Surface Functionalization of Bioactive Hybrid Adsorbents for Enhanced Adsorption of Organic Dyes
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of Mango Seed Kernel Extract (MS-Ext)
2.3. Surface Functionalization of ZnO by the Mango Seed Extract (MS-Ext)
2.4. Surface Functionalization of Cu Doped ZnO by MS-Ext
2.5. Characterization
2.5.1. Adsorbent Surface Charge and Thermogravimetric Analysis (TGA)
2.5.2. FTIR for Detection of Functional Groups in ZnO Samples Modified by BPCs
2.5.3. Morphology and Elemental Analysis
2.5.4. Adsorption and Selectivity Experiments
2.6. Kinetics of the Adsorption
2.7. Adsorption Isotherm
3. Results and Discussion
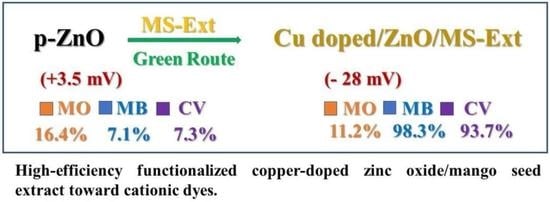
3.1. Surface Functionalization of ZnO with MS Extract
3.2. Characterization of Functionalized Adsorbents
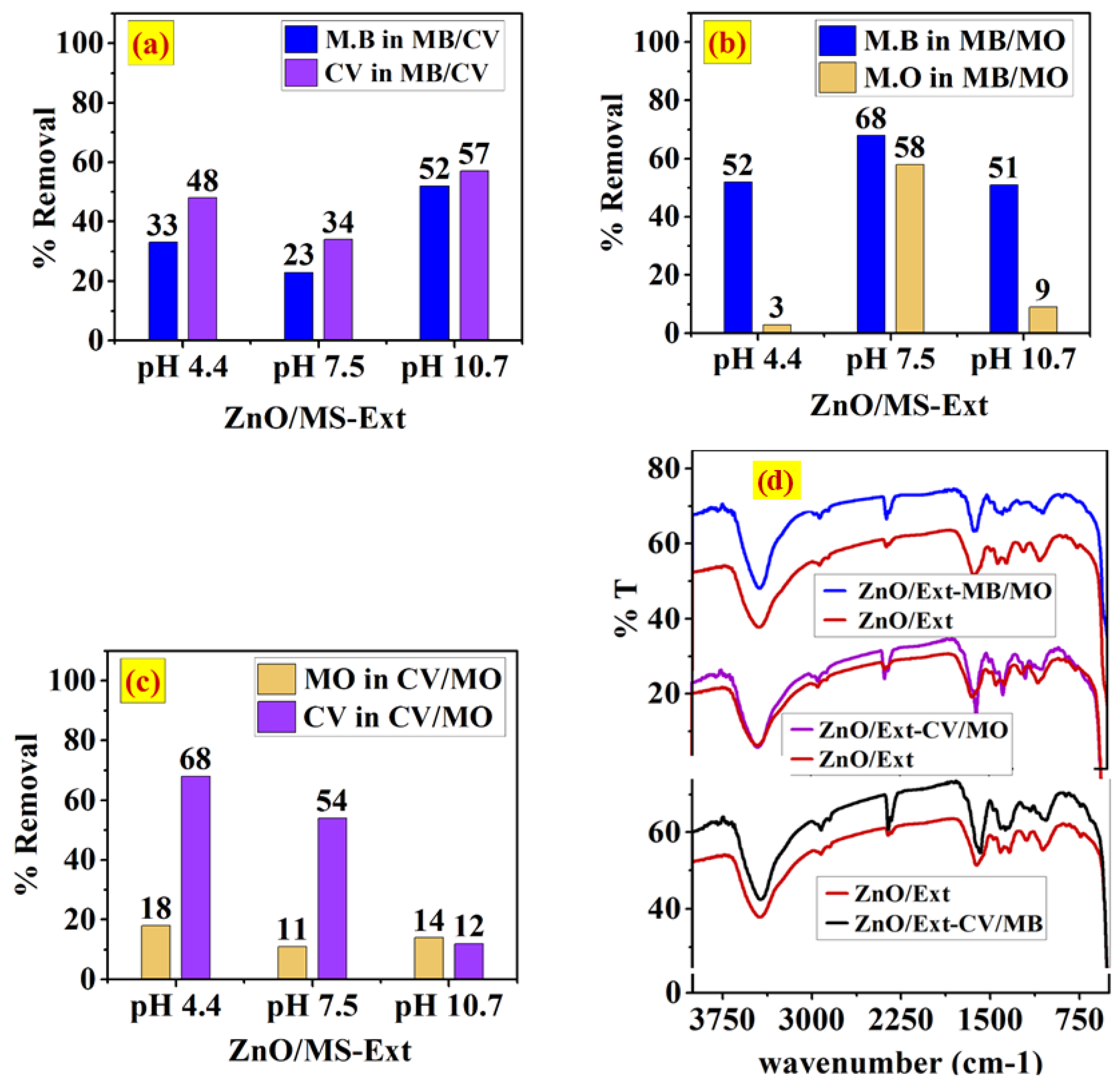
3.2.1. FTIR Spectra for Analyzing the Functional Groups
3.2.2. Adsorbent Surface Charge
3.2.3. Morphology and Elemental Analysis
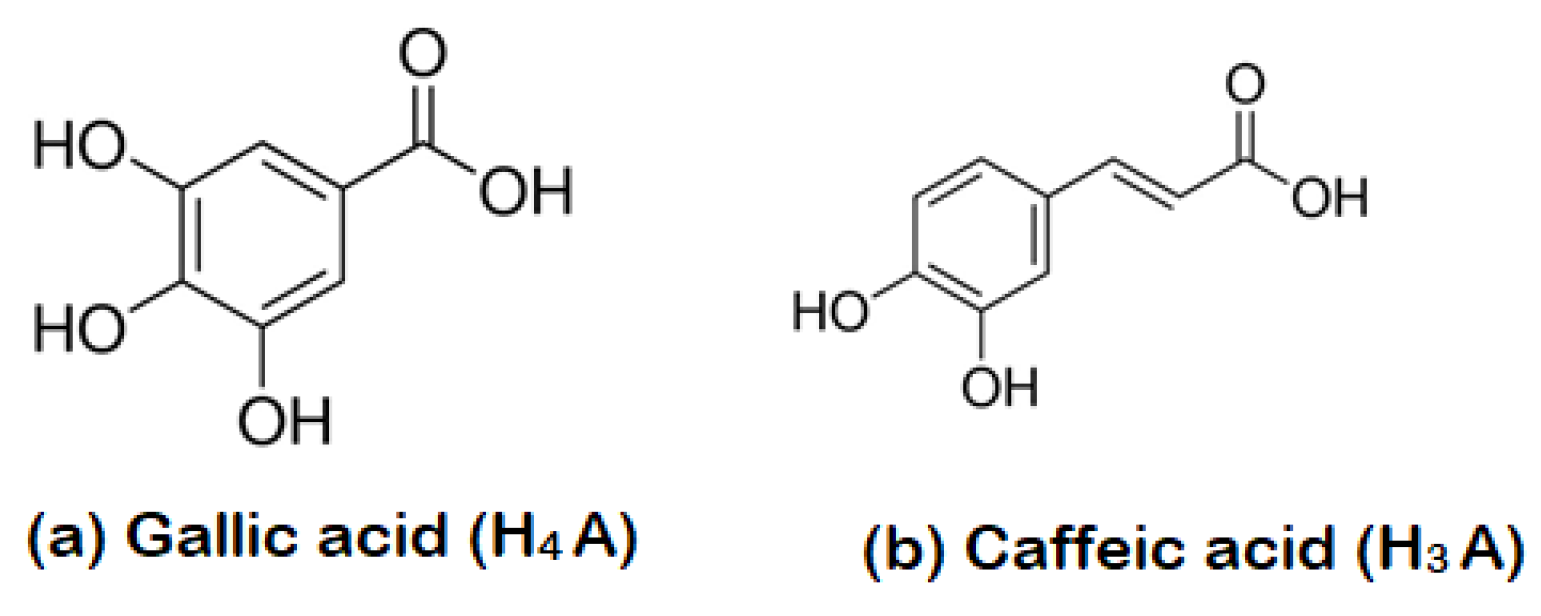
3.3. The Role of MS-Ext in Enhancing Adsorption
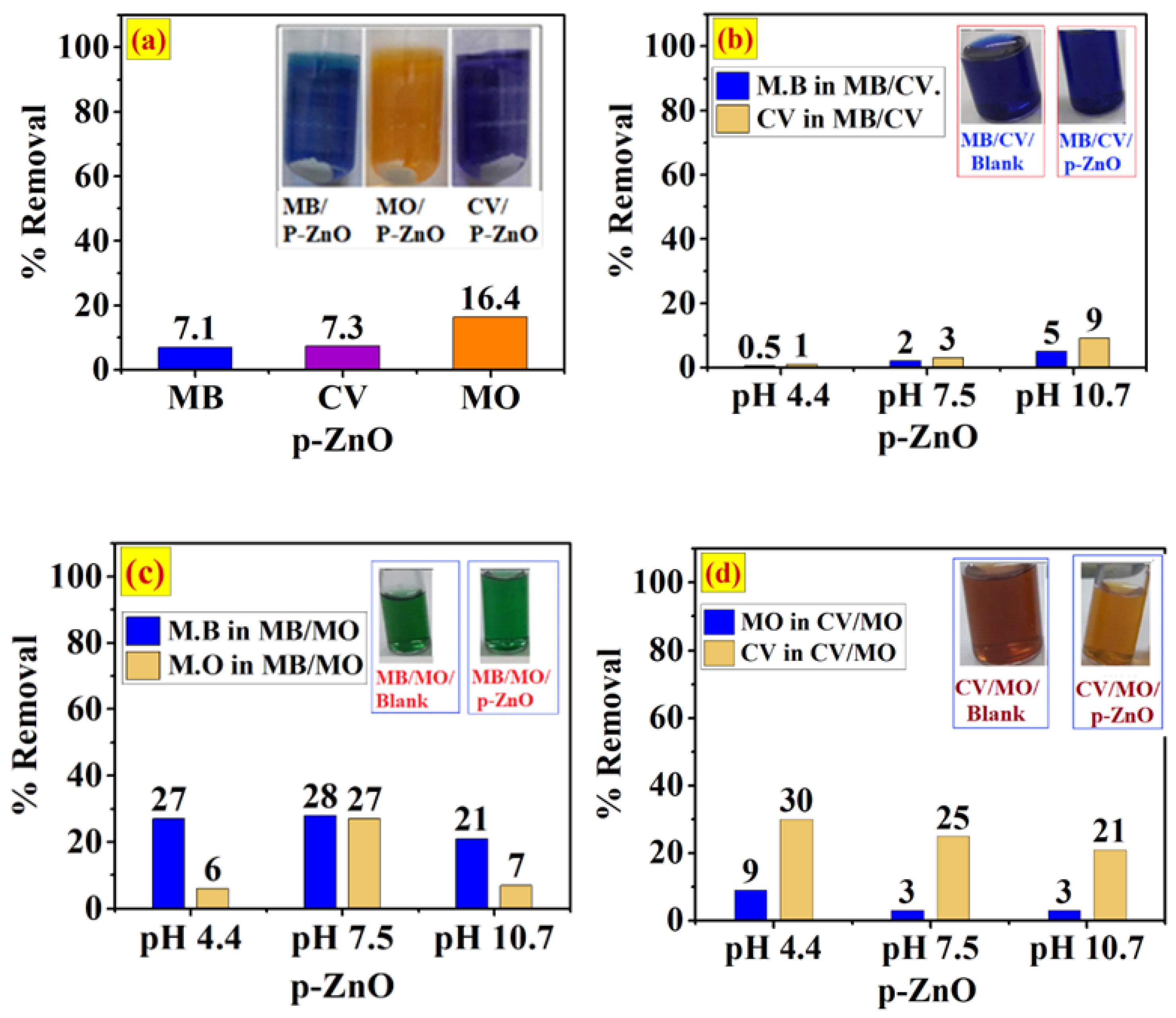
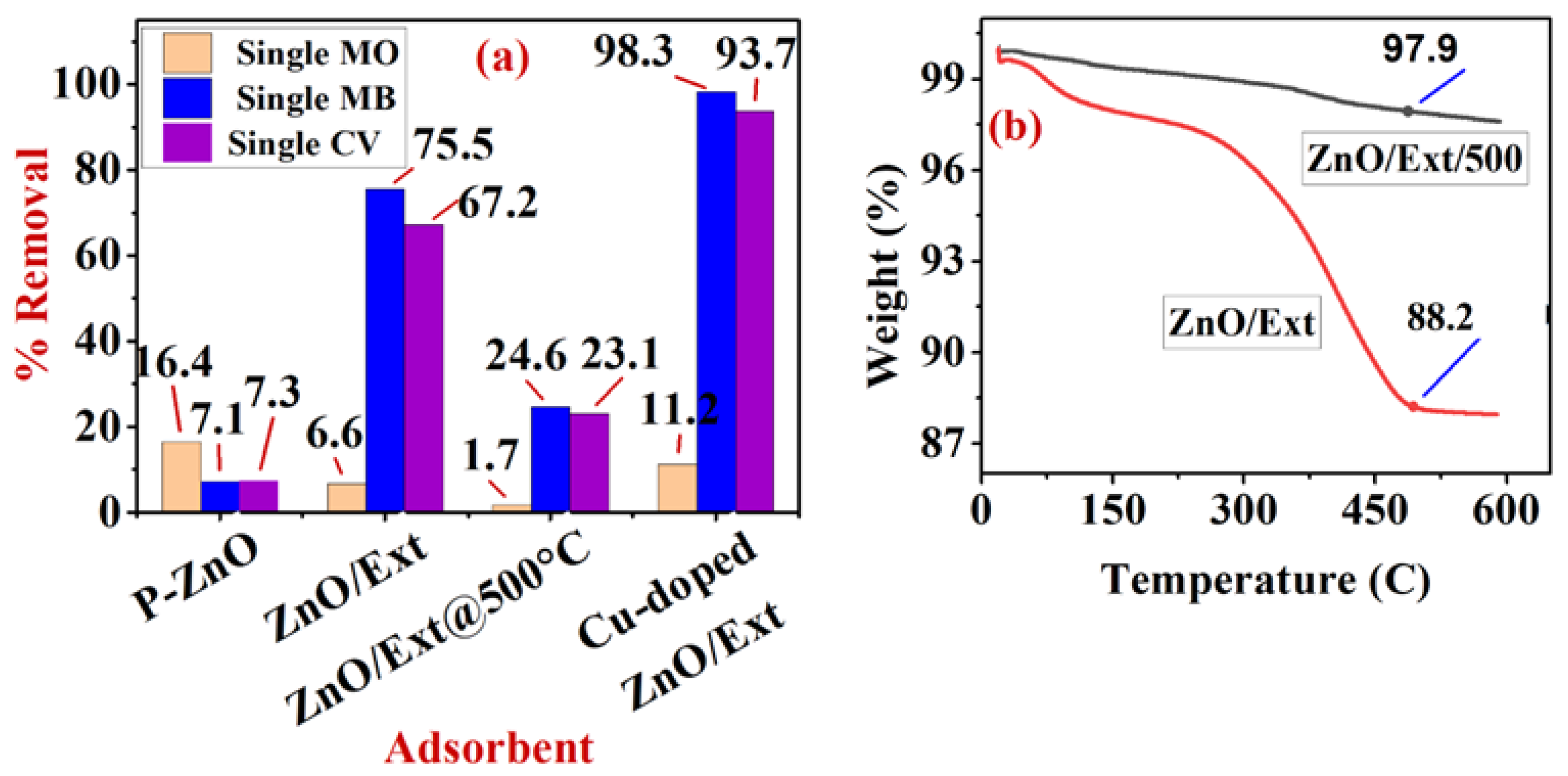
3.4. Adsorption of Single and Binary System onto p-ZnO
The Influence of Competitive Adsorption on Selectivity onto p-ZnO
3.5. Effect of SurfaceFunctionalization of p-ZnO on Enhancing Adsorption Process
3.5.1. Adsorption of Single and Binary Systems onto ZnO/MS-Ext and ZnO/MS-Ext@500 °C
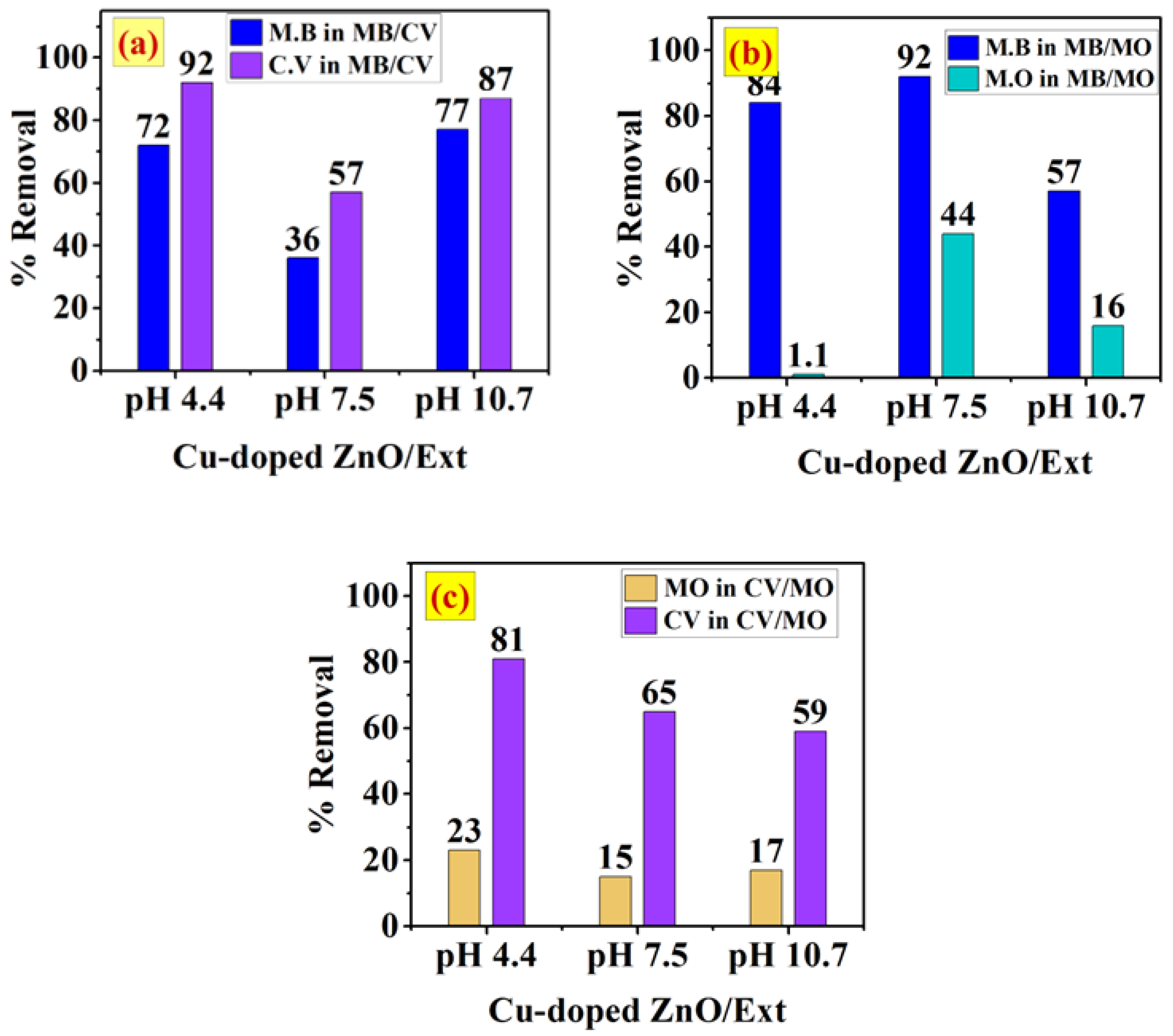
3.5.2. Adsorption of Single and Binary Systems onto Cu-Doped ZnO/MS-Ext
3.6. Adsorption Kinetics of MB/CV Binary System
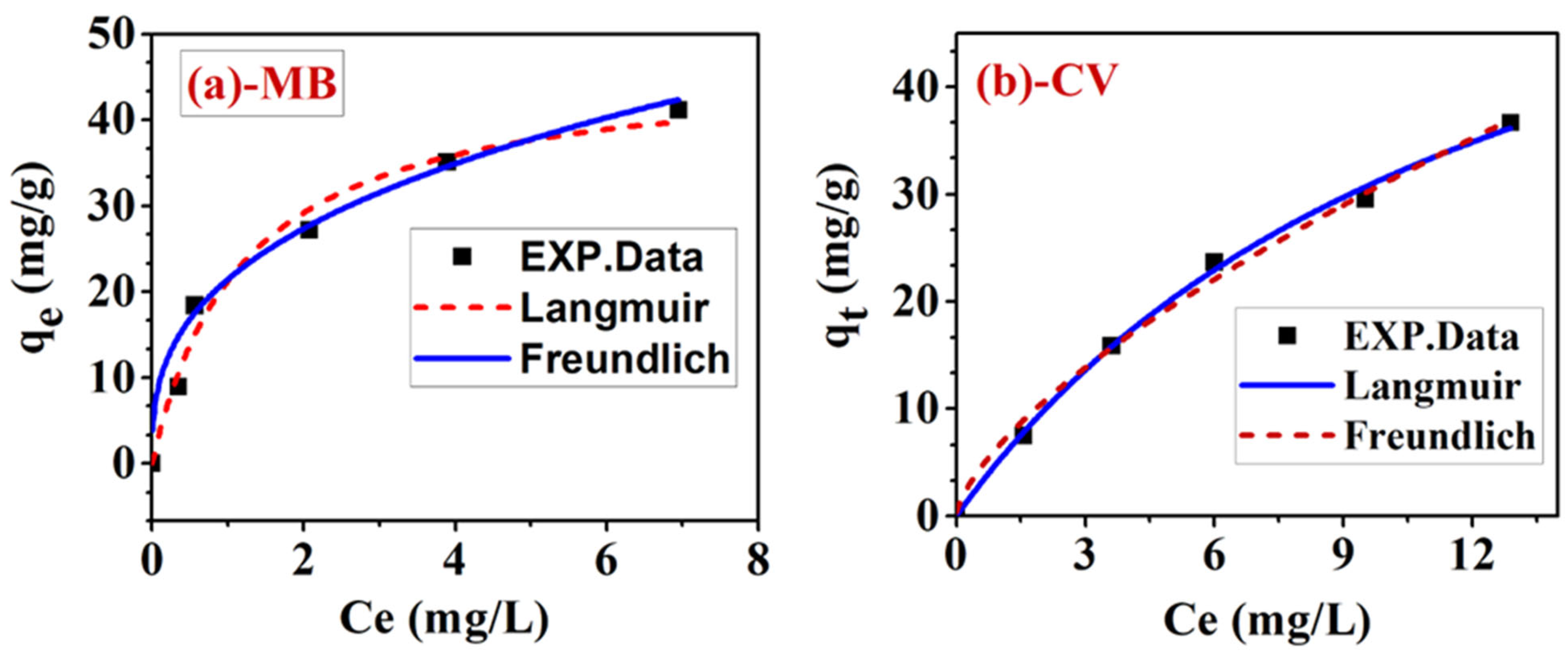
3.7. Adsorption Isotherm of MB/CV Binary System
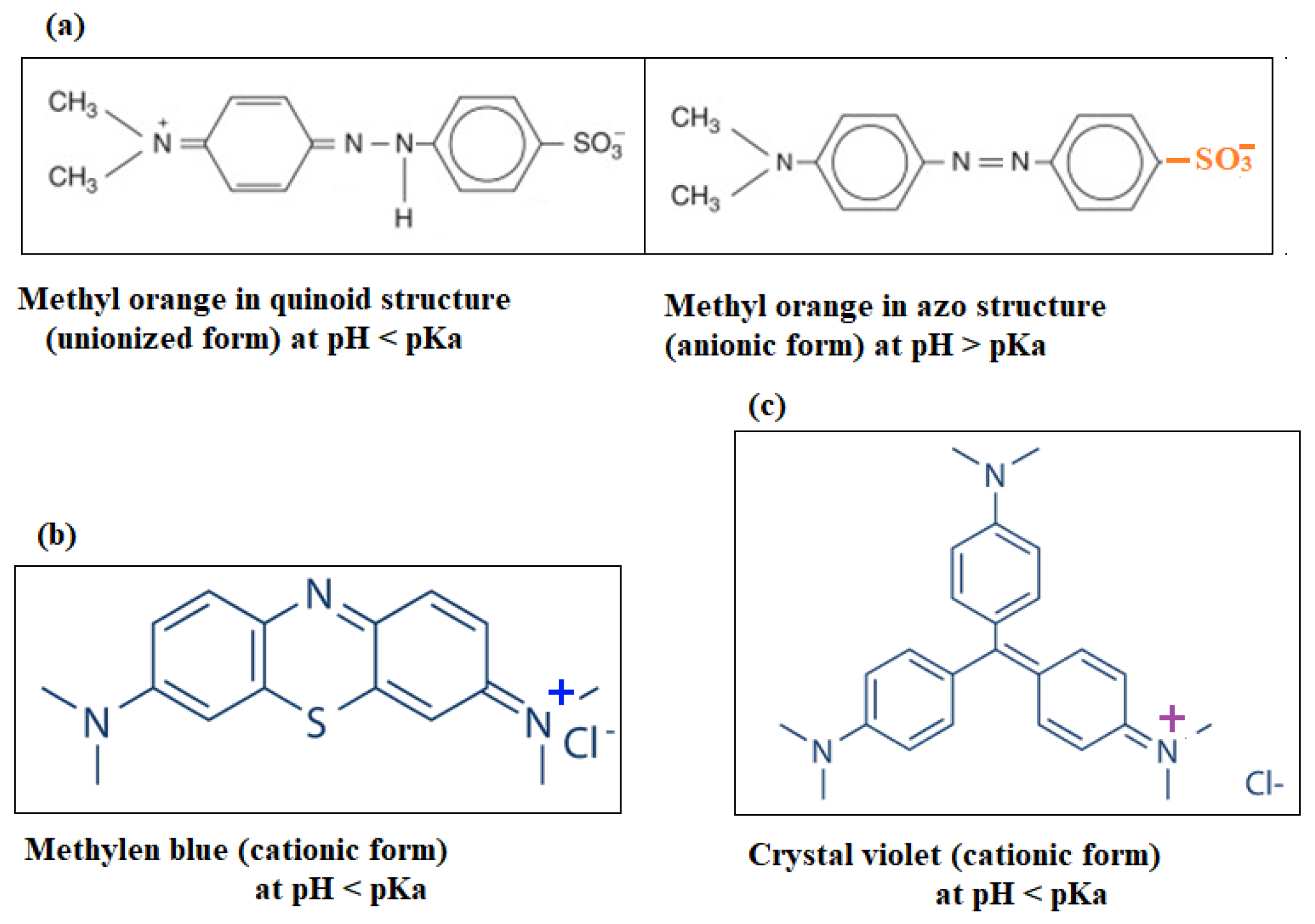
3.8. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani, K.A.; Jangid, N.K.; Rashid, A. Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2020. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bhattacharjee, J. A comparative study between physicochemical and biological methods for effective removal of textile dye from wastewater. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 1; pp. 1–21. [Google Scholar] [CrossRef]
- Singha, K.; Pandit, P.; Maity, S.; Sharma, S.R. Harmful environmental effects for textile chemical dyeing practice. In Green Chemistry for Sustainable Textiles; Woodhead Publishing: Cambridge, UK, 2021; Chapter 11; pp. 153–164. [Google Scholar] [CrossRef]
- Gupta, S.; Mittal, Y.; Tamta, P.; Srivastava, P.; Yadav, A.K. Textile wastewater treatment using microbial fuel cell and coupled technology: A green approach for detoxification and bioelectricity generation. In Integrated Microbial Fuel Cells for Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2020; pp. 73–92. [Google Scholar] [CrossRef]
- Lin, R.; Liang, Z.; Yang, C.; Zhao, Z.; Cui, F. Selective adsorption of organic pigments on inorganically modified mesoporous biochar and its mechanism based on molecular structure. J. Colloid Interface Sci. 2020, 573, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.L.; Huang, Y.; Hansen, L.T.; Yost, C.K.; Lake, C.; Jamieson, R.C. Fate and distribution of determinants of antimicrobial resistance in lateral flow sand filters used for treatment of domestic wastewater. Sci. Total Environ. 2021, 767, 145481. [Google Scholar] [CrossRef] [PubMed]
- Sosthene, K.M.; Gahi, N. Low Cost Filtration of Domestic Wastewater for Irrigation Purpose. World J. Eng. Technol. 2018, 6, 585–602. [Google Scholar] [CrossRef]
- Loktionova, E.V.; Sysa, O.K.; Loktionov, V.A. Extraction of methylene blue coloring agent from model solutions with a plant-origin sorbent. J. Phys. Conf. Ser. 2021, 1926, 012018. [Google Scholar] [CrossRef]
- Koul, B.; Sharma, K.; Shah, M.P. Phycoremediation: A sustainable alternative in wastewater treatment (WWT) regime. Environ. Technol. Innov. 2021, 25, 102040. [Google Scholar] [CrossRef]
- Mostafa, N.G.; Yunnus, A.F.; Elawwad, A. Adsorption of Pb(II) from Water onto ZnO, TiO2, and Al2O3: Process Study, Adsorption Behaviour, and Thermodynamics. Adsorpt. Sci. Technol. 2022, 2022, 7582756. [Google Scholar] [CrossRef]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.-Y.; Tam, K.C. Selective adsorption and separation of organic dyes using functionalized cellulose nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar] [CrossRef]
- Molla, A.; Li, Y.; Mandal, B.; Kang, S.G.; Hur, S.H.; Chung, J.S. Selective adsorption of organic dyes on graphene oxide: Theoretical and experimental analysis. Appl. Surf. Sci. 2019, 464, 170–177. [Google Scholar] [CrossRef]
- Taoufik, N.; Elmchaouri, A.; Anouar, F.; Korili, S.; Gil, A. Improvement of the adsorption properties of an activated carbon coated by titanium dioxide for the removal of emerging contaminants. J. Water Process. Eng. 2019, 31, 100876. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment. In Green Adsorbents for Pollutant Removal; Springer International Publishing: Cham, Switzerland, 2018; pp. 23–71. [Google Scholar] [CrossRef]
- Charazińska, S.; Lochyński, P.; Burszta-Adamiak, E. Removal of heavy metal ions form acidic electrolyte for stainless steel electropolishing via adsorption using Polish peats. J. Water Process. Eng. 2021, 42, 102169. [Google Scholar] [CrossRef]
- Roy, H.; Shakil, R.; Tarek, Y.A.; Firoz, S.H. Study of the Removal of Basic Blue-41 from Simulated Wastewater by Activated Carbon Prepared from Discarded Jute Fibre. ECS Trans. 2022, 107, 8407–8420. [Google Scholar] [CrossRef]
- Praveen, S.; Jegan, J.; Pushpa, T.B.; Gokulan, R.; Bulgariu, L. Biochar for removal of dyes in contaminated water: An overview. Biochar 2022, 4, 10. [Google Scholar] [CrossRef]
- Islam, M.S.; Roy, H.; Afrose, S. Phosphoric acid surface modified Moringa oleifera leaves biochar for the sequestration of methyl orange from aqueous solution: Characterizations, isotherm, and kinetics analysis. Remediat. J. 2022, 32, 281–298. [Google Scholar] [CrossRef]
- Mao, B.; Sidhureddy, B.; Thiruppathi, A.R.; Wood, P.C.; Chen, A. Efficient dye removal and separation based on graphene oxide nanomaterials. New J. Chem. 2020, 44, 4519–4528. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Karaman, C.; Zare, N.; Karimi-Maleh, H.; Vasseghian, Y. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J. Water Process. Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Roy, H.; Islam, M.S.; Arifin, M.T.; Firoz, S.H. Synthesis, Characterization and Sorption Properties of Biochar, Chitosan and ZnO-Based Binary Composites towards a Cationic Dye. Sustainability 2022, 14, 14571. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.A.; Jothirani, R. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalination Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Pivehzhani, O.A.; Johan, M.R. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Fernandez-Lafuente, R.; Berenguer-Murcia, Á.; Meza-Gordillo, R.; Gutiérrez, L.-F.; Pacheco, N.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T. Phenoliccompounds in mango fruit: A review. J. Food Meas. Charact. 2021, 16, 619–636. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Ospina, J.C.G. Chemical Compositionof Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- Gajewska, M.; Skrzypiec, K.; Jóźwiakowski, K.; Mucha, Z.; Wójcik, W.; Karczmarczyk, A.; Bugajski, P. Kinetics of pollutants removal in vertical and horizontal flow constructed wetlands in temperate climate. Sci. Total Environ. 2020, 718, 137371. [Google Scholar] [CrossRef]
- Elmorsi, T.M.; Elsayed, M.H.; Bakr, M.F. Enhancing the removal of methylene blue by modified ZnO nanoparticles: Kinetics and equilibrium studies. Can. J. Chem. 2017, 95, 590–600. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-Solvent Selection for Supercritical Fluid Extraction (SFE) of Phenolic Compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef]
- Ulfa, M.; Nisa, D.; Muhammad, F.P.; Prasetyoko, D. Investigating the Hydrophilicity of Zinc Oxide Nanoparticles Using Xylene and Water for Ibubrofen Adsorption. J. Chem. Technol. Metall. 2021, 56, 761–768. [Google Scholar]
- Dzigbor, A.; Chimphango, A. Production and optimization of NaCl-activated carbon from mango seed using response surface methodology. Biomass Convers. Biorefinery 2019, 9, 421–431. [Google Scholar] [CrossRef]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, antioxidant and anticancer activities of gold nanoparticles green synthesized using Mangifera indica seed aqueous extract. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Verma, S.K.; Das, T.; Panda, P.K.; Parashar, K.; Suar, M.; Parashar, S.K.S. Altered electrical properties with controlled copper doping in ZnO nanoparticles infers their cytotoxicity in macrophages by ROS induction and apoptosis. Chem. Biol. Interact. 2018, 297, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.F.; Özkasikci, D.; Fürtauer, S.; Reinelt, M. The Effect of Deprotonation on the Reaction Kinetics of an Oxygen Scavenger Based on Gallic Acid. Front. Chem. 2019, 7, 680. [Google Scholar] [CrossRef]
- Levy, L.; Gurov, A.; Radian, A. The effect of gallic acid interactions with iron-coated clay on surface redox reactivity. Water Res. 2020, 184, 116190. [Google Scholar] [CrossRef]
- Badhani, B.; Kakkar, R. DFT study of structural and electronic properties of gallic acid and its anions in gas phase and in aqueous solution. Struct. Chem. 2017, 28, 1789–1802. [Google Scholar] [CrossRef]
- Jyothi, N.S.; Ravichandran, K. Optimum pH for effective dye degradation: Mo, Mn, Co and Cu doped ZnO photocatalysts in thin film form. Ceram. Int. 2020, 46, 23289–23292. [Google Scholar] [CrossRef]
- Kumari, H.J.; Krishnamoorthy, P.; Arumugam, T.K.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, W.; Chen, C.; Wang, X. Adsorption of Methyl Orange Dye onto Multiwalled Carbon Nanotubes. Procedia Environ. Sci. 2013, 18, 890–895. [Google Scholar] [CrossRef]
- Chen, K.; Du, L.; Gao, P.; Zheng, J.; Liu, Y.; Lin, H. Super and Selective Adsorption of Cationic Dyes onto Carboxylate-Modified Passion Fruit Peel Biosorbent. Front. Chem. 2021, 9, 646492. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Duan, Y.; Zhou, L. Multi-carboxylic magnetic gel from hyperbranched polyglycerol formed by thiol-ene photopolymerization for efficient and selective adsorption of methylene blue and methyl violet dyes. J. Colloid Interface Sci. 2018, 529, 139–149. [Google Scholar] [CrossRef]
- Idrisi, M.; Saleh, H.A.M.; Qasem, K.M.A.; Shahid, M.; Mehtab, M.; Ahmad, M. Efficient and selective adsorption and separation of methylene blue (MB) from mixture of dyes in aqueous environment employing a Cu(II) based metal organic framework. Inorg. Chim. Acta 2020, 511, 119787. [Google Scholar] [CrossRef]
- Gupta, A.; Viltres, H.; Gupta, N.K. Sono-adsorption of organic dyes onto CoFe2O4/Graphene oxide nanocomposite. Surf. Interfaces 2020, 20, 100563. [Google Scholar] [CrossRef]
- Sharma, P.; Jain, R.; Premi, A.; Kumar, N.; Parashar, A. Linear and non-linear regression analysis for the sorption kinetics of Tropaeoline 000 onto Nano Talc. Int. J. Environ. Anal. Chem. 2020, 102, 2152–2167. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, Q.; Zhang, W.; Zhang, Y.; Zhao, M. Comparative adsorption of methylene blue by magnetic baker’s yeast and EDTAD-modified magnetic baker’s yeast: Equilibrium and kinetic study. Arab. J. Chem. 2019, 12, 2448–2456. [Google Scholar] [CrossRef]
- Chodankar, D.; Vora, A.; Kanhed, A. β-cyclodextrin and its derivatives: Application in wastewater treatment. Environ. Sci. Pollut. Res. 2021, 29, 1585–1604. [Google Scholar] [CrossRef]

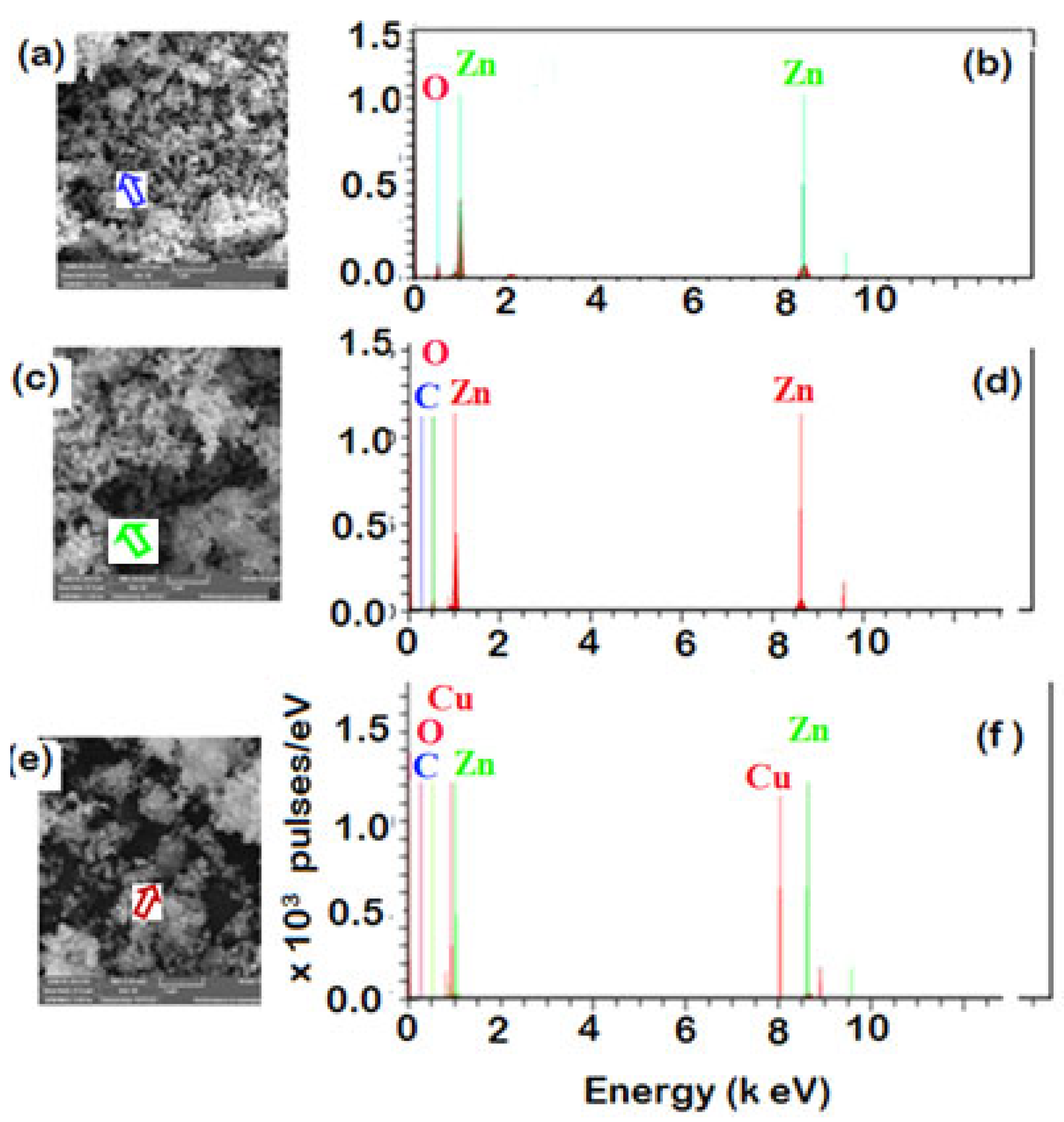



| p-ZnO | ZnO/MS-Ext | Cu-doped ZnO-MS-Ext | ||||
|---|---|---|---|---|---|---|
| Element | Mass [%] | Atomic [%] | Mass [%] | Atomic [%] | Mass [%] | Atomic [%] |
| Zinc | 81.13 | 51.27 | 66.91 | 30.50 | 85.53 | 56.76 |
| Oxygen | 18.87 | 48.73 | 20.40 | 38.00 | 6.02 | 16.32 |
| Carbon | 0 | 0 | 12.70 | 31.50 | 7.22 | 26.08 |
| Copper | 0 | 0 | 0 | 1.23 | 0.84 | |
| Sum | 100 | 100 | 100 | 100 | 100 | |
| Nonlinear Pseudo-First-Order | Nonlinear Pseudo-Second-Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [MB] ppm | (mg/g) | (mg/g) | (min−1) | RSS | qe. (cal) (mg/g) | (min−1) | RSS | ||
| 10 | 8.55 | 7.88 | 0.074 | 0.965 | 0.492 | 8.64 | 0.0135 | 0.990 | 0.492 |
| 20 | 17.2 | 17.10 | 0.038 | 0.998 | 1.74 | 19.94 | 0.0023 | 0.993 | 1.738 |
| 30 | 23.33 | 23.41 | 0.029 | 0.996 | 1.36 | 28.26 | 0.0011 | 0.997 | 1.359 |
| 40 | 35.1 | 33.30 | 0.074 | 0.985 | 1.33 | 36.25 | 0.0034 | 0.999 | 1.316 |
| 50 | 41.15 | 39.08 | 0.111 | 0.989 | 1.67 | 41.36 | 0.0056 | 0.999 | 1.673 |
| Nonlinear Pseudo-First-Order | Nonlinear Pseudo-Second-Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [MB] ppm | (mg/g) | (mg/g) | (min−1) | RSS | qe. (cal) (mg/g) | (min−1) | RSS | ||
| 10 | 7.55 | 6.99 | 0.042 | 0.961 | 1.505 | 8.00 | 0.007 | 0.988 | 0.472 |
| 20 | 15.88 | 15.18 | 0.041 | 0.982 | 3.234 | 17.45 | 0.003 | 0.997 | 0.502 |
| 30 | 23.67 | 23.01 | 0.025 | 0.978 | 8.887 | 28.19 | 0.001 | 0.991 | 3.510 |
| 40 | 29.55 | 28.8 | 0.108 | 0.995 | 3.382 | 30.37 | 0.008 | 1.000 | 0.194 |
| 50 | 41.65 | 38.2 | 0.067 | 0.973 | 31.234 | 42.03 | 0.003 | 0.994 | 7.040 |
| Nonlinear Elovich Model | Linear Elovich Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [MB] ppm | (mg/g) | β (g/mg) | α (mg/g/min) | RSS | β (g/mg) | α (mg/g/min) | RSS | ||
| 10 | 8.55 | 0.227 | 79.071 | 0.997 | 2.542 | 0.585 | 1.709 | 0.974 | 1.137 |
| 20 | 17.2 | 0.889 | 12.25 | 0.999 | 0.052 | 0.272 | 3.672 | 0.975 | 5.099 |
| 30 | 23.33 | 0.246 | 2.080 | 0.976 | 5.780 | 0.2080 | 4.807 | 0.962 | 13.647 |
| 40 | 35.1 | 0.152 | 1.558 | 0.988 | 5.112 | 0.139 | 7.211 | 0.971 | 22.971 |
| 50 | 41.15 | 0.296 | 40.571 | 0.999 | 0.679 | 0.117 | 8.560 | 0.929 | 82.477 |
| Nonlinear Elovich Model | Linear Elovich Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [MB] ppm | (mg/g) | β (g/mg) | α (mg/g/min) | RSS | β (g/mg) | α (mg/g/min) | RSS | ||
| 10 | 8.55 | 1.25 | 0.666 | 0.998 | 0.063 | 0.686 | 1.460 | 0.992 | 0.075 |
| 20 | 17.2 | 2.49 | 0.300 | 0.997 | 0.490 | 0.309 | 3.241 | 0.990 | 0.431 |
| 30 | 23.33 | 1.22 | 0.1462 | 0.997 | 1.291 | 0.166 | 6.032 | 0.987 | 2.109 |
| 40 | 35.1 | 79.071 | 0.227 | 0.996 | 2.411 | 0.429 | 2.331 | 0.950 | 2.410 |
| 50 | 41.15 | 38.25 | 0.171 | 0.999 | 1.049 | 0.171 | 5.835 | 0.993 | 1.037 |
| Dye in Mixture | Langmuir Constants | Freundlich Constants | ||||||
|---|---|---|---|---|---|---|---|---|
(mg/g) | (L/mg) | RSS | (L/mg) | n | RSS | |||
| MB | 46.61 | 0.077 | 0.982 | 22.38 | 21.42 | 0.352 | 0.954 | 35.88 |
| CV | 72.49 | 0.836 | 0.998 | 2.14 | 6.6 | 0.673 | 0.987 | 4.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyad, Y.M.; Elmorsi, T.M.; Alam, M.G.; Abel, B. Surface Functionalization of Bioactive Hybrid Adsorbents for Enhanced Adsorption of Organic Dyes. Int. J. Environ. Res. Public Health 2023, 20, 5750. https://doi.org/10.3390/ijerph20095750
Riyad YM, Elmorsi TM, Alam MG, Abel B. Surface Functionalization of Bioactive Hybrid Adsorbents for Enhanced Adsorption of Organic Dyes. International Journal of Environmental Research and Public Health. 2023; 20(9):5750. https://doi.org/10.3390/ijerph20095750
Chicago/Turabian StyleRiyad, Yasser M., Taha M. Elmorsi, Mohd Gulfam Alam, and Bernd Abel. 2023. "Surface Functionalization of Bioactive Hybrid Adsorbents for Enhanced Adsorption of Organic Dyes" International Journal of Environmental Research and Public Health 20, no. 9: 5750. https://doi.org/10.3390/ijerph20095750