Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Primary Prevention
3.2. CAD Diagnosis—Challenges with Access, Accuracy, and Appropriate Use
3.3. CAD Revascularization—Challenges with Access, Appropriate Use, and Rising Patient Complexity
3.4. Post-Acute Care—Challenges with Infrastructure, Access, and Adherence
3.5. Additional Challenges—Clinician Burnout
3.6. Additional Challenges—Technology Risks
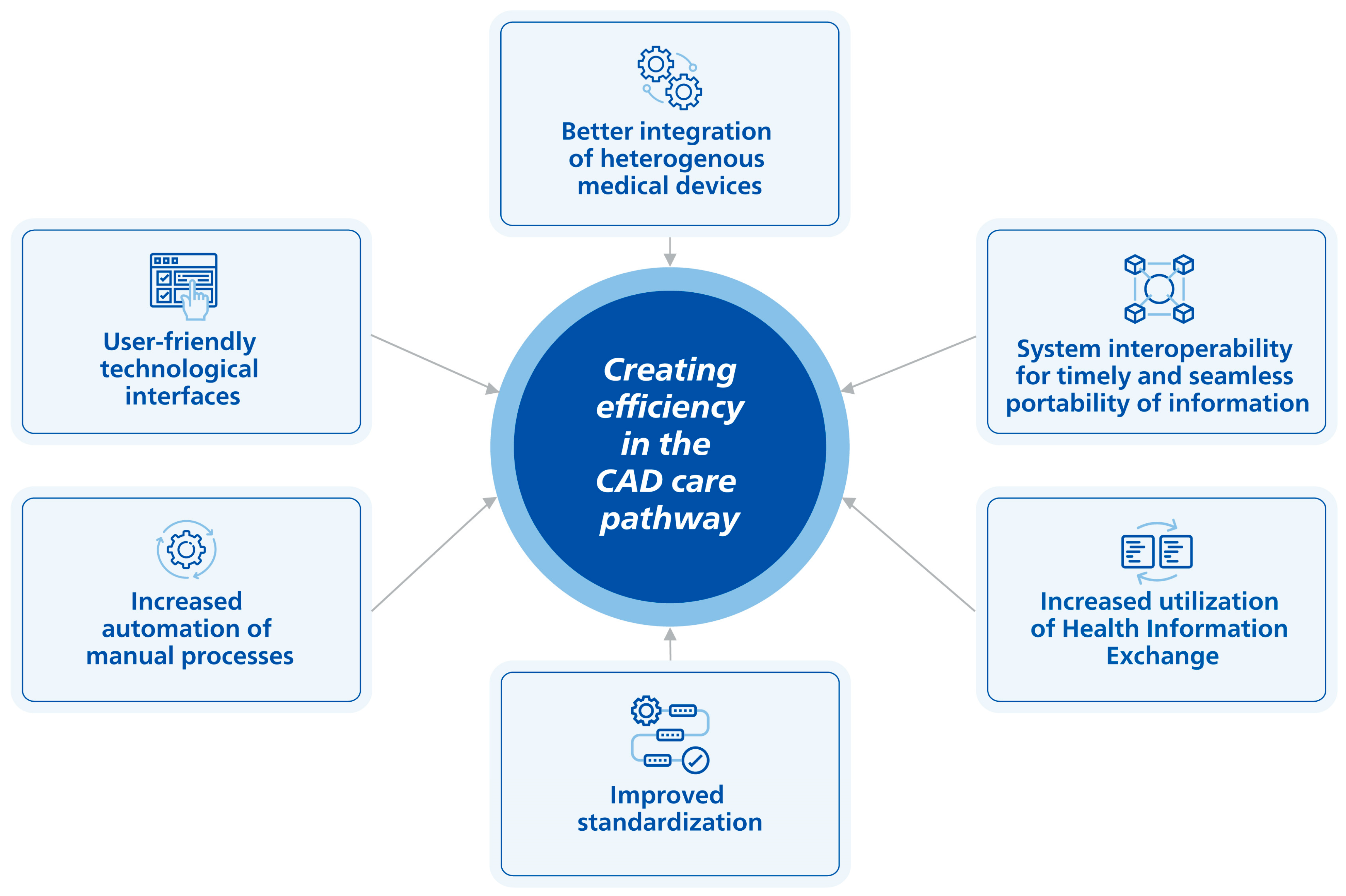
4. Evolving Solutions
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation. Global Burden of Disease (GBD) Results Tool. 2019. Available online: https://ghdx.healthdata.org/gbd-results-tool (accessed on 20 July 2022).
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef]
- Nelson, A.J.; Ardissino, M.; Psaltis, P.J. Current approach to the diagnosis of atherosclerotic coronary artery disease: More questions than answers. Ther. Adv. Chronic Dis. 2019, 10, 2040622319884819. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Singh, A.; Museedi, A.S.; Grossman, S.A. Acute Coronary Syndrome. In StatPearls; Treasure Island; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Shahjehan, R.D.; Bhutta, B.S. Coronary Artery Disease. In StatsPearls; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef]
- Regmi, M.; Siccardi, M.A. Coronary Artery Disease Prevention. In StatsPearls; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Ahmad, M.; Mehta, P.; Reddivari, A.K.R.; Mungee, S. Percutaneous Coronary Intervention. In StatPearls; Treasure Island; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Reed, G.W.; Hantz, S.; Cunningham, R.; Krishnaswamy, A.; Ellis, S.G.; Khot, U.; Rak, J.; Kapadia, S.R. Operational Efficiency and Productivity Improvement Initiatives in a Large Cardiac Catheterization Laboratory. JACC Cardiovasc. Interv. 2018, 11, 329–338. [Google Scholar] [CrossRef]
- Agarwal, S.; Gallo, J.J.; Parashar, A.; Agarwal, K.K.; Ellis, S.G.; Khot, U.N.; Spooner, R.; Murat Tuzcu, E.; Kapadia, S.R. Impact of lean six sigma process improvement methodology on cardiac catheterization laboratory efficiency. Cardiovasc. Revasc. Med. 2016, 17, 95–101. [Google Scholar] [CrossRef]
- Mahadevan, K.; Cowan, E.; Kalsi, N.; Bolam, H.; Arnett, R.; Hobson, A.; Guha, K.; Morton, G.; Brennan, P.A.; Kalra, P.R. Distractions in the cardiac catheterisation laboratory: Impact for cardiologists and patient safety. Open Heart 2020, 7, e001260. [Google Scholar] [CrossRef]
- Doorey, A.J.; Turi, Z.G.; Lazzara, E.H.; Mendoza, E.G.; Garratt, K.N.; Weintraub, W.S. Safety gaps in medical team communication: Results of quality improvement efforts in a cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 2020, 95, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Papin, J. A Suggested Approach for Improving Flow in the Cardiac Catheterization Laboratory. Catheter. Lab. Digest. 2013, 21. Available online: https://www.hmpgloballearningnetwork.com/site/cathlab/articles/suggested-approach-improving-flow-cardiac-catheterization-laboratory (accessed on 20 July 2022).
- Darmoch, F.; Alraies, M.C.; Al-Khadra, Y.; Moussa Pacha, H.; Pinto, D.S.; Osborn, E.A. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e013678. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Davies, J.E.; Sen, S.; Dehbi, H.M.; Al-Lamee, R.; Petraco, R.; Nijjer, S.S.; Bhindi, R.; Lehman, S.J.; Walters, D.; Sapontis, J.; et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N. Engl. J. Med. 2017, 376, 1824–1834. [Google Scholar] [CrossRef]
- Levy, A.G.; Scherer, A.M.; Zikmund-Fisher, B.J.; Larkin, K.; Barnes, G.D.; Fagerlin, A. Prevalence of and Factors Associated with Patient Nondisclosure of Medically Relevant Information to Clinicians. JAMA Netw. Open 2018, 1, e185293. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Thourani, V.H.; Nissen, A.P.; Habib, R.H.; Dearani, J.A.; Ropski, A.; Crestanello, J.A.; Shahian, D.M.; Jacobs, J.P.; Badhwar, V. The Effect of COVID-19 on Adult Cardiac Surgery in the United States in 717 103 Patients. Ann. Thorac. Surg. 2022, 113, 738–746. [Google Scholar] [CrossRef]
- Ad, N.; Luc, J.G.Y.; Nguyen, T.C.; Group, C.-N.A.C.S.S.W. Cardiac surgery in North America and coronavirus disease 2019 (COVID-19): Regional variability in burden and impact. J. Thorac. Cardiovasc. Surg. 2021, 162, 893–903.e894. [Google Scholar] [CrossRef]
- Rodriguez-Leor, O.C.-A.B.; Ojeda, S.; Martin-Moreiras, J.; Rumoroso, J.R.; Lopez-Palop, R.; Serrador, A.; Cequier, A.; Romaguera, R.; Cruz, I.; Perez de Prado, A.; et al. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv. Cardiol. 2020, 2, 82–89. [Google Scholar] [CrossRef]
- De Luca, G.; Verdoia, M.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Ferrer, G.R.; Ganyukov, V.; Wojakowski, W.; et al. Impact of COVID-19 Pandemic on Mechanical Reperfusion for Patients With STEMI. J. Am. Coll. Cardiol. 2020, 76, 2321–2330. [Google Scholar] [CrossRef]
- Mehta, L.S.; Lewis, S.J.; Duvernoy, C.S.; Rzeszut, A.K.; Walsh, M.N.; Harrington, R.A.; Poppas, A.; Linzer, M.; Binkley, P.F.; Douglas, P.S.; et al. Burnout and Career Satisfaction Among U.S. Cardiologists. J. Am. Coll. Cardiol. 2019, 73, 3345–3348. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M. Cardiologists and the Burnout scenario. Eur. Heart J. 2019, 40, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.; Parmenter, B.J.; Baker, M.K.; Mitchell, B.L.; Williams, A.D.; Lyndon, K.; Mair, T.; Maiorana, A.; Smart, N.A.; Levinger, I. Cardiac Rehabilitation for Patients with Coronary Artery Disease: A Practical Guide to Enhance Patient Outcomes Through Continuity of Care. Clin. Med. Insights Cardiol. 2017, 11, 1179546817710028. [Google Scholar] [CrossRef]
- Schrijvers, G.V.H.A.; Huiskes, N. The care pathway: Concepts and theories: An introduction. Int. J. Integr. Care 2012, 12, e192. [Google Scholar] [CrossRef]
- Roberts, R.; Chang, C.C.; Hadley, T. Genetic Risk Stratification: A Paradigm Shift in Prevention of Coronary Artery Disease. JACC Basic. Transl. Sci. 2021, 6, 287–304. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.R.; Lazris, A.; Ganatra, S. Overuse of Cardiac Testing. Am. Fam. Physician 2018, 98, 561–563. [Google Scholar]
- Amor, A.J.; Serra-Mir, M.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Fito, M.; Estruch, R.; Serra-Majem, L.; Aros, F.; Babio, N.; et al. Prediction of Cardiovascular Disease by the Framingham-REGICOR Equation in the High-Risk PREDIMED Cohort: Impact of the Mediterranean Diet Across Different Risk Strata. J. Am. Heart Assoc. 2017, 6, e004803. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Kwok, C.S.; Bennett, S.; Azam, Z.; Welsh, V.; Potluri, R.; Loke, Y.K.; Mallen, C.D. Misdiagnosis of Acute Myocardial Infarction: A Systematic Review of the Literature. Crit. Pathw. Cardiol. 2021, 20, 155–162. [Google Scholar] [CrossRef]
- Quinn, G.R.; Ranum, D.; Song, E.; Linets, M.; Keohane, C.; Riah, H.; Greenberg, P. Missed Diagnosis of Cardiovascular Disease in Outpatient General Medicine: Insights from Malpractice Claims Data. Jt. Comm. J. Qual. Patient Saf. 2017, 43, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Nadal, G.M.O.; Matas, A.; Cepas, P.; Aldea, A.; Izquierdo, M.; Coll-Vinent, B.; Garcia, A.; Carbo, M.; Manuel, O.; Aguilo, S.; et al. An analysis based on sex & gender in the chest pain unit of an emergency department during the last 12 years. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 122. [Google Scholar] [CrossRef]
- France, D.J.; Levin, S.; Ding, R.; Hemphill, R.; Han, J.; Russ, S.; Aronsky, D.; Weinger, M. Factors Influencing Time-Dependent Quality Indicators for Patients with Suspected Acute Coronary Syndrome. J. Patient Saf. 2020, 16, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef]
- Zegre-Hemsey, J.; Sommargren, C.E.; Drew, B.J. Initial ECG acquisition within 10 minutes of arrival at the emergency department in persons with chest pain: Time and gender differences. J. Emerg. Nurs. 2011, 37, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Chahine, A.; Haykal, T.; Kanugula, A.K.; Bachuwa, G.; Alkotob, M.L.; Bhatt, D.L. Meta-analysis of optimal timing of coronary intervention in non-ST-elevation acute coronary syndrome. Catheter. Cardiovasc. Interv. 2020, 95, 185–193. [Google Scholar] [CrossRef]
- Vafaie, M.; Hochadel, M.; Munzel, T.; Hailer, B.; Schumacher, B.; Heusch, G.; Voigtlander, T.; Mudra, H.; Haude, M.; Barth, S.; et al. Guideline-adherence regarding critical time intervals in the German Chest Pain Unit registry. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Garg, L.; Sharma, A.; Mohananey, D.; Bhatia, N.; Singh, A.; Shirani, J.; Dixon, S. Comparison of Inhospital Mortality and Frequency of Coronary Angiography on Weekend Versus Weekday Admissions in Patients with Non-ST-Segment Elevation Acute Myocardial Infarction. Am. J. Cardiol. 2016, 118, 632–634. [Google Scholar] [CrossRef]
- Khoshchehreh, M.; Groves, E.M.; Tehrani, D.; Amin, A.; Patel, P.M.; Malik, S. Changes in mortality on weekend versus weekday admissions for Acute Coronary Syndrome in the United States over the past decade. Int. J. Cardiol. 2016, 210, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Alrawashdeh, A.; Nehme, Z.; Williams, B.; Stub, D. Review article: Impact of 12-lead electrocardiography system of care on emergency medical service delays in ST-elevation myocardial infarction: A systematic review and meta-analysis. Emerg. Med. Australas. 2019, 31, 702–709. [Google Scholar] [CrossRef]
- Arinaga, T.; Suematsu, Y.; Nakamura, A.; Imaizumi, T.; Hanaoka, Y.; Takagi, T.; Koga, H.; Tanaka, H.; Shokyu, Y.; Miura, S.I. The Effectiveness of Mobile Cloud 12-Lead Electrocardiogram Transmission System in Patients with ST-Segment Elevation Myocardial Infarction. Medicina 2022, 58, 247. [Google Scholar] [CrossRef]
- Ward, M.J.; Vogus, T.J.; Munoz, D.; Collins, S.P.; Moser, K.; Jenkins, C.A.; Liu, D.; Kripalani, S. Examining the Timeliness of ST-elevation Myocardial Infarction Transfers. West J. Emerg. Med. 2021, 22, 319–325. [Google Scholar] [CrossRef]
- Walker, S.; Girardin, F.; McKenna, C.; Ball, S.G.; Nixon, J.; Plein, S.; Greenwood, J.P.; Sculpher, M. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: An economic evaluation using data from the CE-MARC study. Heart 2013, 99, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Genders, T.S.; Petersen, S.E.; Pugliese, F.; Dastidar, A.G.; Fleischmann, K.E.; Nieman, K.; Hunink, M.G. The optimal imaging strategy for patients with stable chest pain: A cost-effectiveness analysis. Ann. Intern. Med. 2015, 162, 474–484. [Google Scholar] [CrossRef]
- Min, J.K.; Gilmore, A.; Jones, E.C.; Berman, D.S.; Stuijfzand, W.J.; Shaw, L.J.; O’Day, K.; Danad, I. Cost-effectiveness of diagnostic evaluation strategies for individuals with stable chest pain syndrome and suspected coronary artery disease. Clin. Imaging 2017, 43, 97–105. [Google Scholar] [CrossRef]
- Karady, J.; Mayrhofer, T.; Ivanov, A.; Foldyna, B.; Lu, M.T.; Ferencik, M.; Pursnani, A.; Salerno, M.; Udelson, J.E.; Mark, D.B.; et al. Cost-effectiveness Analysis of Anatomic vs Functional Index Testing in Patients with Low-Risk Stable Chest Pain. JAMA Netw. Open 2020, 3, e2028312. [Google Scholar] [CrossRef]
- Song, Y.B.; Arbab-Zadeh, A.; Matheson, M.B.; Ostovaneh, M.R.; Vavere, A.L.; Dewey, M.; Rochitte, C.; Niinuma, H.; Laham, R.; Schuijf, J.D.; et al. Contemporary Discrepancies of Stenosis Assessment by Computed Tomography and Invasive Coronary Angiography. Circ. Cardiovasc. Imaging 2019, 12, e007720. [Google Scholar] [CrossRef]
- Yi, Y.; Zhao, X.M.; Wu, R.Z.; Wang, Y.; Vembar, M.; Jin, Z.Y.; Wang, Y.N. Low Dose and Low Contrast Medium Coronary CT Angiography Using Dual-Layer Spectral Detector CT. Int. Heart J. 2019, 60, 608–617. [Google Scholar] [CrossRef]
- Rassouli, N.; Etesami, M.; Dhanantwari, A.; Rajiah, P. Detector-based spectral CT with a novel dual-layer technology: Principles and applications. Insights Imaging 2017, 8, 589–598. [Google Scholar] [CrossRef]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.M.; Spertus, J.A.; Kennedy, K.F.; Nallamothu, B.K.; Chan, P.S.; Patel, M.R.; Bryson, C.L.; Malenka, D.J.; Rumsfeld, J.S. Patient selection for diagnostic coronary angiography and hospital-level percutaneous coronary intervention appropriateness: Insights from the National Cardiovascular Data Registry. JAMA Intern. Med. 2014, 174, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijic, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2023, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.; Schmidt, S.E.; Rasmussen, L.D.; Juarez Orozco, L.E.; Steffensen, F.H.; Botker, H.E.; Knuuti, J.; Bottcher, M. Validation of the European Society of Cardiology pre-test probability model for obstructive coronary artery disease. Eur. Heart J. 2021, 42, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, P. Computed Tomography to Replace Invasive Coronary Angiography? Circ. Cardiovasc. Interv. 2019, 12, e008710. [Google Scholar] [CrossRef]
- Baggiano, A.; Guglielmo, M.; Muscogiuri, G.; Guaricci, A.I.; Del Torto, A.; Pontone, G. (Epicardial and microvascular) angina or atypical chest pain: Differential diagnoses with cardiovascular magnetic resonance. Eur. Heart J. Suppl. 2020, 22, E116–E120. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Twisk, J.W.R.; Norgaard, B.L.; Zarins, C.K.; Knaapen, P.; Min, J.K. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur. Heart J. 2017, 38, 991–998. [Google Scholar] [CrossRef]
- Fitzgerald, B.T.; Scalia, W.M.; Scalia, G.M. Female False Positive Exercise Stress ECG Testing—Fact Versus Fiction. Heart Lung. Circ. 2019, 28, 735–741. [Google Scholar] [CrossRef]
- Ladapo, J.A.; Goldfeld, K.S.; Douglas, P.S. Projected morbidity and mortality from missed diagnoses of coronary artery disease in the United States. Int. J. Cardiol. 2015, 195, 250–252. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Seekings, P.J.; Hung, C.L.; Iversen, M.B.; Frost, M.J.; Ouwerkerk, W.; Jiang, Z.; Eisenhaber, F.; Goh, R.S.M.; Zhao, H.; et al. Automated interpretation of systolic and diastolic function on the echocardiogram: A multicohort study. Lancet Digit. Health 2022, 4, e46–e54. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.M.H.T.; de Vasconcellos, H.D. Abstract: Inter- and Intra-reader Reproducibility of Left Ventricular Volumetric and Deformational Assessment By Three-dimensional Echocardiography in a Multi-center Community-based Study: The Coronary Artery Risk Development in young Adults (CARDIA) Study. J. Am. Soc. Echocardiogr. 2016, 30, B133–B161. [Google Scholar]
- Khouri, M.G.; Ky, B.; Dunn, G.; Plappert, T.; Englefield, V.; Rabineau, D.; Yow, E.; Barnhart, H.X.; St John Sutton, M.; Douglas, P.S. Echocardiography Core Laboratory Reproducibility of Cardiac Safety Assessments in Cardio-Oncology. J. Am. Soc. Echocardiogr. 2018, 31, 361–371.e363. [Google Scholar] [CrossRef]
- Zamzmi, G.; Hsu, L.Y.; Li, W.; Sachdev, V.; Antani, S. Harnessing Machine Intelligence in Automatic Echocardiogram Analysis: Current Status, Limitations, and Future Directions. IEEE Rev. Biomed. Eng. 2021, 14, 181–203. [Google Scholar] [CrossRef]
- Foy, A.J.; Dhruva, S.S.; Peterson, B.; Mandrola, J.M.; Morgan, D.J.; Redberg, R.F. Coronary Computed Tomography Angiography vs Functional Stress Testing for Patients with Suspected Coronary Artery Disease: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2017, 177, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, P.N.; Kruk, M.; Kepka, C.; Schoepf, U.J.; Otani, K.; Leonard, T.J.; Debski, M.; Dzielinska, Z.; Pregowski, J.; Witkowski, A.; et al. Assessing the value of coronary artery computed tomography as the first-line anatomical test for stable patients with indications for invasive angiography due to suspected coronary artery disease. Initial cost analysis in the CAT-CAD randomized trial. J. Cardiovasc. Comput. Tomogr. 2020, 14, 75–79. [Google Scholar] [CrossRef] [PubMed]
- England, R.W.; Sheikhbahaei, S.; Solomon, A.J.; Arbab-Zadeh, A.; Solnes, L.B.; Bronner, J.; Johnson, P.T. When More Is Better: Underused Advanced Imaging Exams That Can Improve Outcomes and Reduce Cost of Care. Am. J. Med. 2021, 134, 848–853.e841. [Google Scholar] [CrossRef]
- Hamilton-Craig, C.; Fifoot, A.; Hansen, M.; Pincus, M.; Chan, J.; Walters, D.L.; Branch, K.R. Diagnostic performance and cost of CT angiography versus stress ECG—A randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE). Int. J. Cardiol. 2014, 177, 867–873. [Google Scholar] [CrossRef]
- The SCOT-HEART Investigators; Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef]
- Baggiano, A.; Italiano, G.; Guglielmo, M.; Fusini, L.; Guaricci, A.I.; Maragna, R.; Giacari, C.M.; Mushtaq, S.; Conte, E.; Annoni, A.D.; et al. Changing Paradigms in the Diagnosis of Ischemic Heart Disease by Multimodality Imaging. J. Clin. Med. 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Min, D.B.; Le, V.T.; Meredith, K.G.; Dhar, R.; Biswas, S.; Jensen, K.R.; Mason, S.M.; Ethington, J.D.; Lappe, D.L.; et al. Implementation of a cardiac PET stress program: Comparison of outcomes to the preceding SPECT era. JCI. Insight 2018, 3, e120949. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.; Mavridis, D.; Greenwood, J.P.; Coles, B.; Nikolakopoulou, A.; Jüni, P.; Salanti, G.; Windecker, S. Outcomes of non-invasive diagnostic modalities for the detection of coronary artery disease: Network meta-analysis of diagnostic randomised controlled trials. BMJ 2018, 360, k504. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Deo, S.V.; Kalra, A.; Zia, A.; Altarabsheh, S.E.; Deo, V.S.; Mustafa, R.R.; Younes, A.; Rao, S.V.; Markowitz, A.H.; et al. Stability after Initial Decline in Coronary Revascularization Rates in the United States. Ann. Thorac. Surg. 2019, 108, 1404–1408. [Google Scholar] [CrossRef]
- Kataruka, A.; Maynard, C.C.; Kearney, K.E.; Mahmoud, A.; Bell, S.; Doll, J.A.; McCabe, J.M.; Bryson, C.; Gurm, H.S.; Jneid, H.; et al. Temporal Trends in Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting: Insights from the Washington Cardiac Care Outcomes Assessment Program. J. Am. Heart Assoc. 2020, 9, e015317. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Kwok, C.S.; Amin, A.P.; Shah, B.; Kinnaird, T.; Alkutshan, R.; Balghith, M.; Ratib, K.; Nolan, J.; Bagur, R.; Mamas, M.A. Cost of coronary syndrome treated with percutaneous coronary intervention and 30-day unplanned readmission in the United States. Catheter. Cardiovasc. Interv. 2021, 97, 80–93. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Scholz, K.H.; Maier, S.K.G.; Maier, L.S.; Lengenfelder, B.; Jacobshagen, C.; Jung, J.; Fleischmann, C.; Werner, G.S.; Olbrich, H.G.; Ott, R.; et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: Results from the German prospective, multicentre FITT-STEMI trial. Eur. Heart J. 2018, 39, 1065–1074. [Google Scholar] [CrossRef]
- Meisel, S.R.; Kleiner-Shochat, M.; Abu-Fanne, R.; Frimerman, A.; Danon, A.; Minha, S.; Levi, Y.; Blatt, A.; Mohsen, J.; Shotan, A.; et al. Direct Admission of Patients With ST-Segment-Elevation Myocardial Infarction to the Catheterization Laboratory Shortens Pain-to-Balloon and Door-to-Balloon Time Intervals but Only the Pain-to-Balloon Interval Impacts Short-and Long-Term Mortality. J. Am. Heart Assoc. 2021, 10, e018343. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Normand, S.L.; Wang, Y.; Hofer, T.P.; Brush, J.E., Jr.; Messenger, J.C.; Bradley, E.H.; Rumsfeld, J.S.; Krumholz, H.M. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: A retrospective study. Lancet 2015, 385, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.Y.; Bonsu, K.O.; Nallamothu, B.K.; Reid, C.M.; Dhippayom, T.; Reidpath, D.D.; Chaiyakunapruk, N. Coronary intervention door-to-balloon time and outcomes in ST-elevation myocardial infarction: A meta-analysis. Heart 2018, 104, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.Y.; Andrianopoulos, N.; Brennan, A.; Ajani, A.; Reid, C.M.; Duffy, S.J.; Clark, D.J.; Reidpath, D.D.; Chaiyakunapruk, N. Re-examining the effect of door-to-balloon delay on STEMI outcomes in the context of unmeasured confounders: A retrospective cohort study. Sci. Rep. 2019, 9, 19978. [Google Scholar] [CrossRef]
- Korsnes, J.S.; Davis, K.L.; Ariely, R.; Bell, C.F.; Mitra, D. Health care resource utilization and costs associated with nonfatal major adverse cardiovascular events. J. Manag. Care Spec. Pharm. 2015, 21, 443–450. [Google Scholar] [CrossRef]
- Zeitouni, M.; Al-Khalidi, H.R.; Roettig, M.L.; Bolles, M.M.; Doerfler, S.M.; Fordyce, C.B.; Hellkamp, A.S.; Henry, T.D.; Magdon-Ismail, Z.; Monk, L.; et al. Catheterization Laboratory Activation Time in Patients Transferred With ST-Segment-Elevation Myocardial Infarction: Insights from the Mission: Lifeline STEMI Accelerator-2 Project. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006204. [Google Scholar] [CrossRef]
- Shavadia, J.S.; Roe, M.T.; Chen, A.Y.; Lucas, J.; Fanaroff, A.C.; Kochar, A.; Fordyce, C.B.; Jollis, J.G.; Tamis-Holland, J.; Henry, T.D.; et al. Association Between Cardiac Catheterization Laboratory Pre-Activation and Reperfusion Timing Metrics and Outcomes in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Report from the ACTION Registry. JACC Cardiovasc. Interv. 2018, 11, 1837–1847. [Google Scholar] [CrossRef]
- Wykrzykowska, J.J.; Kerkmeijer, L.S.M. Complex PCI: Time for a universal definition. EuroIntervention 2020, 16, 536–537. [Google Scholar] [CrossRef]
- Kwok, C.S.; Shah, B.; Al-Suwaidi, J.; Fischman, D.L.; Holmvang, L.; Alraies, C.; Bagur, R.; Nagaraja, V.; Rashid, M.; Mohamed, M.; et al. Timing and Causes of Unplanned Readmissions After Percutaneous Coronary Intervention: Insights from the Nationwide Readmission Database. JACC Cardiovasc. Interv. 2019, 12, 734–748. [Google Scholar] [CrossRef]
- Sukul, D.; Seth, M.; Dupree, J.M.; Syrjamaki, J.D.; Ryan, A.M.; Nallamothu, B.K.; Gurm, H.S. Drivers of Variation in 90-Day Episode Payments After Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2019, 12, e006928. [Google Scholar] [CrossRef]
- Huckfeldt, P.J.; Mehrotra, A.; Hussey, P.S. The Relative Importance of Post-Acute Care and Readmissions for Post-Discharge Spending. Health Serv. Res. 2016, 51, 1919–1938. [Google Scholar] [CrossRef]
- Wasfy, J.H.; Yeh, R.W. Future of the PCI Readmission Metric. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Narain, A.; Pacha, H.M.; Lo, T.S.; Holroyd, E.W.; Alraies, M.C.; Nolan, J.; Mamas, M.A. Readmissions to Hospital After Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Factors Associated with Readmissions. Cardiovasc. Revasc. Med. 2020, 21, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehuda, O.; Kazi, D.S.; Bonafede, M.; Wade, S.W.; Machacz, S.F.; Stephens, L.A.; Hlatky, M.A.; Hernandez, J.B. Angina and associated healthcare costs following percutaneous coronary intervention: A real-world analysis from a multi-payer database. Catheter. Cardiovasc. Interv. 2016, 88, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Simpson, A.; Bhagnani, T.; Leeper, N.J.; Murphy, B.; Nordstrom, B.; Ting, W.; Zhao, Q.; Berger, J.S. Incidence and Cost of Major Adverse Cardiovascular Events and Major Adverse Limb Events in Patients with Chronic Coronary Artery Disease or Peripheral Artery Disease. Am. J. Cardiol. 2019, 123, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Yeo, I.; Feldman, D.N.; Cheung, J.W.; Minutello, R.M.; Singh, H.S.; Bergman, G.; Wong, S.C.; Kim, L.K. Associations Between Hospital Length of Stay, 30-Day Readmission, and Costs in ST-Segment-Elevation Myocardial Infarction After Primary Percutaneous Coronary Intervention: A Nationwide Readmissions Database Analysis. J. Am. Heart Assoc. 2020, 9, e015503. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.P.; Patterson, M.; House, J.A.; Giersiefen, H.; Spertus, J.A.; Baklanov, D.V.; Chhatriwalla, A.K.; Safley, D.M.; Cohen, D.J.; Rao, S.V.; et al. Costs Associated with Access Site and Same-Day Discharge Among Medicare Beneficiaries Undergoing Percutaneous Coronary Intervention: An Evaluation of the Current Percutaneous Coronary Intervention Care Pathways in the United States. JACC Cardiovasc. Interv. 2017, 10, 342–351. [Google Scholar] [CrossRef]
- Amin, A.P.; Crimmins-Reda, P.; Miller, S.; Rahn, B.; Caruso, M.; Pierce, A.; Dennis, B.; Pendegraft, M.; Sorensen, K.; Kurz, H.I.; et al. Novel Patient-Centered Approach to Facilitate Same-Day Discharge in Patients Undergoing Elective Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2018, 7, e005733. [Google Scholar] [CrossRef]
- Levitan, E.B.; Muntner, P.; Chen, L.; Deng, L.; Kilgore, M.L.; Becker, D.; Glasser, S.P.; Safford, M.M.; Howard, G.; Kilpatrick, R.; et al. Burden of Coronary Heart Disease Rehospitalizations Following Acute Myocardial Infarction in Older Adults. Cardiovasc. Drugs Ther. 2016, 30, 323–331. [Google Scholar] [CrossRef]
- Kytö, V.A.-O.; Prami, T.; Khanfir, H.; Hasvold, P.; Reissell, E.; Airaksinen, J. Usage of PCI and long-term cardiovascular risk in post-myocardial infarction patients: A nationwide registry cohort study from Finland. BMC Cardiovasc. Disord. 2019, 19, 123. [Google Scholar] [CrossRef]
- Kumar, V.; Weerakoon, S.; Dey, A.K.; Earls, J.P.; Katz, R.J.; Reiner, J.S.; Shaw, L.J.; Blankstein, R.; Mehta, N.N.; Choi, A.D. The evolving role of coronary CT angiography in Acute Coronary Syndromes. J. Cardiovasc. Comput. Tomogr. 2021, 15, 384–393. [Google Scholar] [CrossRef]
- Machta, S.; Gauthier, V.; Ferrieres, J.; Montaye, M.; Huo Yung Kai, S.; Gbokou, S.; Biasch, K.; Moitry, M.; Amouyel, P.; Dallongeville, J.; et al. Comparison of clinical profiles and care for patients with incident versus recurrent acute coronary syndromes in France: Data from the MONICA registries. PLoS ONE 2022, 17, e0263589. [Google Scholar] [CrossRef]
- Cao, C.F.; Li, S.F.; Chen, H.; Song, J.X. Predictors and in-hospital prognosis of recurrent acute myocardial infarction. J. Geriatr. Cardiol. 2016, 13, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Saczynski, J.S.; McManus, D.D.; Waring, M.E.; McManus, R.; Allison, J.; Parish, D.C.; Lessard, D.; Person, S.; Gore, J.M.; et al. Characteristics of contemporary patients discharged from the hospital after an acute coronary syndrome. Am. J. Med. 2015, 128, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Alghrairi, M.; Sulaiman, N.; Mutashar, S. Health Care Monitoring and Treatment for Coronary Artery Diseases: Challenges and Issues. Sensors 2020, 20, 4303. [Google Scholar] [CrossRef]
- Nagaraja, V.; Ooi, S.Y.; Nolan, J.; Large, A.; De Belder, M.; Ludman, P.; Bagur, R.; Curzen, N.; Matsukage, T.; Yoshimachi, F.; et al. Impact of Incomplete Percutaneous Revascularization in Patients with Multivessel Coronary Artery Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e004598. [Google Scholar] [CrossRef]
- Hannan, E.L.; Zhong, Y.; Jacobs, A.K.; Ling, F.S.K.; Berger, P.B.; Walford, G.; Venditti, F.J.; King, S.B., 3rd. Incomplete revascularization for percutaneous coronary interventions: Variation among operators, and association with operator and hospital characteristics. Am. Heart J. 2017, 186, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, J.M.; Koo, B.K.; Nam, C.W.; Shin, E.S.; Doh, J.H.; Rhee, T.M.; Hwang, D.; Park, J.; Zhang, J.; et al. Prognostic Implication of Functional Incomplete Revascularization and Residual Functional SYNTAX Score in Patients with Coronary Artery Disease. JACC Cardiovasc. Interv. 2018, 11, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Serruys, P.W.; Gao, C.; Ono, M.; Wang, R.; Thuijs, D.; Mack, M.J.; Curzen, N.; Mohr, F.W.; Davierwala, P.; et al. Ten-Year All-Cause Death According to Completeness of Revascularization in Patients with Three-Vessel Disease or Left Main Coronary Artery Disease: Insights from the SYNTAX Extended Survival Study. Circulation 2021, 144, 96–109. [Google Scholar] [CrossRef]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef]
- Malik, A.O.; Spertus, J.A.; Patel, M.R.; Dehmer, G.J.; Kennedy, K.; Chan, P.S. Potential Association of the ISCHEMIA Trial with the Appropriate Use Criteria Ratings for Percutaneous Coronary Intervention in Stable Ischemic Heart Disease. JAMA Intern. Med. 2020, 180, 1540–1542. [Google Scholar] [CrossRef]
- Qian, F.; Zhong, Y.; Hannan, E.L. Relationship between operator and hospital volumes and short-term mortality for percutaneous coronary intervention in New York. Int. J. Cardiol. 2019, 293, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Herwig, A.; Dehnen, D.; Weltermann, B. Patient factors driving overuse of cardiac catheterisation: A qualitative study with 25 participants from two German teaching practices. BMJ Open 2019, 9, e024600. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; Ko, D.T.; Nallamothu, B.K. Policing or Learning? Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005484. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Samadashvili, Z.; Cozzens, K.; Gesten, F.; Osinaga, A.; Fish, D.G.; Donahue, C.L.; Bass, R.J.; Walford, G.; Jacobs, A.K.; et al. Changes in Percutaneous Coronary Interventions Deemed "Inappropriate" by Appropriate Use Criteria. J. Am. Coll. Cardiol. 2017, 69, 1234–1242. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Lambert, C.T.; Gordon, N.F.; Fletcher, G.F.; Franklin, B.A.; Wenger, N.K.; Sperling, L. Cardiac rehabilitation and risk reduction: Time to “rebrand and reinvigorate”. J. Am. Coll. Cardiol. 2015, 65, 389–395. [Google Scholar] [CrossRef]
- van Halewijn, G.; Deckers, J.; Tay, H.Y.; van Domburg, R.; Kotseva, K.; Wood, D. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 232, 294–303. [Google Scholar] [CrossRef]
- Brouwers, R.W.M.; van Exel, H.J.; van Hal, J.M.C.; Jorstad, H.T.; de Kluiver, E.P.; Kraaijenhagen, R.A.; Kuijpers, P.; van der Linde, M.R.; Spee, R.F.; Sunamura, M.; et al. Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth. Heart J. 2020, 28, 443–451. [Google Scholar] [CrossRef]
- Ritchey, M.D.; Maresh, S.; McNeely, J.; Shaffer, T.; Jackson, S.L.; Keteyian, S.J.; Brawner, C.A.; Whooley, M.A.; Chang, T.; Stolp, H.; et al. Tracking Cardiac Rehabilitation Participation and Completion Among Medicare Beneficiaries to Inform the Efforts of a National Initiative. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e005902. [Google Scholar] [CrossRef]
- Turk-Adawi, K.I.; Grace, S.L. Narrative review comparing the benefits of and participation in cardiac rehabilitation in high-, middle- and low-income countries. Heart Lung Circ. 2015, 24, 510–520. [Google Scholar] [CrossRef]
- Vilela, E.M.; Ladeiras-Lopes, R.; Joao, A.; Braga, J.; Torres, S.; Viamonte, S.; Ribeiro, J.; Teixeira, M.; Nunes, J.P.; Fontes-Carvalho, R. Current role and future perspectives of cardiac rehabilitation in coronary heart disease. World J. Cardiol. 2021, 13, 695–709. [Google Scholar] [CrossRef]
- Global Health Data Exchange. GBD Results Tool. 2021. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 22 July 2022).
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2022, 19, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Million Hearts. Cardiac Rehabilitation at a Glance. Available online: https://millionhearts.hhs.gov/data-reports/factsheets/cardiac.html (accessed on 22 July 2022).
- Valaker, I.; Norekval, T.M.; Raholm, M.B.; Nordrehaug, J.E.; Rotevatn, S.; Fridlund, B.; Investigators, C. Continuity of care after percutaneous coronary intervention: The patient’s perspective across secondary and primary care settings. Eur. J. Cardiovasc. Nurs. 2017, 16, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Eijsvogels, T.M.H.; Maessen, M.F.H.; Bakker, E.A.; Meindersma, E.P.; van Gorp, N.; Pijnenburg, N.; Thompson, P.D.; Hopman, M.T.E. Association of Cardiac Rehabilitation with All-Cause Mortality Among Patients with Cardiovascular Disease in the Netherlands. JAMA Netw. Open 2020, 3, e2011686. [Google Scholar] [CrossRef] [PubMed]
- Tanguturi, V.K.; Temin, E.; Yeh, R.W.; Thompson, R.W.; Rao, S.K.; Mallick, A.; Cavallo, E.; Ferris, T.G.; Wasfy, J.H. Clinical Interventions to Reduce Preventable Hospital Readmission After Percutaneous Coronary Intervention. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 600–604. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, M.; Cappelletti, E.; Sarini, M.; Greco, A.; Monzani, D.; Pancani, L.; Steca, P. Communication and disease management: A qualitative study on coronary disease. Health Psychol. Behav. Med. 2015, 3, 94–108. [Google Scholar] [CrossRef]
- Pourhabib, S.; Chessex, C.; Murray, J.; Grace, S.L. Elements of patient-health-care provider communication related to cardiovascular rehabilitation referral. J. Health Psychol. 2016, 21, 468–482. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Miller, A.A.-O.; Hajiesmaieli, M.; Kangasniemi, M.; Alhani, F.; Jelvehmoghaddam, H.; Fathi, M.; Farzanegan, B.; Ardehali, S.H.; Hatamian, S.; et al. Cardiac rehabilitation using the Family-Centered Empowerment Model versus home-based cardiac rehabilitation in patients with myocardial infarction: A randomised controlled trial. Open Heart 2016, 3, e000349. [Google Scholar] [CrossRef]
- Maxwell, Y.L. COVID-19 Doubled Burnout Rates in Cardiology; TCTMD: New York, NY, USA, 2021. [Google Scholar]
- Cullen, M.W.; Damp, J.B.; Soukoulis, V.; Keating, F.K.; Abudayyeh, I.; Auseon, A.; Bhakta, D.; Qasim, A.; Seryak, A.; Smith, S.A.; et al. Burnout and Well-Being Among Cardiology Fellowship Program Directors. J. Am. Coll. Cardiol. 2021, 78, 1717–1726. [Google Scholar] [CrossRef]
- Larysz, A.; Prokopowicz, A.; Zakliczynski, M.; Uchmanowicz, I. Occurrence of Professional Burnout and Severity of Depressive Symptoms among Cardiac Nurses: A Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2021, 18, 12038. [Google Scholar] [CrossRef]
- Betsy Lehman Center. The Financial and Human Cost of Medical Error… and How Massachusetts Can Lead the Way on Patient Safety; Betsy Lehman Center for Patient Safety: Boston, MA, USA, 2019.
- Shanafelt, T.D.; Noseworthy, J.H. Executive Leadership and Physician Well-being: Nine Organizational Strategies to Promote Engagement and Reduce Burnout. Mayo Clin. Proc. 2017, 92, 129–146. [Google Scholar] [CrossRef]
- Samra, R. Empathy and Burnout in Medicine-Acknowledging Risks and Opportunities. J. Gen. Intern. Med. 2018, 33, 991–993. [Google Scholar] [CrossRef] [PubMed]
- NSI Nursing Solutions. 2022 NSI National Health Care Retention & RN Staffing Report; NSI Nursing Solutions Inc.: East Petersburg, PA, USA, 2022. [Google Scholar]
- Sandoval, Y.; Lobo, A.S.; Somers, V.K.; Rosenfield, K.; Bradley, S.M.; Sorajja, P.; Tajti, P.; Brilakis, E.S. Sleep deprivation in interventional cardiology: Implications for patient care and physician-health. Catheter. Cardiovasc. Interv. 2018, 91, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.S.; Sandoval, Y.; Burke, M.N.; Sorajja, P.; Mooney, M.; Traverse, J.; Henry, T.D.; Chavez, I.; Gossl, M.; Lips, D.L.; et al. Sleep Deprivation in Cardiology: A Multidisciplinary Survey. J. Invasive. Cardiol. 2019, 31, 195–198. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Piccaluga, E.; Gargani, L.; Sabatino, L.; Borghini, A.; Faita, F.; Bruno, R.M.; Padovani, R.; Guagliumi, G.; Picano, E. Subclinical carotid atherosclerosis and early vascular aging from long-term low-dose ionizing radiation exposure: A genetic, telomere, and vascular ultrasound study in cardiac catheterization laboratory staff. JACC Cardiovasc. Interv. 2015, 8, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.E.; Parikh, P.B. Prevention of Contrast and Radiation Injury During Coronary Angiography and Percutaneous Coronary Intervention. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 32. [Google Scholar] [CrossRef]
- Ciraj-Bjelac, O.; Rehani, M.M.; Sim, K.H.; Liew, H.B.; Vano, E.; Kleiman, N.J. Risk for radiation-induced cataract for staff in interventional cardiology: Is there reason for concern? Catheter. Cardiovasc. Interv. 2010, 76, 826–834. [Google Scholar] [CrossRef]
- Valdenor, C.; McCullough, P.A.; Paculdo, D.; Acelajado, M.C.; Dahlen, J.R.; Noiri, E.; Sugaya, T.; Peabody, J. Measuring the Variation in the Prevention and Treatment of CI-AKI Among Interventional Cardiologists. Curr. Probl. Cardiol. 2021, 46, 100851. [Google Scholar] [CrossRef]
- Arslan, S.; Yildiz, A.; Dalgic, Y.; Batit, S.; Kilicarslan, O.; Ser, O.S.; Dalgic, S.N.; Kocas, C.; Abaci, O. Avoiding the emergence of contrast-induced acute kidney injury in acute coronary syndrome: Routine hydration treatment. Coron. Artery. Dis. 2021, 32, 397–402. [Google Scholar] [CrossRef]
- Azzalini, L.; Kalra, S. Contrast-Induced Acute Kidney Injury-Definitions, Epidemiology, and Implications. Interv. Cardiol. Clin. 2020, 9, 299–309. [Google Scholar] [CrossRef]
- Valle, J.A.; McCoy, L.A.; Maddox, T.M.; Rumsfeld, J.S.; Ho, P.M.; Casserly, I.P.; Nallamothu, B.K.; Roe, M.T.; Tsai, T.T.; Messenger, J.C. Longitudinal Risk of Adverse Events in Patients with Acute Kidney Injury after Percutaneous Coronary Intervention: Insights From the National Cardiovascular Data Registry. Circ. Cardiovasc. Interv. 2017, 10, e004439. [Google Scholar] [CrossRef]
- Doyle, P.A.; Vockley, M. Overcoming User-Centered Challenges with Complex Health Technology. Biomed. Instrum. Technol. 2018, 52, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.A.; Jones, P.G.; Cannon, L.; Spertus, J.A. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Pujadas, S.; Serra, A.; Bajo, E.; Carreras, F.; Barros, A.; Cinca, J.; Pons-Llado, G.; Vaquerizo, B. Assessment of Inducible Myocardial Ischemia, Quality of Life, and Functional Status After Successful Percutaneous Revascularization in Patients With Chronic Total Coronary Occlusion. Am. J. Cardiol. 2016, 117, 720–726. [Google Scholar] [CrossRef]
- Young, M.N.; Secemsky, E.A.; Kaltenbach, L.A.; Jaffer, F.A.; Grantham, J.A.; Rao, S.V.; Yeh, R.W. Examining the Operator Learning Curve for Percutaneous Coronary Intervention of Chronic Total Occlusions. Circ. Cardiovasc. Interv. 2019, 12, e007877. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Banerjee, S.; Karmpaliotis, D.; Lombardi, W.L.; Tsai, T.T.; Shunk, K.A.; Kennedy, K.F.; Spertus, J.A.; Holmes, D.R., Jr.; Grantham, J.A. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: A report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2015, 8, 245–253. [Google Scholar] [CrossRef]
- McInerney, A.; Escaned, J.; Gonzalo, N. Online coregistration of intravascular ultrasound and optical coherence tomography. Minerva. Cardiol. Angiol. 2021, 69, 641–654. [Google Scholar] [CrossRef]
- Bajaj, R.; Parasa, R.; Ramasamy, A.; Makariou, N.; Foin, N.; Prati, F.; Lansky, A.; Mathur, A.; Baumbach, A.; Bourantas, C.V. Computerized technologies informing cardiac catheterization and guiding coronary intervention. Am. Heart J. 2021, 240, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Zago, E.I.; Samdani, A.J.; Pereira, G.T.R.; Vergara-Martel, A.; Alaiti, M.A.; Dallan, L.A.; Ely Pizzato, P.; Zimin, V.; Fares, A.; Bezerra, H.G. An assessment of the quality of optical coherence tomography image acquisition. Int. J. Cardiovasc. Imaging 2020, 36, 1013–1020. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- Hollander, J.E.; Carr, B.G. Virtually Perfect? Telemedicine for COVID-19. N. Engl. J. Med. 2020, 382, 1679–1681. [Google Scholar] [CrossRef]
- Woitek, F.J.; Haussig, S.; Mierke, J.; Linke, A.; Mangner, N. Remote proctoring for high-risk coronary interventions with mechanical circulatory support during COVID-19 pandemic and beyond. Clin. Res. Cardiol. 2021, 110, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Procter, S.; Andresen, D.; Hatcliff, J.; Warren, S.; Spees, W.; Weininger, S. Demonstration of a medical device integration and coordination framework. In Proceedings of the 31st International Conference on Software Engineering-Companion Volume, Vancouver, BC, Canada, 16–24 May 2009; pp. 433–434. [Google Scholar]
- Touahria, I.E.K.A. A component based framework to enable medical devices communication. Ing. Des. Syst. D’information 2021, 26, 295–302. [Google Scholar] [CrossRef]
- Whitelaw, S.; Pellegrini, D.M.; Mamas, M.A.; Cowie, M.; Van Spall, H.G.C. Barriers and facilitators of the uptake of digital health technology in cardiovascular care: A systematic scoping review. Eur. Heart J. Digit. Health 2021, 2, 62–74. [Google Scholar] [CrossRef]
- Warty, R.R.S.V.; Salih, M.; Fox, D.; McArthur, S.L.; Mol, B.M. Barriers to the Diffusion of Medical Technologies Within Healthcare: A Systematic Review. IEEE Access 2021, 9, 139043–139058. [Google Scholar] [CrossRef]
- American Hospital Association. Massive Growth in Expenses and Rising Inflation Fuel Continued Financial Challenges for America’s Hospitals and Health Systems; American Hospital Association: Chicago, IL, USA, 2022. [Google Scholar]
- European Society of Radiology (ESR). Renewal of radiological equipment. Insights Imaging 2014, 5, 543–546. [Google Scholar] [CrossRef]
- Fernandez Lozano, I.; Pozo Osinalde, E.; Garcia Bolao, I.; Ojeda Pineda, S.; Rodriguez Padial, L.; Iniguez Romo, A. Criteria for the Management of Technological Assets in Cardiovascular Imaging. Rev. Esp. Cardiol. 2018, 71, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Durbin, J.; Barnsley, J.; Finlayson, B.; Jaakkimainen, L.; Lin, E.; Berta, W.; McMurray, J. Quality of communication between primary health care and mental health care: An examination of referral and discharge letters. J. Behav. Health Serv. Res. 2012, 39, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Vermeir, P.; Vandijck, D.; Degroote, S.; Peleman, R.; Verhaeghe, R.; Mortier, E.; Hallaert, G.; Van Daele, S.; Buylaert, W.; Vogelaers, D. Communication in healthcare: A narrative review of the literature and practical recommendations. Int. J. Clin. Pract. 2015, 69, 1257–1267. [Google Scholar] [CrossRef]
- HIMSS. Interoperability in Healthcare. 2022. Available online: https://www.himss.org/resources/interoperability-healthcare (accessed on 22 July 2022).
- The West Health Institute. The Value of Medical Device Interoperability; The West Health Institute: San Diego, CA, USA, 2013. [Google Scholar]
- Jha, A.K.; Chan, D.C.; Ridgway, A.B.; Franz, C.; Bates, D.W. Improving safety and eliminating redundant tests: Cutting costs in U.S. hospitals. Health Aff. 2009, 28, 1475–1484. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Suter, E.; Oelke, N.D.; Adair, C.E.; Armitage, G.D. Ten key principles for successful health systems integration. Healthc. Q. 2009, 13, 16–23. [Google Scholar] [CrossRef]
- Shameer, K.; Badgeley, M.A.; Miotto, R.; Glicksberg, B.S.; Morgan, J.W.; Dudley, J.T. Translational bioinformatics in the era of real-time biomedical, health care and wellness data streams. Brief. Bioinform. 2017, 18, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.E.; Johnston, D.; Adler-Milstein, J.; Bates, D.W. The value of health care information exchange and interoperability. Health Aff. 2005, 24, W5-10–W5-18. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Vest, J.R.; Unruh, M.A.; Kern, L.M.; Kaushal, R.; Investigators, H. Use of Health Information Exchange and Repeat Imaging Costs. J. Am. Coll. Radiol. 2015, 12, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Lee, J.; Kim, T.; Choi, J.S.; Kang, M.; Chang, D.K.; Cha, W.C. An Automated Fast Healthcare Interoperability Resources-Based 12-Lead Electrocardiogram Mobile Alert System for Suspected Acute Coronary Syndrome. Yonsei Med. J. 2020, 61, 416–422. [Google Scholar] [CrossRef]
- Lyon, A.; Minchole, A.; Martinez, J.P.; Laguna, P.; Rodriguez, B. Computational techniques for ECG analysis and interpretation in light of their contribution to medical advances. J. R. Soc. Interface 2018, 15, 20170821. [Google Scholar] [CrossRef]
- Chen, M.; Guo, S.; Tan, X. Does Health Information Exchange Improve Patient Outcomes? Empirical Evidence from Florida Hospitals. Health Aff. 2019, 38, 197–204. [Google Scholar] [CrossRef]
- Peeters, P.; Verbist, J.; Deloose, K.; Bosiers, M. The Catheterization Lab of the Future. Endovasc. Today 2008, 3, 94–96. [Google Scholar]
- Jia, R.; Jin, Z.; Li, H.; Han, J. Re-crossing the distal cell in bifurcation verified by using an enhanced stent visualization system and optical coherence tomography: A report of two cases. J. Thorac. Dis. 2017, 9, E197–E201. [Google Scholar] [CrossRef]
- Bajaj, R.; Garcia-Garcia, H.M.; Courtney, B.K.; Ramasamy, A.; Tufaro, V.; Erdogan, E.; Khan, A.H.; Alves, N.; Rathod, K.S.; Onuma, Y.; et al. Multi-modality intravascular imaging for guiding coronary intervention and assessing coronary atheroma: The Novasight Hybrid IVUS-OCT system. Minerva. Cardiol. Angiol. 2021, 69, 655–670. [Google Scholar] [CrossRef]
- Kumar, P.; Jino, B.; Roy, S.; Shafeeq, A.; Rajendran, M. Absolute zero-contrast percutaneous coronary intervention under intravascular ultrasound guidance in chronic kidney disease patients—From despair to hope? Int. J. Cardiol. Heart Vasc. 2022, 40, 101052. [Google Scholar] [CrossRef]
- Mariani, J., Jr.; Guedes, C.; Soares, P.; Zalc, S.; Campos, C.M.; Lopes, A.C.; Spadaro, A.G.; Perin, M.A.; Filho, A.E.; Takimura, C.K.; et al. Intravascular ultrasound guidance to minimize the use of iodine contrast in percutaneous coronary intervention: The MOZART (Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasTy) randomized controlled trial. JACC Cardiovasc. Interv. 2014, 7, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Angiolillo, D.J.; Tannenbaum, M.; Driesman, M.; Chu, A.; Patterson, J.; Kuehl, W.; Battaglia, J.; Dabbons, S.; Shamoon, F.; et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am. J. Cardiol. 2008, 101, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.S.; Bohm, F.; Blum, K.; Riedel, M.; Abdelwahed, Y.S.; Klotsche, J.; Steiner, J.K.; Heuberger, A.; Skurk, C.; Mochmann, H.C.; et al. Impact of real-time angiographic co-registered optical coherence tomography on percutaneous coronary intervention: The OPTICO-integration II trial. Clin. Res. Cardiol. 2021, 110, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.; Javadzadegan, A.; Mohammadi, M.; Ravipudi, L.; Cheng, S.; Martins, R. Development of an innovative technology to segment luminal borders of intravascular ultrasound image sequences in a fully automated manner. Comput. Biol. Med. 2019, 108, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalishahi, S.; Miotto, R.; Dudley, J.T.; Lavelli, A.; Rinaldi, F.; Osmani, V. Natural Language Processing of Clinical Notes on Chronic Diseases: Systematic Review. JMIR Med. Inform. 2019, 7, e12239. [Google Scholar] [CrossRef]
- Sanborn, T.A.; Tcheng, J.E.; Anderson, H.V.; Chambers, C.E.; Cheatham, S.L.; DeCaro, M.V.; Durack, J.C.; Everett, A.D.; Gordon, J.B.; Hammond, W.E.; et al. ACC/AHA/SCAI 2014 health policy statement on structured reporting for the cardiac catheterization laboratory: A report of the American College of Cardiology Clinical Quality Committee. J. Am. Coll. Cardiol. 2014, 63, 2591–2623. [Google Scholar] [CrossRef]
- Nan, S.; Van Gorp, P.; Lu, X.; Kaymak, U.; Korsten, H.; Vdovjak, R.; Duan, H. A meta-model for computer executable dynamic clinical safety checklists. BMC Med. Inform. Decis. Mak. 2017, 17, 170. [Google Scholar] [CrossRef]
- Pageler, N.M.; Longhurst, C.A.; Wood, M.; Cornfield, D.N.; Suermondt, J.; Sharek, P.J.; Franzon, D. Use of electronic medical record-enhanced checklist and electronic dashboard to decrease CLABSIs. Pediatrics 2014, 133, e738–e746. [Google Scholar] [CrossRef]
- Garg, T.; Lee, J.Y.; Evans, K.H.; Chen, J.; Shieh, L. Development and evaluation of an electronic health record-based best-practice discharge checklist for hospital patients. Jt. Comm. J. Qual. Patient. Saf. 2015, 41, 126–131. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Harrison, A.M.; O’Horo, J.C.; Berrios, R.A.; Pickering, B.W.; Herasevich, V. The Effect of an Electronic Checklist on Critical Care Provider Workload, Errors, and Performance. J. Intensive Care Med. 2016, 31, 205–212. [Google Scholar] [CrossRef]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatston, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. CAD-RADS(TM) Coronary Artery Disease—Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J. Cardiovasc. Comput. Tomogr. 2016, 10, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Bunck, A.C.; Baessler, B.; Ritter, C.; Kroger, J.R.; Persigehl, T.; Pinto Santos, D.; Steinmetz, M.; Niehaus, A.; Bamberg, F.; Beer, M.; et al. Structured Reporting in Cross-Sectional Imaging of the Heart: Reporting Templates for CMR Imaging of Cardiomyopathies (Myocarditis, Dilated Cardiomyopathy, Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Siderosis). Rofo 2020, 192, 27–37. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, C.; Zhuang, N.; Gao, Y.; Li, Z.; Ren, X.; Yang, L.; Zhang, J.; Budoff, M.J.; Lu, B. Current utilization of cardiac computed tomography in mainland China: A national survey. J. Cardiovasc. Comput. Tomogr. 2016, 10, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Szilveszter, B.; Kolossvary, M.; Karady, J.; Jermendy, A.L.; Karolyi, M.; Panajotu, A.; Bagyura, Z.; Vecsey-Nagy, M.; Cury, R.C.; Leipsic, J.A.; et al. Structured reporting platform improves CAD-RADS assessment. J. Cardiovasc. Comput. Tomogr. 2017, 11, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, Y.; Luo, N.; Guo, N.; Hong, M.; Jia, X.; Wang, Z.; Yang, Z. Diagnostic Accuracy and Generalizability of a Deep Learning-Based Fully Automated Algorithm for Coronary Artery Stenosis Detection on CCTA: A Multi-Centre Registry Study. Front. Cardiovasc. Med. 2021, 8, 707508. [Google Scholar] [CrossRef]
- Thoenes, M.; Agarwal, A.; Grundmann, D.; Ferrero, C.; McDonald, A.; Bramlage, P.; Steeds, R.P. Narrative review of the role of artificial intelligence to improve aortic valve disease management. J. Thorac. Dis. 2021, 13, 396–404. [Google Scholar] [CrossRef]
- Lin, A.; Kolossvary, M.; Isgum, I.; Maurovich-Horvat, P.; Slomka, P.J.; Dey, D. Artificial intelligence: Improving the efficiency of cardiovascular imaging. Expert. Rev. Med. Devices 2020, 17, 565–577. [Google Scholar] [CrossRef]
- Alsharqi, M.; Woodward, W.J.; Mumith, J.A.; Markham, D.C.; Upton, R.; Leeson, P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018, 5, R115–R125. [Google Scholar] [CrossRef]
| Primary Prevention | Emergency Care | Diagnosis | Treatment | Secondary Prevention | Follow-Up |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |||||
|
| Research Theme | Key Search Terms Used |
|---|---|
| General | (coronary artery disease [tw] or catheterization lab [tw] or cath lab [tw] (cardiologist [tw] or cardiologists [MeSH])) AND |
| Overview of coronary artery disease pathway | (review [tw] or systematic review [tw]) AND |
| Diagnostic burdens | (diagnostic error [tw] or delay [tw] or wait times [tw] or (suboptimal diagnosis [tw] or diagnostic challenges [MeSH]) or accuracy [tw])) OR |
| Treatment burdens | (undertreatment [tw] or (incomplete [tw] or treatment gap [MeSH]) or (delay [tw] or wait time [MeSH]) or overuse [tw] or underuse [tw]) OR |
| Workflow efficiencies | (workflow [tw] or ((in)efficiency [tw] or productivity [MeSH]) or turnover time [tw] or procedure time [tw] or electronic medical record [tw] or integration [tw] or standard * [tw] or streamline [tw] or error [tw] or administrative [tw] or (user-friendly [tw] or ease of use [MeSH]) or automat * [tw] or data integration [MeSH] or artificial intelligence [tw] or data access [tw] or real-time data [tw] and (interoperability [tw] or (integration [tw] or integrated [MeSH]))) OR |
| Post-acute care | (rehabilitation [tw] or follow-up [tw]) OR |
| Healthcare worker burdens | ((healthcare worker [MeSH] or healthcare provider [MeSH] or technologist [tw] or clinician [tw] or nurse [tw] or cardiologist [tw]) and (stress [tw] or burnout [tw] or satisfaction [tw] or workload [tw] or radiation [tw] or learning curve [tw]) OR |
| Economic burdens | (cost [tw] or economics [tw] or cost-effectiveness [tw] or economic burden [tw]) OR |
| Patient burdens | (contrast [tw] or radiation [tw] or (acute kidney injury [tw] or complication [tw] or readmission [tw])) |
| Modality | Strengths | Limitations |
|---|---|---|
| Functional testing | ||
| Stress ECG |
|
|
| Stress Echo |
|
|
| SPECT |
|
|
| Stress PET |
|
|
| Stress CMR |
|
|
| Anatomical testing | ||
| CCTA |
|
|
| Spectral detector CT |
|
|
| ICA |
|
|
| Burden | Key Impacts on the Healthcare System, Providers, and Patients |
|---|---|
| Suboptimal diagnosis (e.g., false negatives) |
|
| Delays in accessing emergent diagnostic care | |
| Increasing complexity in clinician decision-making with expansion of test strategies and options |
|
| Overuse of invasive technologies (e.g., inaccuracies, false positives) |
|
| Underuse of emergent PCI |
|
| Increasing use of PCI in complex cases and associated burdens |
|
| Overuse of PCI |
|
| Underutilization of cardiac rehabilitation |
|
| Inefficiencies in the catheterization lab |
|
| Clinician burnout and sleep deprivation |
|
| Manual processes |
|
| Technology risks |
|
| COVID-19 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodeboina, M.; Piayda, K.; Jenniskens, I.; Vyas, P.; Chen, S.; Pesigan, R.J.; Ferko, N.; Patel, B.P.; Dobrin, A.; Habib, J.; et al. Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 5633. https://doi.org/10.3390/ijerph20095633
Kodeboina M, Piayda K, Jenniskens I, Vyas P, Chen S, Pesigan RJ, Ferko N, Patel BP, Dobrin A, Habib J, et al. Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review. International Journal of Environmental Research and Public Health. 2023; 20(9):5633. https://doi.org/10.3390/ijerph20095633
Chicago/Turabian StyleKodeboina, Monika, Kerstin Piayda, Inge Jenniskens, Pearl Vyas, Sara Chen, Ramon Julian Pesigan, Nicole Ferko, Barkha P. Patel, Annamaria Dobrin, Jayson Habib, and et al. 2023. "Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review" International Journal of Environmental Research and Public Health 20, no. 9: 5633. https://doi.org/10.3390/ijerph20095633
APA StyleKodeboina, M., Piayda, K., Jenniskens, I., Vyas, P., Chen, S., Pesigan, R. J., Ferko, N., Patel, B. P., Dobrin, A., Habib, J., & Franke, J. (2023). Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review. International Journal of Environmental Research and Public Health, 20(9), 5633. https://doi.org/10.3390/ijerph20095633







