Patterns and Predictors of HIV Comorbidity among Adolescents and Young Adults in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Description
2.2. Included Disease Conditions
2.3. Analysis
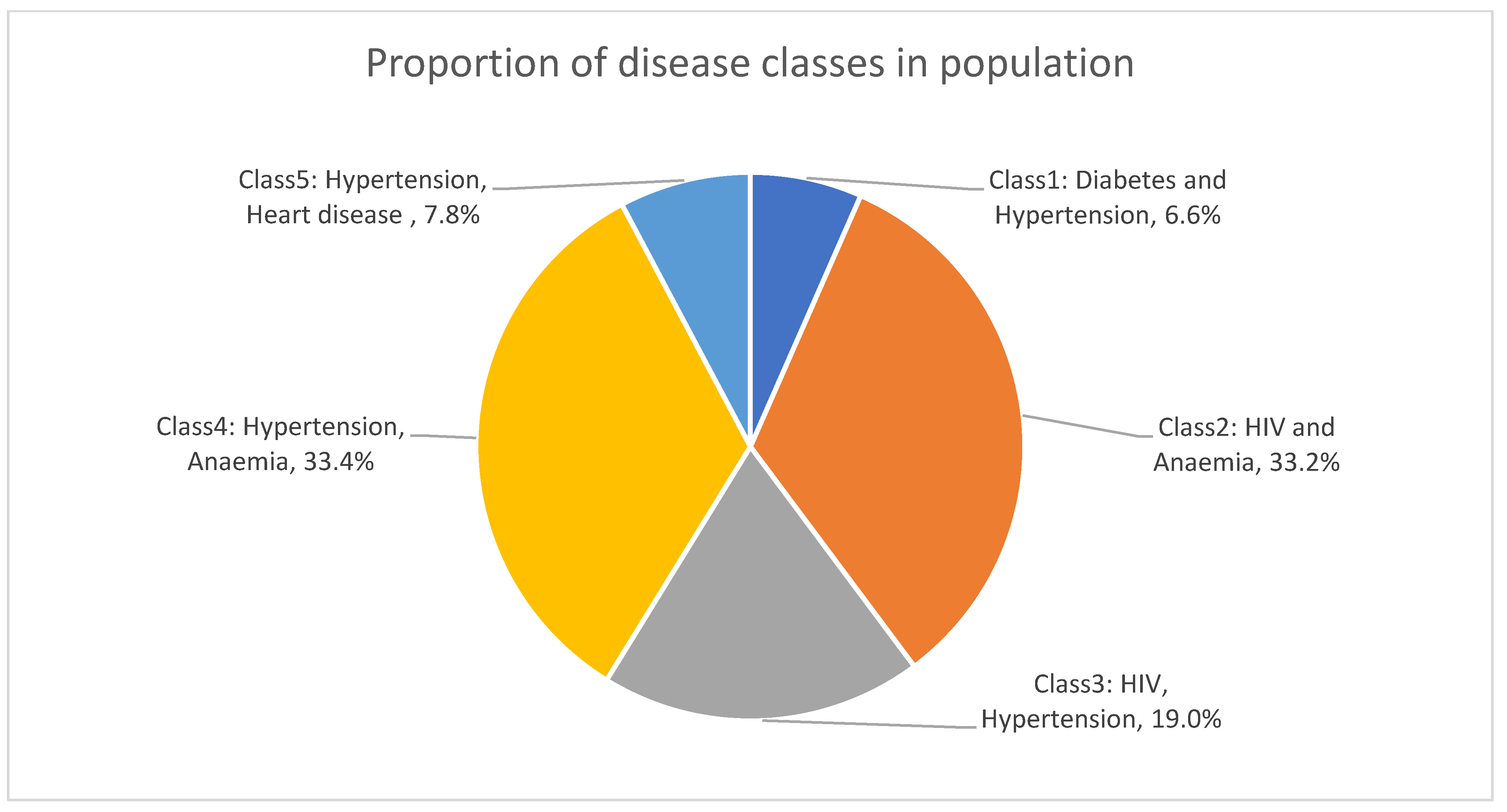
3. Results
3.1. Sample Description
3.2. Prevalence of Single Diseases within Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Young People and HIV. 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/young-people-and-hiv_en.pdf (accessed on 26 October 2023).
- U.S. Agency for International Development, the Joint United Nations Programme on HIV/AIDS (UNAIDS) Inter-Agency Task Team on HIV and Young People, FHI. Young People Most at Risk of HIV: A Meeting Report and Discussion Paper from the Interagency Youth Working Group. 2010. Available online: https://data.unaids.org/pub/basedocument/2010/2010_ypmar_en.pdf (accessed on 24 October 2023).
- Pettifor, A.; Stoner, M.; Pike, C.; Bekker, L.-G. Adolescent lives matter: Preventing HIV in adolescents. Curr. Opin. HIV AIDS 2018, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Simbayi LC, Z.K.; Zuma, K.; Zungu, N.; Moyo, S.; Marinda, E.; Jooste, S.; Naidoo, I. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017: Towards Achieving the UNAIDS 90-90-90 Targets. Human Sciences Research Council. 2019. Available online: http://hdl.handle.net/20.500.11910/15052 (accessed on 2 October 2023).
- Haas, A.D.; Lienhard, R.; Didden, C.; Cornell, M.; Folb, N.; Boshomane, T.M.G.; Salazar-Vizcaya, L.; Ruffieux, Y.; Nyakato, P.; Wettstein, A.E.; et al. Mental Health, ART Adherence, and Viral Suppression Among Adolescents and Adults Living with HIV in South Africa: A Cohort Study. AIDS Behav. 2023, 27, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Nojilana, B.; Bradshaw, D.; Pillay-Van Wyk, V.; Msemburi, W.; Somdyala, N.; Joubert, J.D.; Groenewald, P.; Laubscher, R.; Dorrington, R.E. Persistent burden from non-communicable diseases in South Africa needs strong action. S. Afr. Med. J. 2016, 106, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.B.; Olivier, S.; Gunda, R.; Koole, O.; Surujdeen, A.; Gareta, D.; Munatsi, D.; Modise, T.H.; Dreyer, J.; Nxumalo, S.; et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: A cross-sectional, population-based multimorbidity study. Lancet Glob. Health 2021, 9, e967–e976. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.E.; Griffith, D.; Agwu, A.L. Preventing and diagnosing HIV-related comorbidities in adolescents. Top. Antivir. Med. 2022, 30, 537–544. [Google Scholar] [PubMed]
- Akker, M.v.D.; Dieckelmann, M.; Hussain, M.A.; Bond-Smith, D.; Muth, C.; Pati, S.; Saxena, S.; Silva, D.; Skoss, R.; Straker, L.; et al. Children and adolescents are not small adults: Toward a better understanding of multimorbidity in younger populations. J. Clin. Epidemiol. 2022, 149, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fabic, M.S.; Choi, Y.; Bird, S. A systematic review of Demographic and Health Surveys: Data availability and utilization for research. Bull. World Health Organ. 2012, 90, 604–612. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health, Statistics South Africa, South African Medical Research Council, and ICF. South Africa Demographic and Health Survey 2016 Pretoria, South Africa, and Rockville, Maryland, USA: NDoH, Stats SA, SAMRC, and ICF. 2019. Available online: https://dhsprogram.com/pubs/pdf/FR337/FR337.pdf (accessed on 11 September 2023).
- Xu, X.; Mishra, G.D.; Jones, M. Evidence on multimorbidity from definition to intervention: An overview of systematic reviews. Ageing Res. Rev. 2017, 37, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Pillay-van Wyk, V.; Msemburi, W.; Laubscher, R.; Dorrington, R.E.; Groenewald, P.; Glass, T.; Nojilana, B.; Joubert, J.D.; Matzopoulos, R.; Prinsloo, M.; et al. Mortality trends and differentials in South Africa from 1997 to 2012: Second National Burden of Disease Study. Lancet Glob. Health 2016, 4, e642–e653. [Google Scholar] [CrossRef]
- ICF. Demographic and Health Surveys Standard Recode Manual for DHS7 Rockville, Maryland, U.S.A.: ICF: The Demographic and Health Surveys Program. 2018. Available online: https://dhsprogram.com/pubs/pdf/DHSG4/Recode7_DHS_10Sep2018_DHSG4.pdf (accessed on 11 September 2023).
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus: World Health Organization. 1999. Available online: https://apps.who.int/iris/handle/10665/66040 (accessed on 1 September 2023).
- Bradshaw, D.; Van-Wyk, V.P.; Neethling, I.; Roomaney, R.A.; Cois, A.; Joubert, J.D.; Nannan, N.; Abdelatief, N.; Awotiwon, O.F.; Turawa, E.B.; et al. Overview: Second Comparative Risk Assessment for South Africa (SACRA2) highlights need for health promotion and strengthened surveillance. S. Afr. Med. J. 2022, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Statistics South Africa. Mid-Year Population Estimates 2016. 2016. Available online: https://www.statssa.gov.za/publications/P0302/P03022016.pdf (accessed on 1 September 2023).
- Huntley, A.L.; Johnson, R.; Purdy, S.; Valderas, J.M.; Salisbury, C. Measures of Multimorbidity and Morbidity Burden for Use in Primary Care and Community Settings: A Systematic Review and Guide. Ann. Fam. Med. 2012, 10, 134–141. [Google Scholar] [CrossRef]
- Sinnige, J.; Braspenning, J.; Schellevis, F.; Stirbu-Wagner, I.; Westert, G.; Korevaar, J. The prevalence of disease clusters in older adults with multiple chronic diseases-A systematic literature review. PLoS ONE 2013, 8, e79641. [Google Scholar] [CrossRef] [PubMed]
- Roomaney, R.A. Burden of Multimorbidity in South Africa: Implications for Health Policy and Service Delivery. Ph.D. Thesis, University of the Western Cape, Cape Town, South Africa, 2022. Available online: https://etd.uwc.ac.za/handle/11394/9524 (accessed on 23 February 2024).
- LCA Stata Plugin, Version 1.2; Methodology Center, Penn State University: University Park, PA, USA, 2015.
- Lanza, S.T.; Dziak, J.J.; Huang, L.; Wagner, A.T.; Collins, L.M. LCA Stata Plugin Users’ Guide (Version 1.2). 2015. Available online: https://www.methodology.psu.edu/files/2019/03/Stata-LCA-Plugin-v1.2c-2e00dl9.pdf (accessed on 2 September 2023).
- Weller, B.E.; Bowen, N.K.; Faubert, S.J. Latent Class Analysis: A Guide to Best Practice. J. Black Psychol. 2020, 46, 287–311. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Akaike, H. Information theory as an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 276–281. [Google Scholar]
- Sclove, S.L. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika 1987, 52, 333–343. [Google Scholar] [CrossRef]
- Kamkuemah, M.; Gausi, B.; Oni, T. High prevalence of multimorbidity and non-communicable disease risk factors in South African adolescents and youth living with HIV: Implications for integrated prevention. S. Afr. Med. J. 2022, 112, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Migisha, R.; Ario, A.R.; Kadobera, D.; Bulage, L.; Katana, E.; Ndyabakira, A.; Elyanu, P.; Kalamya, J.N.; Harris, J.R. High blood pressure and associated factors among HIV-infected young persons aged 13 to 25 years at selected health facilities in Rwenzori region, western Uganda, September–October 2021. Clin. Hypertens. 2023, 29, 6. [Google Scholar] [CrossRef]
- Shakil, S.S.; Ojji, D.; Longenecker, C.T.; Roth, G.A. Early Stage and Established Hypertension in Sub-Saharan Africa: Results From Population Health Surveys in 17 Countries, 2010–2017. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e009046. [Google Scholar] [CrossRef]
- Roomaney, R.A.; van Wyk, B.; Cois, A.; Van-Wyk, V.P. Multimorbidity patterns in South Africa: A latent class analysis. Front. Public Health 2023, 10, 1082587. [Google Scholar] [CrossRef] [PubMed]
- Akseer, N.; Mehta, S.; Wigle, J.; Chera, R.; Brickman, Z.J.; Al-Gashm, S.; Sorichetti, B.; Vandermorris, A.; Hipgrave, D.B.; Schwalbe, N.; et al. Non-communicable diseases among adolescents: Current status, determinants, interventions and policies. BMC Public Health 2020, 20, 1908. [Google Scholar] [CrossRef] [PubMed]
- Frigati, L.J.; Ameyan, W.; Cotton, M.F.; Gregson, C.L.; Hoare, J.; Jao, J.; Majonga, E.D.; Myer, L.; Penazzato, M.; Rukuni, R.; et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc. Health 2020, 4, 688–698. [Google Scholar] [CrossRef] [PubMed]
| Total % (n) (n = 1787) | HIV Status % (n) | p-Value | ||
|---|---|---|---|---|
| HIV Positive (n = 153) | HIV Negative (n = 1634) | |||
| Age * (Median years and IQR) | 19 (17–22) | 21 (18–23) | 19 (17–22) | <0.001 *,∞ |
| Gender | <0.001 * | |||
| 47.7 (852) | 19.0 (29) | 50.4 (823) | |
| 52.3 (935) | 81.1 (124) | 49.6 (811) | |
| Locality | 0.028 * | |||
| 54.8 (980) | 46.4 (71) | 55.6 (909) | |
| 45.2 (807) | 53.6 (82) | 44.4 (725) | |
| Province | <0.001 * | |||
| 3.5 (63) | 2 (3) | 3.7 (60) | |
| 16.0 (285) | 19 (29) | 15.7 (256) | |
| 6.0 (107) | 3.3 (5) | 6.2 (102) | |
| 11.2 (201) | 13.1 (20) | 11.1 (181) | |
| 16.8 (300) | 29.4 (45) | 15.6 (255) | |
| 11.8 (211) | 8.5 (13) | 12.1 (198) | |
| 6.4 (114) | 5.9 (9) | 6.4 (105) | |
| 14.7 (263) | 15.7 (24) | 14.6 (239) | |
| 13.6 (243) | 3.3 (5) | 14.6 (238) | |
| Education level | 0.225 | |||
| 11.8 (210) | 12.4 (19) | 11.7 (191) | |
| 83.4 (1 491) | 85.6 (131) | 83.2 (1360) | |
| 4.8 (86) | 2 (3) | 5.1 (83) | |
| Employment status | 0.115 | |||
| 87.5 (1 563) | 91.5 (140) | 87.1 (1423) | |
| 12.5 (224) | 8.5 (13) | 12.9 (211) | |
| Wealth index | 0.062 | |||
| 26.5 (473) | 35.3 (54) | 25.6 (419) | |
| 22.6 (404) | 21.6 (33) | 22.7 (371) | |
| 24.9 (444) | 24.8 (38) | 24.9 (406) | |
| 18.9 (337) | 13.7 (21) | 19.3 (316) | |
| 7.2 (129) | 4.6 (7) | 7.5 (122) | |
| BMI | 0.092 | |||
| 12.1 (214) | 8.7 (13) | 12.4 (201) | |
| 61.9 (1095) | 56.0 (84) | 62.4 (1011) | |
| 17.6 (311) | 24.0 (36) | 17.0 (275) | |
| 5.5 (97) | 6.7 (10) | 5.4 (87) | |
| 2.2 (38) | 4.0 (6) | 2.0 (32) | |
| 0.9 (15) | 0.7 (1) | 0.9 (14) | |
| Current Smoker (Yes) | 12.1 (216) | 5.9 (9) | 12.7 (207) | 0.014 |
| Current Alcohol Consumption (Yes) | 35 (625) | 28.8 (44) | 35.6 (581) | 0.092 |
| Disease Condition | Total % (95% CI) | Prevalence by HIV Status % (95% CI) | ||
|---|---|---|---|---|
| HIV Positive | HIV Negative | |||
| Self-reported | Diabetes | 0.22 (0.09–0.52) | 0.44 (0.06–3.07) | 0.20 (0.08–0.50) |
| Bronchitis/COPD | 0.27 (0.14–0.53) | 0 | 0.25 (0.10–0.62) | |
| Heart disease | 0.51 (0.29–0.88) | 1.51 (0.46–4.89) | 0.45 (0.18–1.10) | |
| High cholesterol | 0.47 (0.2–1.09) | 0 | 0.71 (0.27–1.86) | |
| Stroke | 0.1 (0.04–0.28) | 0.30 (0.04–2.16) | 0.07 (0.02–0.32) | |
| TB (in past 12 months) | 0.47 (0.26–0.84) | 1.62 (0.58–4.47) | 0.44 (0.18–1.04) | |
| Measured | HIV | 8.37 (6.8–10.27) | 100 | - |
| Hypertension | 18.56 (16.33–21.03) | 30.54 (21.49–41.39) | 17.04 (14.68–19.70) | |
| Anaemia | 23.27 (20.98–25.73) | 35.84 (27.36–45.30) | 21.14 (18.65–23.87) | |
| Diabetes | 1.61 (1.04–2.49) | 2.03 (0.69–5.80) | 1.12 (0.69–1.82) | |
| Self-reported and measured | Diabetes | 1.18 (0.78–1.76) | 2.03 (0.69–5.80) | 1.28 (0.82–2.00) |
| Number of Diseases | Total | Prevalence by HIV Status % (95% CI) | |
|---|---|---|---|
| HIV Positive | HIV Negative | ||
| No diseases | 69.64 (67.11–72.05) | NA | 63.79 (60.70–66.76) |
| One disease | 25.13 (23.04–27.35) | 46.71 (37.52–56.13) | 32.62 (29.91–35.46) |
| Two or more diseases | 5.23 (4.25–6.41) | 53.29 (43.87–62.48) | 3.59 (2.59–4.97) |
| Covariates | Odds Ratio | Standard Error | z | p > z | (95% CI) |
|---|---|---|---|---|---|
| Age 20–24 years (Ref: 15–19 years) | 2.54 * | 0.49 | 4.78 | 0 | 1.73–3.72 |
| Sex (Ref: male) | 3.84 * | 0.93 | 5.54 | 0 | 2.38–6.17 |
| Locality (Ref: rural) | 2.17 * | 0.45 | 3.74 | 0 | 1.45–3.26 |
| Education (Ref: primary) | |||||
| 0.80 | 0.22 | −0.81 | 0.418 | 0.46–1.38 |
| 0.26 * | 0.17 | −2.03 | 0.042 | 0.07–0.95 |
| Wealth Index (Ref: poorest) | |||||
| 0.70 | 0.17 | −1.46 | 0.144 | 0.43–1.13 |
| 0.62 | 0.15 | −1.92 | 0.055 | 0.38–1.01 |
| 0.33 * | 0.10 | −3.55 | 0 | 0.18–0.61 |
| 0.31 * | 0.14 | −2.58 | 0.01 | 0.13–0.75 |
| Employed | 0.52 * | 0.17 | −2.04 | 0.042 | 0.28–0.98 |
| BMI Category | |||||
| 0.79 | 0.26 | −0.74 | 0.462 | 0.42–1.49 |
| 0.78 | 0.29 | −0.67 | 0.503 | 0.38–1.60 |
| 0.63 | 0.30 | −0.99 | 0.324 | 0.25–1.58 |
| 1.00 | 0.57 | −0.01 | 0.994 | 0.33–3.03 |
| 0.41 | 0.45 | −0.81 | 0.417 | 0.05–3.57 |
| Current alcohol use | 1.10 | 0.23 | 0.47 | 0.64 | 0.73–1.65 |
| Current smoker | 0.62 | 0.24 | −1.23 | 0.218 | 0.29–1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wyk, B.; Roomaney, R.A. Patterns and Predictors of HIV Comorbidity among Adolescents and Young Adults in South Africa. Int. J. Environ. Res. Public Health 2024, 21, 457. https://doi.org/10.3390/ijerph21040457
van Wyk B, Roomaney RA. Patterns and Predictors of HIV Comorbidity among Adolescents and Young Adults in South Africa. International Journal of Environmental Research and Public Health. 2024; 21(4):457. https://doi.org/10.3390/ijerph21040457
Chicago/Turabian Stylevan Wyk, Brian, and Rifqah Abeeda Roomaney. 2024. "Patterns and Predictors of HIV Comorbidity among Adolescents and Young Adults in South Africa" International Journal of Environmental Research and Public Health 21, no. 4: 457. https://doi.org/10.3390/ijerph21040457
APA Stylevan Wyk, B., & Roomaney, R. A. (2024). Patterns and Predictors of HIV Comorbidity among Adolescents and Young Adults in South Africa. International Journal of Environmental Research and Public Health, 21(4), 457. https://doi.org/10.3390/ijerph21040457







