II | 1 | 4.5 | 0 | 0.0 | |
| Obesity Class III | 0 | 0.0 | 0 | 0.0 | |
| Body composition | Mean | SD | Mean | SD | p-value |
| Weight (kg) | 68.0 | 9.7 | 66.0 | 7.9 | 0.451 |
| BMI (kg/m2) | 26.3 | 4.3 | 26.2 | 3.1 | 0.921 |
| % BF | 39.7 | 4.9 | 39.5 | 3.8 | 0.921 |
| Waist circumference (cm) | 85.4 | 8.8 | 85.2 | 7.8 | 0.961 |
| Waist hip ratio | 0.84 | 0.06 | 0.83 | 0.07 | 0.855 |
| Triceps skin fold (mm) | 20.4 | 6.6 | 20.1 | 4.4 | 0.842 |
| SAT (cm2) | 206.1 | 81.9 | 205.6 | 56.7 | 0.982 |
| VAT (cm2) | 111.0 | 57.3 | 99.9 | 39.4 | 0.501 |
| AMC (cm) | 23.8 | 2.3 | 23.7 | 1.7 | 0.849 |
| AMA (cm2) | 38.9 | 8.0 | 38.27 | 6.4 | 0.792 |
| MT (cm2) | 45.0 | 7.1 | 41.2 | 5.6 | 0.088 |
| Level of physical activity (METs/h/week) | 42.5 | 25.6 | 26.2 | 20.2 | 0.027 * |
| Control Group (CG) | Intervention Group (IG) | CG vs. IG | |||||
|---|---|---|---|---|---|---|---|
| Mean Difference † | SD | p-Value | Mean Difference † | SD | p-Value | p Mean Difference Cg-IG ‡ | |
| Weight (Kg) | 0.7 | 3.0 | 0.302 | −1.2 | 2.4 | 0.042 * | 0.033 * |
| BMI (kg/m2) | 0.3 | 1.1 | 0.244 | −0.4 | 1.0 | 0.057 | 0.031 * |
| % BF | 0.4 | 1.3 | 0.067 | −0.4 | 1.2 | 0.147 | 0.031 * |
| Waist circumference (cm) | 1.0 | 3.3 | 0.172 | −2.1 | 4.0 | 0.026 * | 0.008 ** |
| Waist Hip Ratio | 0.0 | 0.0 | 0.449 | −0.0 | 0.0 | 0.128 | 0.085 |
| Triceps skinfold (mm) | 1.2 | 3.8 | 0.286 | −1.5 | 3.1 | 0.039 * | 0.014 * |
| SAT (cm2) | 0.9 | 35.8 | 0.912 | −11.7 | 17.1 | 0.010 * | 0.188 |
| VAT (cm2) | 7.8 | 26.5 | 0.227 | −7.9 | 15.3 | 0.043 * | 0.036 * |
| AMC (cm) | −0.2 | 1.2 | 0.431 | 0.1 | 1.0 | 0.704 | 0.395 |
| AMA (cm2) | −0.9 | 4.9 | 0.406 | 0.3 | 3.7 | 0.731 | 0.386 |
| MT (cm2) | −0.4 | 6.0 | 0.760 | 1.6 | 3.5 | 0.078 | 0.121 |
| PG-SGA Score | 0 | 3 | 1.000 | −2 | 3 | 0.019 * | 0.067 |
| METs/h/week | −11.8 | 29.2 | 0.073 | 3.4 | 23.2 | 0.516 | 0.068 |
| Control Group (CG) | Intervention Group (IG) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | (a) | Initial | Final | (a) | (b) | ||||||||||||||
| Foods | SENC | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p | P ♯ |
| Cereals | 4–6 | 2.4 | 1.6 | 0.001 ** | 3.4 | 3.3 | 0.017 * | 1.0 | 3.1 | 0.664 | 2.4 | 2.1 | 0.002 * | 2.6 | 1.8 | 0.007 * | 0.3 | 2.5 | 0.115 | 0.771 |
| Vegetables | ≥2 | 2.2 | 0.9 | 0.211 | 2.4 | 1.2 | 0.094 | 0.2 | 1.0 | 0.832 | 2.2 | 0.8 | 0.346 | 2.7 | 1.6 | 0.664 | 0.5 | 1.6 | 0.503 | 0.568 |
| Fruit fresh | ≥3 | 2.7 | 1.8 | 0.053 | 2..8 | 2.2 | 0.053 | 0.1 | 1.7 | 1.000 | 2.2 | 1.1 | 0.004 * | 2.4 | 1.5 | 0.069 | 0.1 | 1.4 | 0.664 | 0.961 |
| Pulses | 2–3 | 2.5 | 0.9 | 0.015 * | 2.2 | 0.9 | 0.436 | −0.4 | 1.0 | 0.085 | 2.0 | 1.1 | 0.263 | 2.0 | 1.0 | 0.078 | −0.1 | 1.4 | 0.860 | 0.376 |
| Nuts | 3–7 | 2.0 | 2.2 | 0.078 | 3.1 | 4.2 | 0.064 | 1.0 | 4.3 | 0.077 | 1.8 | 2.8 | 0.007 * | 2.3 | 2.4 | 0.144 | 0.5 | 3.3 | 0.332 | 0.854 |
| Dairy | 2–4 | 2.8 | 1.8 | 0.524 | 2.8 | 1.9 | 0.832 | −0.1 | 2.0 | 0.908 | 2.7 | 1.7 | 0.189 | 2.5 | 1.2 | 0.087 | −0.2 | 1.9 | 0.664 | 0.961 |
| Fish | 2–3 | 5.6 | 2.3 | 0.001 * | 6.0 | 3.1 | 0.001 * | 0.4 | 2.6 | 0.495 | 5.8 | 2.9 | ˂0.001 * | 5.3 | 2.1 | ˂0.001 * | −0.5 | 2.5 | 0.399 | 0.281 |
| Poultry | 3–4 | 2.5 | 1.4 | 0.132 | 2.4 | 1.3 | 0.051 | −0.1 | 1.5 | 0.791 | 2.6 | 1.8 | 0.272 | 2.7 | 1.7 | 0.420 | 0.1 | 1.1 | 0.585 | 0.595 |
| Red meat | ≤1 | 2.7 | 1.6 | 0.001 * | 2.5 | 2.5 | 0.383 | −0.2 | 2.1 | 0.049 * | 2.0 | 1.3 | 0.001 * | 2.1 | 1.6 | 0.019 * | 0.1 | 1.5 | 1.000 | 0.208 |
| Eggs | 3–5 | 2.8 | 1.5 | 0.576 | 2.5 | 1.2 | 0.125 | −0.3 | 1.1 | 1.000 | 2.5 | 1.0 | 0.063 | 3.5 | 3.5 | 1.000 | 1.1 | 3.7 | 0.625 | 0.252 |
| Fast food, pastries, soft drinks | ≤1 | 3.8 | 4.0 | 0.286 | 3.7 | 4.4 | 0.286 | −0.1 | 4.1 | 0.824 | 4.4 | 4.4 | 0.012 * | 5.0 | 4.4 | 0.007 * | 0.5 | 5.4 | 0.629 | 0.618 |
| Alcoholic drinks | 1 | 0.3 | 0.4 | 0.001 * | 0.4 | 0.5 | 0.001 * | 0.1 | 0.3 | 0.267 | 0.4 | 0.5 | ˂0.001 * | 0.5 | 0.8 | ˂0.001 * | 0.1 | 0.4 | 0.581 | 0.871 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Unciti, M.; Palacios Samper, N.; Méndez-Sandoval, S.; Idoate, F.; Ibáñez-Santos, J. Effect of Combining Impact-Aerobic and Strength Exercise, and Dietary Habits on Body Composition in Breast Cancer Survivors Treated with Aromatase Inhibitors. Int. J. Environ. Res. Public Health 2023, 20, 4872. https://doi.org/10.3390/ijerph20064872
Garcia-Unciti M, Palacios Samper N, Méndez-Sandoval S, Idoate F, Ibáñez-Santos J. Effect of Combining Impact-Aerobic and Strength Exercise, and Dietary Habits on Body Composition in Breast Cancer Survivors Treated with Aromatase Inhibitors. International Journal of Environmental Research and Public Health. 2023; 20(6):4872. https://doi.org/10.3390/ijerph20064872
Chicago/Turabian StyleGarcia-Unciti, Marisol, Natalia Palacios Samper, Sofía Méndez-Sandoval, Fernando Idoate, and Javier Ibáñez-Santos. 2023. "Effect of Combining Impact-Aerobic and Strength Exercise, and Dietary Habits on Body Composition in Breast Cancer Survivors Treated with Aromatase Inhibitors" International Journal of Environmental Research and Public Health 20, no. 6: 4872. https://doi.org/10.3390/ijerph20064872
APA StyleGarcia-Unciti, M., Palacios Samper, N., Méndez-Sandoval, S., Idoate, F., & Ibáñez-Santos, J. (2023). Effect of Combining Impact-Aerobic and Strength Exercise, and Dietary Habits on Body Composition in Breast Cancer Survivors Treated with Aromatase Inhibitors. International Journal of Environmental Research and Public Health, 20(6), 4872. https://doi.org/10.3390/ijerph20064872






