Immune Checkpoint Inhibitor Related Rheumatological Complications: Cooperation between Rheumatologists and Oncologists
Abstract
1. Introduction: The Role of Immune Checkpoint Inhibitors in Oncology
2. Methods
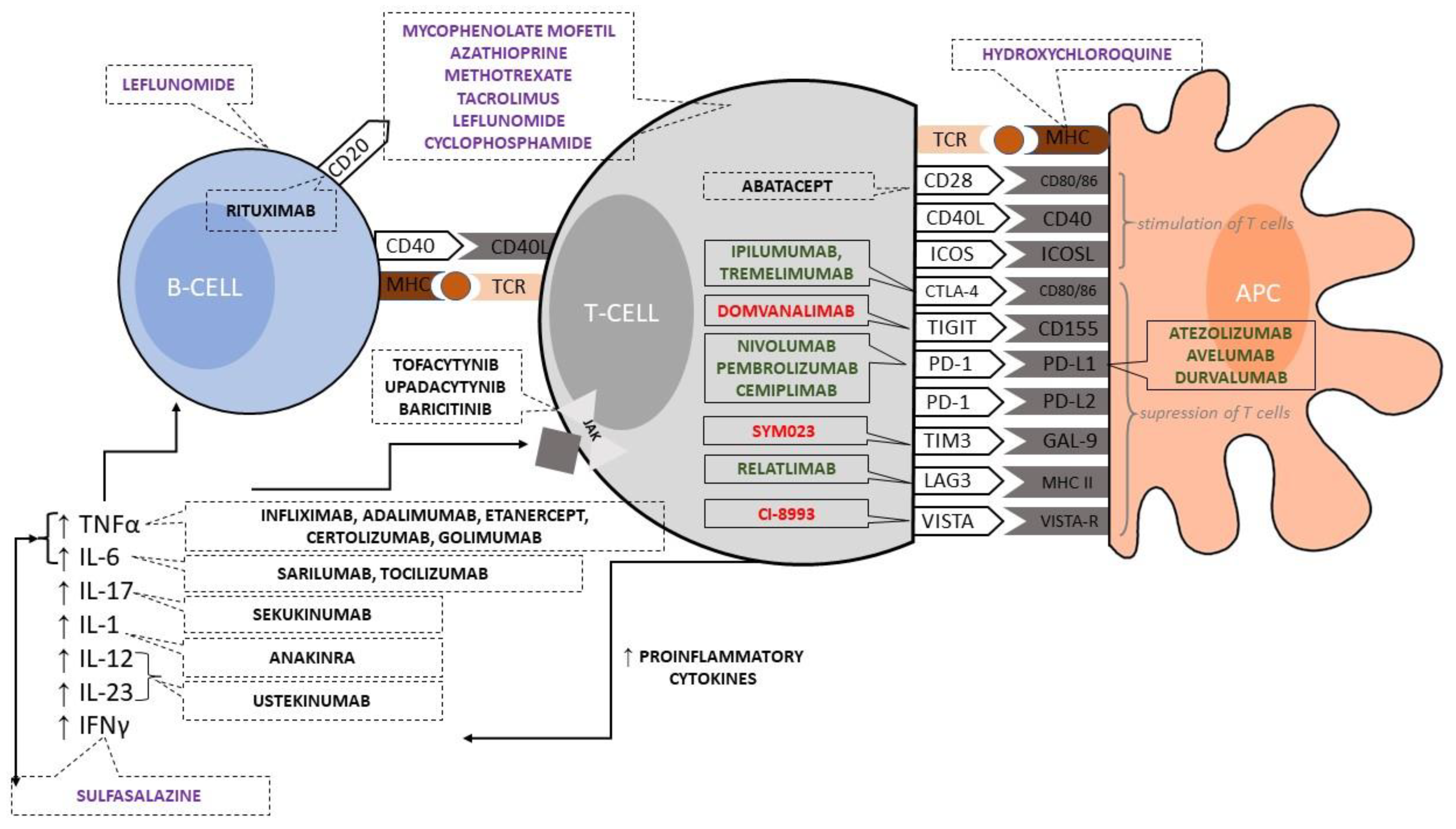
3. Suppressor Modulation of T Cell and Antigen-Presenting Cell Cooperation by Immune Checkpoint Inhibitors
4. Risk Factors for-Rheumatic Adverse Events Related to Immune Checkpoint Inhibitors Are Unknown
5. General Principles and Initial Approach to Rheumatic Adverse Events in Patients on Immune Checkpoint Inhibitors
6. Clinical Characteristics and Management of the Rheumatic Adverse Events Related to Immune Checkpoint Inhibitors
6.1. Inflammatory Arthritis
6.2. Inflammatory Myopathies
6.3. Polymyalgia Rheumatica (PMR)
6.4. Sicca Syndrome and Ocular Disorders
6.5. Others: Vasculitis, Sarcoidosis, and Lupus Erythematosus
| Rheumatic Immune-Related Adverse Events | Signs and Symptoms | Confirmatory Tests | Treatment |
|---|---|---|---|
| Rheumatoid-arthritis-like | Symmetric peripheral polyarticular phenotype. Joint pain accompanied by swelling, morning stiffness with improvement after anti-inflammatory agents. |
| G1: First line: NSAIDs/intra-articular corticosteroid, prednisone up to 20 mg/d for 4 weeks. Second line: DMARDs (hydroxychloroquine, sulfasalazine, methotrexate, and leflunomide). Continuation of ICIs G2: First line: NSAIDs/intra-articular corticosteroid, prednisone up to 0.5 mg/kg/d for 2–3 weeks with tapering. Second line: csDMARDs (sulfasalazine, methotrexate, and leflunomide) or bDMARDs (anti-TNFα and anti-IL-6). Temporal discontinuation of ICIs until G0/G1 G3–4: First line: NSAIDs/intra-articular corticosteroid, prednisone up to 1 mg/kg/d for 2–3 weeks with tapering. Second line: csDMARDs (methotrexate in doses ≥15 mg weekly) with bDMARDs (anti-TNFα and anti-IL-6). ICIs cessation |
| Psoriatic-arthritis-like | Mono/oligoarticular phenotype. Joint pain accompanied by swelling, morning stiffness with improvement after anti-inflammatory agents. |
| G1: NSAIDs/intra-articular corticosteroid, consider methotrexate. Continuation of ICIs G2: NSAIDs/intra-articular corticosteroids with or without csDMARDs (sulfasalazine/methotrexate and azathioprine) or bDMARDs (anti-TNFα, antiIL-17, anti-IL-12/23, and JAK inhibitors). Temporal discontinuation of ICIs until G0/G1 G3–4: NSAIDs/intra-articular corticosteroid with csDMARDs (sulfasalazine/methotrexate and Azathioprine) or bDMARDs (anti-TNFα,anti-IL-17, anti-IL-12/23, andJAK inhibitors). ICIs cessation |
| Spondylarthritis -like | Peripheral/axial spondyloarthropathy with or without a colitis phenotype. Joint pain accompanied by swelling, morning stiffness with improvement after anti-inflammatory agents. |
| G1: NSAIDs/sacroiliac corticosteroid. Continuation of ICIs G2: NSAIDs/sacroiliac corticosteroid with or without bDMARDs (anti-TNF, anti-IL-12/23, and JAKi; avoid anti-IL-17 when colitis). Temporal discontinuation of ICIs until G0/G1 G3–4: NSAIDs/sacroiliac corticosteroid with bDMARDs (TNFi, anti-IL-12/23, and JAKi; avoid anti-IL-17 when colitis). ICIs cessation |
| Inflammatory myopathies | Muscle weakness and pain. Typically, weakness of the proximal limbs. Possible presence of bulbar symptoms (dysphagia, dyspnea, and diplopia), myocarditis, and interstitial lung disease. Typical for dermatomyositis skin lesions to occur. |
| G1: First line: NSAIDs with or without prednisone 0.5 mg/kg/d. Second line: DMARDs (mycophenolate mofetil, methotrexate, and Azathioprine). Continuation of ICIs G2: First line: NSAIDs with or without prednisone 1 mg/kg/d Second line: DMARDs (mycophenolate mofetil, methotrexate, and azathioprine). Temporal discontinuation of ICIs until G0/G1 G3: High-dose systemic steroids (1–2 mg/kg/d) with or without immunosuppressive intravenous immune globulin dose. Second line: rituximab, tacrolimus, and abatacept ICIs cessation G4: Pulse-dose systemic steroids with or without immunosuppressive intravenous immune globulin dose and plasmapheresis. Second line: rituximab, tacrolimus, and abatacept. ICIs cessation |
| Polymyalgia rheumatica | Symmetrical pain and stiffness of the proximal upper/lower extremities without muscle injury. In rare cases associated with large vessel vasculitis. |
| G1: First line: prednisone up to 20 mg/d for 6 weeks/intra-articular corticosteroid. Second line: methotrexate. Continuation of ICIs G2: First line: prednisone up to 30 mg/d for 6 weeks/intra-articular corticosteroids. Second line: methotrexate and anti-IL-6. Temporal discontinuation of ICIs until G0/G1 G3–4: First line: pulse-dose systemic steroids. Second line: anti-IL-6 biologics. ICIs cessation |
| Sicca syndrome and ocular disorders | Sjogren-like syndrome. Eyes/mouth/genital area dryness. Joints and muscle pain possible. Uveitis, peripheral ulcerative keratitis, and other forms of ocular inflammation. |
| G1: First line: saliva substitute/artificial tears. Second line: pilocarpine or other sialagogues and hydroxychloroquine. Continuation of ICIs G2–4: Prednisone up to 40 mg/d for 4 weeks. Temporal discontinuation of ICIs until G0/G1 or ICIs cessation |
| Vasculitis | Skin lesions: purpura, ulcers, bloody nasal discharge, edema, dyspnea, cough, hemoptysis, hematuria, proteinuria, and polyneuropathy. |
| G1–G2: Prednisone up to 1–2 mg/kg/d or pulses and hydroxychloroquine for skin vasculitis). Second line: cyclophosphamide and methotrexate. Temporal discontinuation of ICIs until G0/G1 G3-G4: Rituximab or plasma exchange. ICIs cessation |
| Systemic sclerosis * | Skin thickening, sclerodactylitis, phalangeal ulcers, Raynaud’s phenomenon, calcinosis, dyspnea, and cough. |
| G2–4: Prednisone up to 1 mg/kg/d with or without hydroxychloroquine. Second line: mycophenolate mofetil, azathioprine, and methotrexate (cyclophosphamide in interstitial lung disease). Temporal discontinuation of ICIs until G0/G1 or ICIs cessation |
| Sarcoidosis * | Arthralgia, uveitis, cough, dyspnea, and lymphadenopathy. |
| G2–4: Prednisone up to 1 mg/kg/d. Taper steroids over 2–4 months. Second line: methotrexate, azathioprine, and mycophenolate mofetil. Temporal discontinuation of ICIs until G0/G1 or ICIs cessation |
- Raynaud’s phenomenon—avoidance of cold exposure and vasoconstricting drugs, smoking cessation, calcium channel blockers/phosphodiesterase type 5 inhibitors/topical nitrate/angiotensin II receptor blockers [60];
- Arthralgia—non-opioid analgesics such as nonsteroidal anti-inflammatory drugs or acetaminophen [59]; physical therapy—exercise is generally a safe option for all cancer patients, but for those with active disease, some forms of physical modalities are contraindicated, including heat, ultrasound, transcutaneous electrical nerve stimulation, functional electrical stimulation, low-level light laser, and manual therapy (for more information see [61]);
- Kidney disease—blood pressure control using angiotensin-converting enzyme inhibitors, avoiding values lower than 120/80 mmHG [62];
- Neuropathy—rehabilitation and orthoses, pain control using amitriptyline/nortriptyline, duloxetine, gabapentin, or pregabalin with avoidance of opioids [63];
- Osteoporosis prevention—for patients receiving any dose of steroids for ≥3 months, a total calcium intake of 1000–1200 mg/day and vitamin D intake of 600–2000 international units/day through either diet and/or supplements maintained with occasional vitamin D and calcium serum concentration controlling [64];
- Prevention of opportunistic infections—prophylactic vaccinations (optimal before immunosuppressive therapy begins) for patients receiving high-dose steroids and/or other immunosuppressive agents consider pneumocystis pneumonia prophylaxis using trimethoprim-sulfamethoxazole [54].
7. Oncologic Immunotherapy with Checkpoint Inhibitors in Patients with Pre-Existing Rheumatic Diseases Seem to Be Safe, Albeit Needing Vigilance
7.1. Pre-Existing Rheumatic Diseases Might Worsen during Immune Checkpoint Inhibitor Therapy
| Population | Year | Type of Study | No of Patients with Autoimmunity | No of Patients with Rheumatic Diseases | Outcome * | Ref. |
|---|---|---|---|---|---|---|
| Italian patients with pre-existing autoimmunity and advanced cancer receiving anti-PD-1 mAbs. | 2019 | Retrospective observationalMulticenter | 85 | 10 |
| [66] |
| French population with pre-existing autoimmunity and cancer treaded with ICIs. | 2019 | Retrospective observationalmulticenter | 112 | 20 |
| [69] |
| Patients with autoimmune disorders and cancer receiving ICIs (49 publications). | 2018 | Systematic review | 123 | 68 |
| [68] |
| Cancer patients with pre-existing rheumatic conditions receiving ICIs. | 2018 | Single-center case series | N/A | 16 |
| [67] |
| Greek cancer patients with pre-existing autoimmune conditions receiving ICIs. | 2022 | Retrospective observationalmulticenter | 123 | 54 |
| [65] |
| Cancer patients with pre-existing rheumatic conditions receiving ICIs. | 2022 | Single-center case series | N/A | 45 |
| [77] |
| Cancer patients with pre-existing inflammatory or autoimmune conditions receiving anti-PD-1 and registered in REISAMIC registry. | 2018 | Prospective, multicenter national registry (real-world database) | 45 | 11 |
| [78] |
| NSCLC patients treated in the USA with pre-existing autoimmune conditions receiving anti-PD-1 mAbs. | 2018 | Retrospective observationalmulticenter | 56 | 25 |
| [70] |
| Melanoma patients with pre-existing autoimmune conditions receiving anti-PD-1 mAbs. | 2017 | Retrospective observationalmulticenter | 52 | 27 |
| [76] |
| Melanoma patients with pre-existing autoimmune conditions receiving ipilimumab. | 2016 | Retrospective observationalmulticenter | 30 | 10 |
| [71] |
7.2. Patients with Pre-Existing Autoimmune Disorders Treated with Immune Checkpoint Inhibitors Likely Have a Similar Risk of All Immune-Related Adverse Events to the General Population
7.3. Targeted Treatments Managing Immune-Related Adverse Events Do Not Compromise Immunotherapy Efficacy
8. Surprisingly, Patients Who Develop Immune-Related Adverse Events on Immune Checkpoint Inhibitors Have Better Cancer Clinical Outcomes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Gutierrez, A.K.; Bingham, C.O.; Shah, A.A. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. 2017, 69, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Previously Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.H.; Calabrese, C.; Cappelli, L.C. Rheumatic Immune-Related Adverse Events from Cancer Immunotherapy. Nat. Rev. Rheumatol. 2018, 14, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Wicky, A.; Bouchaab, H.; Imbimbo, M.; Delyon, J.; Gautron Moura, B.; Gérard, C.L.; Latifyan, S.; Özdemir, B.C.; Caikovski, M.; et al. Late-Onset and Long-Lasting Immune-Related Adverse Events from Immune Checkpoint-Inhibitors: An Overlooked Aspect in Immunotherapy. Eur. J. Cancer 2021, 149, 153–164. [Google Scholar] [CrossRef]
- Chatzidionysiou, K.; Liapi, M.; Tsakonas, G.; Gunnarsson, I.; Catrina, A. Treatment of Rheumatic Immune-Related Adverse Events Due to Cancer Immunotherapy with Immune Checkpoint Inhibitors—Is It Time for a Paradigm Shift? Clin. Rheumatol. 2021, 40, 1687–1695. [Google Scholar] [CrossRef]
- Calabrese, C.; Kirchner, E.; Kontzias, K.; Velcheti, V.; Calabrese, L.H. Rheumatic Immune-Related Adverse Events of Checkpoint Therapy for Cancer: Case Series of a New Nosological Entity. RMD Open 2017, 3, e000412. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, J.; Xu, D.; Zeng, X. Rheumatic Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors. Asia Pac. J. Clin. Oncol. 2021, 17, 178–185. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Kostine, M.; Finckh, A.; Bingham, C.O.; Visser, K.; Leipe, J.; Schulze-Koops, H.; Choy, E.H.; Benesova, K.; Radstake, T.R.D.J.; Cope, A.P.; et al. EULAR Points to Consider for the Diagnosis and Management of Rheumatic Immune-Related Adverse Events Due to Cancer Immunotherapy with Checkpoint Inhibitors. Ann. Rheum. Dis. 2021, 80, 36–48. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2021; p. 600. [Google Scholar]
- Chen, L. Co-Inhibitory Molecules of the B7-CD28 Family in the Control of T-Cell Immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Placek, K.; Coffre, M.; Maiella, S.; Bianchi, E.; Rogge, L. Genetic and Epigenetic Networks Controlling T Helper 1 Cell Differentiation. Immunology 2009, 127, 155. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.; Surh, C.D. Normal T Cell Homeostasis: The Conversion of Naive Cells into Memory-Phenotype Cells. Nat.Immunol. 2011, 12, 478–484. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Schwartz, R.S. Immunosuppression--the Promise of Specificity. N. Engl. J. Med. 2005, 353, 836–839. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Z. Tissue-Resident Memory T Cells and Their Biological Characteristics in the Recurrence of Inflammatory Skin Disorders. Cell. Mol. Immunol. 2020, 17, 64–75. [Google Scholar] [CrossRef]
- Murphy, K.M.; Stockinger, B. Effector T Cell Plasticity: Flexibility in the Face of Changing Circumstances. Nat. Immunol. 2010, 11, 674–680. [Google Scholar] [CrossRef]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X.; et al. BTLA Is a Lymphocyte Inhibitory Receptor with Similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel Immune Checkpoint Targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Van Der Vlist, M.; Kuball, J.; Radstake, T.R.D.; Meyaard, L. Immune Checkpoints and Rheumatic Diseases: What Can Cancer Immunotherapy Teach Us? Nat. Rev. Rheumatol. 2016, 12, 593–604. [Google Scholar] [CrossRef]
- Olde Nordkamp, M.J.M.; Koeleman, B.P.; Meyaard, L. Do Inhibitory Immune Receptors Play a Role in the Etiology of Autoimmune Disease? Clin. Immunol. 2014, 150, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Raptopoulou, A.P.; Bertsias, G.; Makrygiannakis, D.; Verginis, P.; Kritikos, I.; Tzardi, M.; Klareskog, L.; Catrina, A.I.; Sidiropoulos, P.; Boumpas, D.T. The Programmed Death 1/Programmed Death Ligand 1 Inhibitory Pathway Is up-Regulated in Rheumatoid Synovium and Regulates Peripheral T Cell Responses in Human and Murine Arthritis. Arthritis Rheum. 2010, 62, 1870–1880. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Gao, L.; Wang, X.; Hu, X.; Wu, M.; Wu, J.; Xu, T.; Shi, Q.; Zhang, X. Soluble PD-1 Aggravates Progression of Collagen-Induced Arthritis through Th1 and Th17 Pathways. Arthritis Res. 2015, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Rheumatologic Complications of Checkpoint Inhibitor Immunotherapy-UpToDate. Available online: https://www.uptodate.com/contents/rheumatologic-complications-of-checkpoint-inhibitor-immunotherapy#H3841094854 (accessed on 30 December 2022).
- Cunningham-Bussel, A.; Wang, J.; Prisco, L.C.; Martin, L.W.; Vanni, K.M.M.; Zaccardelli, A.; Nasrallah, M.; Gedmintas, L.; MacFarlane, L.A.; Shadick, N.A.; et al. Predictors of Rheumatic Immune-Related Adverse Events and De Novo Inflammatory Arthritis after Immune Checkpoint Inhibitor Treatment for Cancer. Arthritis Rheumatol. 2022, 74, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wahren-Herlenius, M.; Dörner, T. Immunopathogenic Mechanisms of Systemic Autoimmune Disease. Lancet 2013, 382, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J.; et al. Five Amino Acids in Three HLA Proteins Explain Most of the Association between MHC and Seropositive Rheumatoid Arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Dorak, M.T.; Bettinotti, M.P.; Bingham, C.O.; Shah, A.A. Association of HLA-DRB1 Shared Epitope Alleles and Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis. Rheumatology 2019, 58, 476. [Google Scholar] [CrossRef]
- Chowell, D.; Morris, L.G.T.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N.; et al. Patient HLA Class I Genotype Influences Cancer Response to Checkpoint Blockade Immunotherapy. Science 2018, 359, 582. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, R.; Le Burel, S.; Dunogeant, L.; Marabelle, A.; Hollebecque, A.; Besse, B.; Leary, A.; Voisin, A.L.; Pontoizeau, C.; Coutte, L.; et al. Rheumatoid Arthritis and Polymyalgia Rheumatica Occurring after Immune Checkpoint Inhibitor Treatment. Ann. Rheum. Dis. 2017, 76, 1747–1750. [Google Scholar] [CrossRef]
- Kostine, M.; Rouxel, L.; Barnetche, T.; Veillon, R.; Martin, F.; Dutriaux, C.; Dousset, L.; Pham-Ledard, A.; Prey, S.; Beylot-Barry, M.; et al. Rheumatic Disorders Associated with Immune Checkpoint Inhibitors in Patients with Cancer-Clinical Aspects and Relationship with Tumour Response: A Single-Centre Prospective Cohort Study. Ann. Rheum. Dis. 2018, 77, 393–398. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 21 July 2022).
- Woodworth, T.; Furst, D.E.; Alten, R.; Bingham, C.; Yocum, D.; Sloan, V.; Tsuji, W.; Stevens, R.; Fries, J.; Witter, J.; et al. Rheumatology Common Toxicity Criteria v.2.0 Personal Non-Commercial Use Only. J. Rheumatol. 2007, 34, 1401–1415. [Google Scholar] [PubMed]
- Braaten, T.J.; Brahmer, J.R.; Forde, P.M.; Le, D.; Lipson, E.J.; Naidoo, J.; Schollenberger, M.; Zheng, L.; Bingham, C.O.; Shah, A.A.; et al. Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Persists after Immunotherapy Cessation. Ann. Rheum. Dis. 2020, 79, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Brahmer, J.R.; Forde, P.M.; Le, D.T.; Lipson, E.J.; Naidoo, J.; Zheng, L.; Bingham, C.O.; Shah, A.A. Clinical Presentation of Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Differs by Immunotherapy Regimen. Semin. Arthritis Rheum. 2018, 48, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Albayda, J.; Dein, E.; Shah, A.A.; Bingham, C.O.; Cappelli, L. Sonographic Findings in Inflammatory Arthritis Secondary to Immune Checkpoint Inhibition: A Case Series. ACR Open Rheumatol. 2019, 1, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Cappelli, L.C.; Forde, P.M.; Marrone, K.A.; Lipson, E.J.; Hammers, H.J.; Sharfman, W.H.; Le, D.T.; Baer, A.N.; Shah, A.A.; et al. Inflammatory Arthritis: A Newly Recognized Adverse Event of Immune Checkpoint Blockade. Oncologist 2017, 22, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Gutierrez, A.K.; Baer, A.N.; Albayda, J.; Manno, R.L.; Haque, U.; Lipson, E.J.; Bleich, K.B.; Shah, A.A.; Naidoo, J.; et al. Inflammatory Arthritis and Sicca Syndrome Induced by Nivolumab and Ipilimumab. Ann. Rheum. Dis. 2017, 76, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Draghi, A.; Borch, T.H.; Radic, H.D.; Chamberlain, C.A.; Gokuldass, A.; Svane, I.M.; Donia, M. Differential Effects of Corticosteroids and Anti-TNF on Tumor-Specific Immune Responses: Implications for the Management of IrAEs. Int. J. Cancer 2019, 145, 1408–1413. [Google Scholar] [CrossRef]
- Thủ, T.Y.; Maria, A.T.J.; Ladhari, C.; Palassin, P.; Quantin, X.; Lesage, C.; Taïeb, G.; Ayrignac, X.; Rullier, P.; Hillaire-Buys, D.; et al. Rheumatic Disorders Associated with Immune Checkpoint Inhibitors: What about Myositis? An Analysis of the WHO’s Adverse Drug Reactions Database. Ann. Rheum. Dis. 2022, 81, E32. [Google Scholar] [CrossRef]
- Liewluck, T.; Kao, J.C.; Mauermann, M.L. PD-1 Inhibitor-Associated Myopathies: Emerging Immune-Mediated Myopathies. J. Immunother. 2018, 41, 208–211. [Google Scholar] [CrossRef]
- Ali, S.S.; Goddard, A.L.; Luke, J.J.; Donahue, H.; Todd, D.J.; Werchniak, A.; Vleugels, R.A. Drug-Associated Dermatomyositis Following Ipilimumab Therapy: A Novel Immune-Mediated Adverse Event Associated with Cytotoxic T-Lymphocyte Antigen 4 Blockade. JAMA Derm. 2015, 151, 195–199. [Google Scholar] [CrossRef]
- Hunter, G.; Voll, C.; Robinson, C.A. Autoimmune Inflammatory Myopathy after Treatment with Ipilimumab. Can. J. Neurol. Sci. 2009, 36, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Albarrán-Artahona, V.; Laguna, J.C.; Gorría, T.; Torres-Jiménez, J.; Pascal, M.; Mezquita, L. Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy. Diagnostics 2022, 12, 2091. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, N.S.; Wetter, D.A.; Wieland, C.N.; Shenoy, N.K.; Markovic, S.N.; Thanarajasingam, U. Scleroderma Induced by Pembrolizumab: A Case Series. Mayo Clin. Proc. 2017, 92, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Shikano, K.; Kaneko, K.; Kaburaki, K.; Isobe, K.; Kawabe, K.; Homma, S.; Kawai, S.; Nanki, T. Nivolumab-Induced Anti-Aminoacyl-TRNA Synthetase Antibody-Positive Polymyositis Complicated by Interstitial Pneumonia in a Patient with Lung Adenocarcinoma. Scand. J. Rheumatol. 2019, 49, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Cappelli, L.C.; Kostine, M.; Kirchner, E.; Braaten, T.; Calabrese, L. Polymyalgia Rheumatica-like Syndrome from Checkpoint Inhibitor Therapy: Case Series and Systematic Review of the Literature. RMD Open 2019, 5, e000906. [Google Scholar] [CrossRef]
- Goldstein, B.L.; Gedmintas, L.; Todd, D.J. Drug-Associated Polymyalgia Rheumatica/Giant Cell Arteritis Occurring in Two Patients after Treatment with Ipilimumab, an Antagonist of Ctla-4. Arthritis Rheumatol. 2014, 66, 768–769. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Davies, M.; Elshoury, A.; Gesthalter, Y.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Warner, B.M.; Baer, A.N.; Lipson, E.J.; Allen, C.; Hinrichs, C.; Rajan, A.; Pelayo, E.; Beach, M.; Gulley, J.L.; Madan, R.A.; et al. Sicca Syndrome Associated with Immune Checkpoint Inhibitor Therapy. Oncologist 2019, 24, 1259–1269. [Google Scholar] [CrossRef]
- Lomax, A.J.; McGuire, H.M.; McNeil, C.; Choi, C.J.; Hersey, P.; Karikios, D.; Shannon, K.; van Hal, S.; Carr, U.; Crotty, A.; et al. Immunotherapy-Induced Sarcoidosis in Patients with Melanoma Treated with PD-1 Checkpoint Inhibitors: Case Series and Immunophenotypic Analysis. Int. J. Rheum. Dis. 2017, 20, 1277–1285. [Google Scholar] [CrossRef]
- Gallan, A.J.; Alexander, E.; Reid, P.; Kutuby, F.; Chang, A.; Henriksen, K.J. Renal Vasculitis and Pauci-Immune Glomerulonephritis Associated With Immune Checkpoint Inhibitors. Am. J. Kidney Dis. 2019, 74, 853–856. [Google Scholar] [CrossRef]
- Clinical Manifestations and Diagnosis of Vasculitic Neuropathies-UpToDate. Available online: https://www-1uptodate-1com-1v54045na046f.hanproxy.cm-uj.krakow.pl/contents/clinical-manifestations-and-diagnosis-of-vasculitic-neuropathies?search=rheumatic%20complications%20immunotherapy&topicRef=8226&source=see_link#H2000456337 (accessed on 29 January 2023).
- Collins, M.P.; Dyck, P.J.B.; Gronseth, G.S.; Guillevin, L.; Hadden, R.D.M.; Heuss, D.; Léger, J.M.; Notermans, N.C.; Pollard, J.D.; Said, G.; et al. Peripheral Nerve Society Guideline on the Classification, Diagnosis, Investigation, and Immunosuppressive Therapy of Non-Systemic Vasculitic Neuropathy: Executive Summary. J. Peripher. Nerv. Syst. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Fadel, F.; Karoui, K.E.; Knebelmann, B. Anti-CTLA4 Antibody-Induced Lupus Nephritis. N. Engl. J. Med. 2009, 361, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Overview of the Treatment and Prognosis of Systemic Sclerosis (Scleroderma) in Adults-UpToDate. Available online: https://www-1uptodate-1com-1v54045na046f.hanproxy.cm-uj.krakow.pl/contents/overview-of-the-treatment-and-prognosis-of-systemic-sclerosis-scleroderma-in-adults?search=rheumatic%20complications%20immunotherapy&topicRef=7539&source=see_link#H13 (accessed on 29 January 2023).
- Treatment of Pulmonary Sarcoidosis: Initial Approach to Treatment-UpToDate. Available online: https://www-1uptodate-1com-1v54045na046f.hanproxy.cm-uj.krakow.pl/contents/treatment-of-pulmonary-sarcoidosis-initial-approach-to-treatment?search=sarcoidosis&topicRef=4353&source=related_link (accessed on 29 January 2023).
- Shen, P.; Deng, X.; Hu, Z.; Chen, Z.; Huang, Y.; Wang, K.; Qin, K.; Huang, Y.; Ba, X.; Yan, J.; et al. Rheumatic Manifestations and Diseases From Immune Checkpoint Inhibitors in Cancer Immunotherapy. Front. Med. 2021, 8, 762247. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Raynaud Phenomenon: Initial Management-UpToDate. Available online: https://www-1uptodate-1com-1v54045na046f.hanproxy.cm-uj.krakow.pl/contents/treatment-of-raynaud-phenomenon-initial-management?search=rheumatic%20complications%20immunotherapy&topicRef=7542&source=see_link (accessed on 29 January 2023).
- Maltser, S.; Cristian, A.; Silver, J.K.; Morris, G.S.; Stout, N.L. A Focused Review of Safety Considerations in Cancer. PM&R 2017, 9, S415. [Google Scholar] [CrossRef]
- Kidney Disease in Systemic Sclerosis (Scleroderma), Including Scleroderma Renal Crisis-UpToDate. Available online: https://www-1uptodate-1com-1v54045na046f.hanproxy.cm-uj.krakow.pl/contents/kidney-disease-in-systemic-sclerosis-scleroderma-including-scleroderma-renal-crisis?sectionName=Surveillance&search=rheumatic%20complications%20immunotherapy&topicRef=7542&anchor=H14&source=see_link#H152417808 (accessed on 29 January 2023).
- Treatment and Prognosis of Nonsystemic Vasculitic Neuropathy-UpToDate. Available online: https://www-1uptodate-1com-1v54045na046f.hanproxy.cm-uj.krakow.pl/contents/treatment-and-prognosis-of-nonsystemic-vasculitic-neuropathy?search=rheumatic%20complications%20immunotherapy&topicRef=8236&source=see_link (accessed on 29 January 2023).
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, E.; Lampaki, S.; Koliou, G.A.; Koumarianou, A.; Levva, S.; Vagionas, A.; Christopoulou, A.; Laloysis, A.; Psyrri, A.; Binas, I.; et al. Real-World Safety and Efficacy Data of Immunotherapy in Patients with Cancer and Autoimmune Disease: The Experience of the Hellenic Cooperative Oncology Group. Cancer Immunol. Immunother. 2022, 71, 327. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Buti, S.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Bersanelli, M.; Michiara, M.; Grassadonia, A.; Brocco, D.; et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019, 24, e327–e337. [Google Scholar] [CrossRef]
- Richter, M.D.; Pinkston, O.; Kottschade, L.A.; Finnes, H.D.; Markovic, S.N.; Thanarajasingam, U. Brief Report: Cancer Immunotherapy in Patients With Preexisting Rheumatic Disease: The Mayo Clinic Experience. Arthritis Rheumatol. 2018, 70, 356–360. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef]
- Tison, A.; Quéré, G.; Misery, L.; Funck-Brentano, E.; Danlos, F.X.; Routier, E.; Robert, C.; Loriot, Y.; Lambotte, O.; Bonniaud, B.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019, 71, 2100–2111. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Gainor, J.F.; Altan, M.; Kravets, S.; Dahlberg, S.E.; Gedmintas, L.; Azimi, R.; Rizvi, H.; Riess, J.W.; Hellmann, M.D.; et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J. Clin. Oncol. 2018, 36, 1905–1912. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kong, X.; Li, Y.; Wang, Z.; Zhang, L.; Xuan, L. PD-1/PD-L1 Inhibitors in Patients With Preexisting Autoimmune Diseases. Front Pharm. 2022, 13, 854967. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Ernstoff, M.S.; Wang, Y.; Menzies, A.M.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.A.; Obeid, M. Autoimmune Diseases and Immune-Checkpoint Inhibitors for Cancer Therapy: Review of the Literature and Personalized Risk-Based Prevention Strategy. Ann. Oncol. 2020, 31, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Hogan, S.; Menzies, A.M.; Dummer, R.; Long, G.V. Interleukin-6 Blockade for Prophylaxis and Management of Immune-Related Adverse Events in Cancer Immunotherapy. Eur. J. Cancer 2021, 157, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Frohne, C.C.; Llano, E.M.; Perkovic, A.; Cohen, R.D.; Luke, J.J. Complete Response of Metastatic Melanoma in a Patient with Crohn’s Disease Simultaneously Receiving Anti-A4β7 and Anti-PD1 Antibodies. J. Immunother. Cancer 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 Therapy in Patients with Advanced Melanoma and Preexisting Autoimmune Disorders or Major Toxicity with Ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Lusa, A.; Alvarez, C.; Saxena Beem, S.; Schwartz, T.A.; Ishizawar, R. Immune-Related Adverse Events in Patients with Pre-Existing Autoimmune Rheumatologic Disease on Immune Checkpoint Inhibitor Therapy. BMC Rheumatol. 2022, 6, 64. [Google Scholar] [CrossRef]
- Danlos, F.X.; Voisin, A.L.; Dyevre, V.; Michot, J.M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and Efficacy of Anti-Programmed Death 1 Antibodies in Patients with Cancer and Pre-Existing Autoimmune or Inflammatory Disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886. [Google Scholar] [CrossRef]
- Paik, P.K.; Luo, J.; Ai, N.; Kim, R.; Ahn, L.; Biswas, A.; Coker, C.; Ma, W.; Wong, P.; Buonocore, D.J.; et al. Phase I Trial of the TNF-α Inhibitor Certolizumab plus Chemotherapy in Stage IV Lung Adenocarcinomas. Nat. Commun. 2022, 13, 6095. [Google Scholar] [CrossRef] [PubMed]
- Tocilizumab, Ipilimumab, and Nivolumab for the Treatment of Advanced Melanoma, Non-Small Cell Lung Cancer, or Urothelial Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04940299?term=NCT04940299&draw=2&rank=1 (accessed on 1 December 2022).
- Montfort, A.; Filleron, T.; Virazels, M.; Dufau, C.; Milhes, J.; Pages, C.; Olivier, P.; Ayyoub, M.; Mounier, M.; Lusque, A.; et al. Combining Nivolumab and Ipilimumab with Infliximab or Certolizumab in Patients with Advanced Melanoma: First Results of a Phase Ib Clinical Trial. Clin. Cancer Res. 2021, 27, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα Blockade Overcomes Resistance to Anti-PD-1 in Experimental Melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Alvarez, M.; Otano, I.; Minute, L.; Ochoa, M.C.; Perez-Ruiz, E.; Melero, I.; Berraondo, P. Impact of Prophylactic TNF Blockade in the Dual PD-1 and CTLA-4 Immunotherapy Efficacy and Toxicity. Cell Stress 2019, 3, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, E.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; de Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF Blockade Uncouples Efficacy and Toxicity in Dual CTLA-4 and PD-1 Immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Fujieda, K.; Hirayama, M.; Ikeda, T.; Yuno, A.; Matsumura, K.; Fukuma, D.; Araki, K.; Mizuta, H.; Nakayama, H.; et al. Soluble IL6R Expressed by Myeloid Cells Reduces Tumor-Specific Th1 Differentiation and Drives Tumor Progression. Cancer Res. 2017, 77, 2279–2291. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Yu, Y.; Tan, K.; Cui, H. Changes of IL-6 And IFN-γ before and after the Adverse Events Related to Immune Checkpoint Inhibitors: A Retrospective Study. Medicine 2022, 101, e31761. [Google Scholar] [CrossRef]
- Rossi, J.F.; Lu, Z.Y.; Jourdan, M.; Klein, B. Interleukin-6 as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1248–1257. [Google Scholar] [CrossRef]
- Melchor, L.; Brioli, A.; Wardell, C.P.; Murison, A.; Potter, N.E.; Kaiser, M.F.; Fryer, R.A.; Johnson, D.C.; Begum, D.B.; Wilson, S.H.; et al. Single-Cell Genetic Analysis Reveals the Composition of Initiating Clones and Phylogenetic Patterns of Branching and Parallel Evolution in Myeloma. Leukemia 2014, 28, 1705–1715. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 Blockade Abrogates Immunotherapy Toxicity and Promotes Tumor Immunity. Cancer Cell 2022, 40, 509–523.e6. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.v.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ellen Maher, V.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Pacholczak-Madej, R.; Grela-Wojewoda, A.; Puskulluoglu, M.; Lompart, J.; Las-Jankowska, M.; Krawczak, K.; Wrona, E.; Zaręba, L.; Żubrowska, J.; Walocha, J.; et al. Early Effects of Nivolumab and Ipilimumab Combined Immunotherapy in the Treatment of Metastatic Melanoma in Poland: A Multicenter Experience. Biomedicines 2022, 10, 2528. [Google Scholar] [CrossRef]
- Liew, D.F.L.; Leung, J.L.Y.; Liu, B.; Cebon, J.; Frauman, A.G.; Buchanan, R.R.C. Association of Good Oncological Response to Therapy with the Development of Rheumatic Immune-Related Adverse Events Following PD-1 Inhibitor Therapy. Int. J. Rheum. Dis. 2019, 22, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Buder-Bakhaya, K.; Benesova, K.; Schulz, C.; Anwar, H.; Dimitrakopoulou-Strauss, A.; Weber, T.F.; Enk, A.; Lorenz, H.M.; Hassel, J.C. Characterization of Arthralgia Induced by PD-1 Antibody Treatment in Patients with Metastasized Cutaneous Malignancies. Cancer Immunol. Immunother. 2018, 67, 175–182. [Google Scholar] [CrossRef]
| Common Terminology Criteria for Adverse Events Version 5.0 * | Rheumatology Common Toxicity Criteria Version 2.0 ** | |
|---|---|---|
| Grade 1—mild | Asymptomatic or mild symptoms, clinical or diagnostic observations only, intervention not indicated. | Asymptomatic, or transient Short duration (<1 week) No change in lifestyle No medication or over-the-counter |
| Grade 2—moderate | Minimal, local, or noninvasive intervention indicated, limiting age-appropriate instrumental activities of daily living (preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc.) | Symptomatic Duration 1–2 weeks Alter lifestyle occasionally Medications give relief |
| Grade 3—severe | Hospitalization or prolongation of hospitalization indicated, disabling, limiting self-care activities of daily living (bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden.) | Prolonged symptoms, reversible Major functional impairment Prescription medications/partial relief; hospitalized < 24 h Temporary or permanent study drug discontinuation |
| Grade 4—life-threatening | Life-threatening consequences, urgent intervention indicated. | At risk of death Substantial disability, especially if permanent Hospitalized > 24 h Permanent study drug discontinuation |
| Grade 5—death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacholczak-Madej, R.; Kosałka-Węgiel, J.; Kuszmiersz, P.; Mituś, J.W.; Püsküllüoğlu, M.; Grela-Wojewoda, A.; Korkosz, M.; Bazan-Socha, S. Immune Checkpoint Inhibitor Related Rheumatological Complications: Cooperation between Rheumatologists and Oncologists. Int. J. Environ. Res. Public Health 2023, 20, 4926. https://doi.org/10.3390/ijerph20064926
Pacholczak-Madej R, Kosałka-Węgiel J, Kuszmiersz P, Mituś JW, Püsküllüoğlu M, Grela-Wojewoda A, Korkosz M, Bazan-Socha S. Immune Checkpoint Inhibitor Related Rheumatological Complications: Cooperation between Rheumatologists and Oncologists. International Journal of Environmental Research and Public Health. 2023; 20(6):4926. https://doi.org/10.3390/ijerph20064926
Chicago/Turabian StylePacholczak-Madej, Renata, Joanna Kosałka-Węgiel, Piotr Kuszmiersz, Jerzy W. Mituś, Mirosława Püsküllüoğlu, Aleksandra Grela-Wojewoda, Mariusz Korkosz, and Stanisława Bazan-Socha. 2023. "Immune Checkpoint Inhibitor Related Rheumatological Complications: Cooperation between Rheumatologists and Oncologists" International Journal of Environmental Research and Public Health 20, no. 6: 4926. https://doi.org/10.3390/ijerph20064926
APA StylePacholczak-Madej, R., Kosałka-Węgiel, J., Kuszmiersz, P., Mituś, J. W., Püsküllüoğlu, M., Grela-Wojewoda, A., Korkosz, M., & Bazan-Socha, S. (2023). Immune Checkpoint Inhibitor Related Rheumatological Complications: Cooperation between Rheumatologists and Oncologists. International Journal of Environmental Research and Public Health, 20(6), 4926. https://doi.org/10.3390/ijerph20064926









