Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Chronic Kidney Disease
Abstract
:1. Introduction
1.1. Chronic Kidney Disease
1.2. Per- and Polyfluoroalkyl Substances (PFASs)
1.3. Metals and Nephrotoxicity
1.3.1. Lead
1.3.2. Cadmium
1.3.3. Mercury
1.4. Problem Statement
1.5. Significance of the Study
- This study is aimed at providing an understanding of the environmental risk factors, particularly the combined effect of contaminants on CKD, which is one of the leading causes of death.
- The present study also helps address the scarcity of studies exploring the combined effects of PFAS and metals on CKD.
- The findings of this study may help regulatory bodies make policies for limiting exposure to these environmental pollutants and provide public health interventions.
1.6. Research Objectives
- We aimed to explore the association between PFAS (PFOA and PFOS) exposure and CKD.
- Examine the relationship between heavy metal (Lead, Cadmium, Mercury) exposure and CKD.
- Investigate the potential synergistic effects of combined PFAS and metal exposure on CKD.
2. Materials and Methods
2.1. Study Population
2.2. PFAS Extraction and Quantitation
2.3. Plasma Heavy Metals (Lead, Cadmium and Mercury) Measurement
2.4. CKD Biomarkers
2.4.1. Albuminuria (Urine Albumin)
2.4.2. Serum and Urine Creatinine
2.4.3. Urine Albumin-to-Creatinine Ratio
2.4.4. Estimated Glomerular Filtration Rate
- Stage 1: eGFR is 90 mL/min/1.73 m2 or higher;
- Stage 2: eGFR ranges from 60 to 89 mL/min/1.73 m2;
- Stage 3: eGFR falls between 30 and 59 mL/min/1.73 m2;
- Stage 4: eGFR is within 15 to 29 mL/min/1.73 m2;
- Stage 5: eGFR is less than 15 mL/min/1.73 m m2.
2.4.5. Chronic Kidney Disease
- Stage 1: Persistent albuminuria with eGFR equal to or greater than 90 mL/min/1.73 m2;
- Stage 2: Persistent albuminuria with eGFR ranging from 60 to 89 mL/min/1.73 m2;
- Stage 3: eGFR between 30 and 59 mL/min/1.73 m2;
- Stage 4: eGFR between 15 and 29 mL/min/1.73 m2;
- Stage 5: eGFR less than 15 mL/min/1.73 m2.
- Negative: no CKD;
- Mild CKD: Stage 1 and 2;
- Moderate-to-severe CKD: Stages 3, 4, and 5.
2.5. Variables and Covariates for Model Adjustment
2.6. Statistical Analysis
2.6.1. Descriptive Statistics, Correlations, and Regression
2.6.2. Bayesian Kernel Machine Regression (BKMR)
3. Results
3.1. Demographics and Health Characteristics
3.2. Kidney Biomarkers and CKD
3.3. T-Test and Binary Logistic Regression Analysis
3.4. BKMR
3.4.1. Quantifying the PFAS and Metal-Related Factors in CKD: PIP and BKMR Analysis
3.4.2. Univariate Analysis: Examining the Isolated Effects of PFOA, PFOS, Hg, Cd, and Pb on CKD
3.5. Visualizing Bivariate Exposure–Response Functions with Fixed Percentile Values
3.6. Overall Risk Summary of CKD with Exposure Percentiles
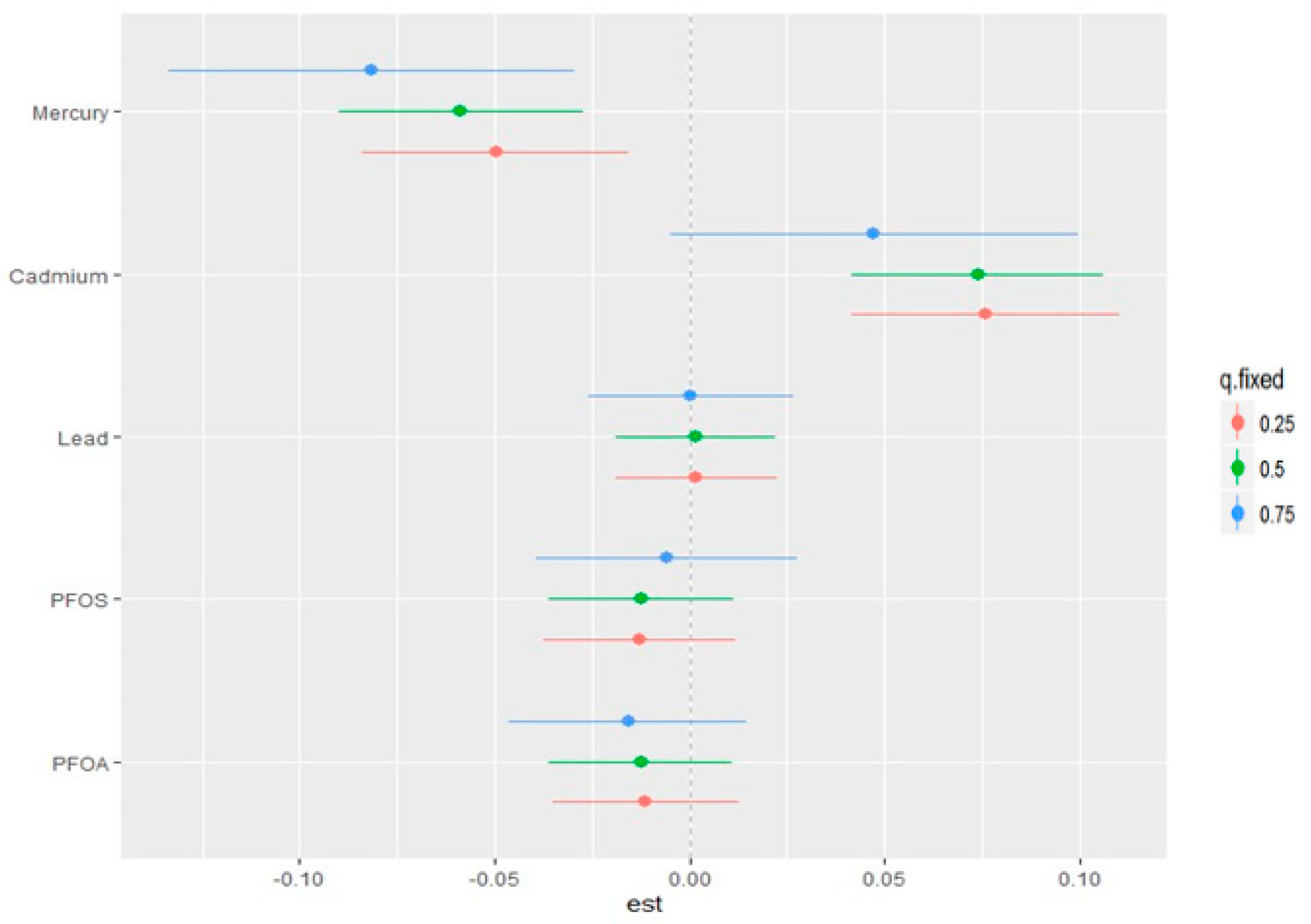
3.7. Single-Variable Effects of PFAS and Metals on CKD
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Zeba, Z.; Fatema, K.; Sumit, A.F.; Zinnat, R.; Ali, L. Early screening of chronic kidney disease patients among the asymptomatic adult population in Bangladesh. J. Prev. Epidemiol. 2020, 5, e10. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hou, N.; Chen, Z.; Liu, J.; Li, X.; Sun, X.; Liu, Y. Secular trends of epidemiologic patterns of chronic kidney disease over three decades: An updated analysis of the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e064540. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States. 2023. Available online: https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html (accessed on 6 October 2023).
- Lamb, E.J.; Levey, A.S.; Stevens, P.E. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: Evolution not revolution. Clin. Chem. 2013, 59, 462–465. [Google Scholar] [CrossRef]
- American Kidney Fund. Stages of Chronic Kidney Disease (CKD). Available online: https://www.kidneyfund.org/all-about-kidneys/stages-kidney-disease (accessed on 18 October 2023).
- Hu, J.; Ke, R.; Teixeira, W.; Dong, Y.; Ding, R.; Yang, J.; Ai, X.; Ye, D.W.; Shang, J. Global, Regional, and National Burden of CKD due to Glomerulonephritis from 1990 to 2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Clin. J. Am. Soc. Nephrol. 2023, 18, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.N.; Wang, X.C.; Su, L.Q.; Ji, S.S.; Dong, X.J.; Zhu, H.J.; Hou, S.S.; Wang, C.; Li, Z.H.; Dong, B.; et al. Serum concentrations of per-/polyfluoroalkyl substances and its association with renal function parameters among teenagers near a Chinese fluorochemical industrial plant: A cross-sectional study. Environ. Pollut. 2022, 302, 119020. [Google Scholar] [CrossRef]
- Boafo, Y.S.; Mostafa, S.; Obeng-Gyasi, E. Association of Per- and Polyfluoroalkyl Substances with Allostatic Load Stratified by Herpes Simplex Virus 1 and 2 Exposure. Toxics 2023, 11, 745. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.F.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; Oken, E. Per- and polyfluoroalkyl substances and kidney function: Follow-up results from the Diabetes Prevention Program trial. Environ. Int. 2021, 148, 106375. [Google Scholar] [CrossRef]
- Shearer, J.J.; Callahan, C.L.; Calafat, A.M.; Huang, W.Y.; Jones, R.R.; Sabbisetti, V.S.; Freedman, N.D.; Sampson, J.N.; Silverman, D.T.; Purdue, M.P.; et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. J. Natl. Cancer Inst. 2021, 113, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Hivert, M.F.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Lin, P.D.; Fleisch, A.F.; Calafat, A.M.; Ye, X.; Webster, T.F.; et al. Associations of Perfluoroalkyl and Polyfluoroalkyl Substances with Incident Diabetes and Microvascular Disease. Diabetes Care 2019, 42, 1824–1832. [Google Scholar] [CrossRef]
- Khalil, N.; Chen, A.; Lee, M.; Czerwinski, S.A.; Ebert, J.R.; DeWitt, J.C.; Kannan, K. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environ. Health Perspect. 2016, 124, 81–87. [Google Scholar] [CrossRef]
- Jian, J.M.; Chen, D.; Han, F.J.; Guo, Y.; Zeng, L.; Lu, X.; Wang, F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 636, 1058–1069. [Google Scholar] [CrossRef]
- Wen, L.L.; Lin, C.Y.; Chou, H.C.; Chang, C.C.; Lo, H.Y.; Juan, S.H. Perfluorooctanesulfonate Mediates Renal Tubular Cell Apoptosis through PPARgamma Inactivation. PLoS ONE 2016, 11, e0155190. [Google Scholar] [CrossRef]
- Rashid, F.; Ramakrishnan, A.; Fields, C.; Irudayaraj, J. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol. Rep. 2020, 7, 125–132. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Disease through Healthy Environments: Ten Chemicals of Major Public Health Concern; Public Environment WHO: Geneva, Switzerland, 2010; Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 3 March 2024).
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Hung, C.H.; Wang, C.W.; Tu, H.P.; Li, C.H.; Tsai, C.C.; Lin, W.Y.; Chen, S.C.; Kuo, C.H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics 2021, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.H.; Jhuang, M.J.; Yeh, Y.P.; Chen, Y.H.; Lu, S.; Chan, C.C. Relationship between renal function and metal exposure of residents living near the No. 6 Naphtha Cracking Complex: A cross-sectional study. J. Formos. Med. Assoc. 2021, 120, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health Risk Assessment of Dietary Cadmium Intake: Do Current Guidelines Indicate How Much is Safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef]
- World Health Organization. Exposure to Lead: A Major Public Health Concern. Available online: https://www.who.int/publications/i/item/9789240037632 (accessed on 18 November 2023).
- United States Environmental Protection Agency. Lead: Learn about Lead. Available online: https://www.epa.gov/lead/learn-about-lead#effects (accessed on 18 October 2023).
- Benfer, E.A. Contaminated Childhood: How the United States Failed to Prevent the Chronic Lead Poisoning of Low-Income Children and Communities of Color. Harv. Environ. Law Rev. 2017, 41, 493. [Google Scholar]
- Dignam, T.; Kaufmann, R.B.; LeStourgeon, L.; Brown, M.J. Control of Lead Sources in the United States, 1970–2017: Public Health Progress and Current Challenges to Eliminating Lead Exposure. J. Public Health Manag. Pract. 2019, 25 (Suppl. S1), S13–S22. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recycling Used Lead-Acid Batteries: Health Considerations; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Centers for Disease Control and Prevention. Childhood Lead Poisoning Prevention: Health Effects of Lead Exposure. Available online: https://www.cdc.gov/nceh/lead/prevention/health-effects.htm (accessed on 3 March 2024).
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.B.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Sarajuddin; Brehman, K.D.; Panhwar, H.; Arain, S.S. Co-exposure of arsenic and cadmium through drinking water and tobacco smoking: Risk assessment on kidney dysfunction. Environ. Sci. Pollut. Res. Int. 2015, 22, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Sun, G.F.; Hu, W.T.; Yuan, Z.H.; Zhang, B.A.; Lu, H. Characteristics of Mercury Intoxication Induced by Skin-lightening Products. Chin. Med. J. 2017, 130, 3003–3004. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, N.; Du, X.; Li, H.; Mei, X.; Song, Y. Toxic Nephropathy Secondary to Chronic Mercury Poisoning: Clinical Characteristics and Outcomes. Kidney Int. Rep. 2022, 7, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bensefa-Colas, L.; Andujar, P.; Descatha, A. Intoxication par le mercure Mercury poisoning. Rev. Med. Interne 2011, 32, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, K.; Etzel, R.; Zhang, X.; Tian, Y.; Zhang, J. Co-exposure to environmental endocrine disruptors in the US population. Environ. Sci. Pollut. Res. Int. 2019, 26, 7665–7676. [Google Scholar] [CrossRef]
- Park, S.K.; Peng, Q.; Ding, N.; Mukherjee, B.; Harlow, S.D. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ. Res. 2019, 175, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Wöckner, M.; Roscher, E.; Völkel, W. ADONA and perfluoroalkylated substances in plasma samples of German blood donors living in South Germany. Int. J. Hyg. Environ. Health 2017, 220, 455–460. [Google Scholar] [CrossRef]
- Herrick, R.L.; Buckholz, J.; Biro, F.M.; Calafat, A.M.; Ye, X.; Xie, C.; Pinney, S.M. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991–2012. Environ. Pollut. 2017, 228, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B. FPF Dossier: Per and polyfluroalkyl substances (PFASs). Food Packag. Forum 2016. [Google Scholar] [CrossRef]
- Langberg, H.A.; Arp, H.P.H.; Breedveld, G.D.; Slinde, G.A.; Hoiseter, A.; Gronning, H.M.; Jartun, M.; Rundberget, T.; Jenssen, B.M.; Hale, S.E. Paper product production identified as the main source of per- and polyfluoroalkyl substances (PFAS) in a Norwegian lake: Source and historic emission tracking. Environ. Pollut. 2020, 273, 116259. [Google Scholar] [CrossRef] [PubMed]
- Goosey, E.; Harrad, S. Perfluoroalkyl substances in UK indoor and outdoor air: Spatial and seasonal variation, and implications for human exposure. Environ. Int. 2012, 45, 86–90. [Google Scholar] [CrossRef] [PubMed]
- USEPA. PFOA & PFOS Drinking Water Health Advisories. Available online: https://www.epa.gov/sites/default/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf (accessed on 10 November 2023).
- USEPA. Contaminant Candidate List (CCL) and Regulatory Determination. Available online: https://www.epa.gov/ccl/regulatory-determination-4 (accessed on 13 November 2023).
- Liu, D.; Yan, S.; Wang, P.; Chen, Q.; Liu, Y.; Cui, J.; Liang, Y.; Ren, S.; Gao, Y. Perfluorooctanoic acid (PFOA) exposure in relation to the kidneys: A review of current available literature. Front. Physiol. 2023, 14, 1103141. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Tsai, H.J.; Wu, P.Y.; Huang, J.C.; Chen, S.C. Environmental Pollution and Chronic Kidney Disease. Int. J. Med. Sci. 2021, 18, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Manera, M.; Mongodi, S.; Esposito, P.; Ronco, C. The Role of Perfluorinated Compound Pollution in the Development of Acute and Chronic Kidney Disease. Contrib. Nephrol. 2021, 199, 285–296. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PFAS_I_MET.pdf (accessed on 12 December 2023).
- Centers for Disease Control and Prevention. Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/PFAS_J.htm (accessed on 12 December 2023).
- Mottaleb, M.A.; Petriello, M.C.; Morris, A.J. High-Throughput UHPLC-MS/MS Measurement of Per- and Poly-Fluorinated Alkyl Substances in Human Serum. J. Anal. Toxicol. 2020, 44, 339–347. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/PBCD-J-PBY-J-R-MET-508.pdf (accessed on 12 December 2023).
- Chavers, B.M.; Simonson, J.; Michael, A.F. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984, 25, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Laboratory Procedure Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/alcr_g_r_met_albumin.pdf (accessed on 15 December 2023).
- Centers for Disease Control and Prevention. Laboratory Procedeure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/BIOPRO-J-MET-Creatinine-508.pdf (accessed on 15 December 2023).
- Centers for Disease Control and Prevention. Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/ALB-CR-J-MET-Urine-Creatinine-508.pdf (accessed on 16 December 2023).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/ALB_CR_J.htm (accessed on 16 December 2023).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Estimating Glomerular Filtration Rate. Available online: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate/estimating#the-mdrd-equation (accessed on 16 December 2023).
- Mendivil, C.O.; Gnecco-Gonzalez, S.; Herrera-Parra, L.J.; Hernandez Vargas, J.A.; Ramirez-Garcia, N.; Acuna-Merchan, L. MDRD is the eGFR equation most strongly associated with 4-year mortality among patients with diabetes in Colombia. BMJ Open Diabetes Res. Care 2023, 11, e003495. [Google Scholar] [CrossRef]
- National Kidney Foundation. Chronic Kidney Disease (CKD). Available online: https://www.kidney.org/atoz/content/about-chronic-kidney-disease (accessed on 20 February 2024).
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The Association of Toxoplasma gondii IgG Antibody and Chronic Kidney Disease Biomarkers. Microorganisms 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- American Kidney Fund. Stages of Kidney Disease (CKD). Available online: https://www.kidneyfund.org/all-about-kidneys/stages-kidney-disease (accessed on 31 December 2023).
- Wu, W.; Zhang, K.; Jiang, S.; Liu, D.; Zhou, H.; Zhong, R.; Zeng, Q.; Cheng, L.; Miao, X.; Tong, Y.; et al. Association of co-exposure to heavy metals with renal function in a hypertensive population. Environ. Int. 2018, 112, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, C.; Brockman, J.; Shikany, J.M.; He, K. Low- and moderate- levels of arsenic exposure in young adulthood and incidence of chronic kidney disease: Findings from the CARDIA Trace Element Study. J. Trace Elem. Med. Biol. 2021, 63, 126657. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Tan, J.; Fang, G.; Ji, H.; Miao, M.; Tian, Y.; Hu, H.; Cao, W.; Liang, H.; Yuan, W. Associations between prenatal exposure to perfluoroalkyl substances and neurobehavioral development in early childhood: A prospective cohort study. Ecotoxicol. Environ. Saf. 2022, 241, 113818. [Google Scholar] [CrossRef]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 16 January 2024).
- Lin, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 20 February 2024).
- Yun, J.; Jang, E.C.; Kwon, S.C.; Min, Y.S.; Lee, Y.J. The association of perfluoroalkyl substances (PFAS) exposure and kidney function in Korean adolescents using data from Korean National Environmental Health Survey (KoNEHS) cycle 4 (2018–2020): A cross-sectional study. Ann. Occup. Environ. Med. 2023, 35, e5. [Google Scholar] [CrossRef]
- Qian, Y.; Ducatman, A.; Ward, R.; Leonard, S.; Bukowski, V.; Lan Guo, N.; Shi, X.; Vallyathan, V.; Castranova, V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: Role in endothelial permeability. J. Toxicol. Environ. Health A 2010, 73, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.X.; Dong, P.; Zhou, Y.; Zhang, L.; Qian, Z.; Geiger, S.D.; Bingheim, E.; Tang, X.; Wu, Y.; Lv, J.; et al. Joint effects of per- and polyfluoroalkyl substance alternatives and heavy metals on renal health: A community-based population study in China. Environ. Res. 2023, 219, 115057. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudlak, B.; Williams, M.J.; Jonsson, J.; Schioth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Co-exposures to toxic metals cadmium, lead, and mercury and their impact on unhealthy kidney function. Environ. Sci. Pollut. Res. Int. 2019, 26, 30112–30118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Flaws, J.A.; Spinella, M.J.; Irudayaraj, J. The Relationship between Typical Environmental Endocrine Disruptors and Kidney Disease. Toxics 2022, 11, 32. [Google Scholar] [CrossRef] [PubMed]

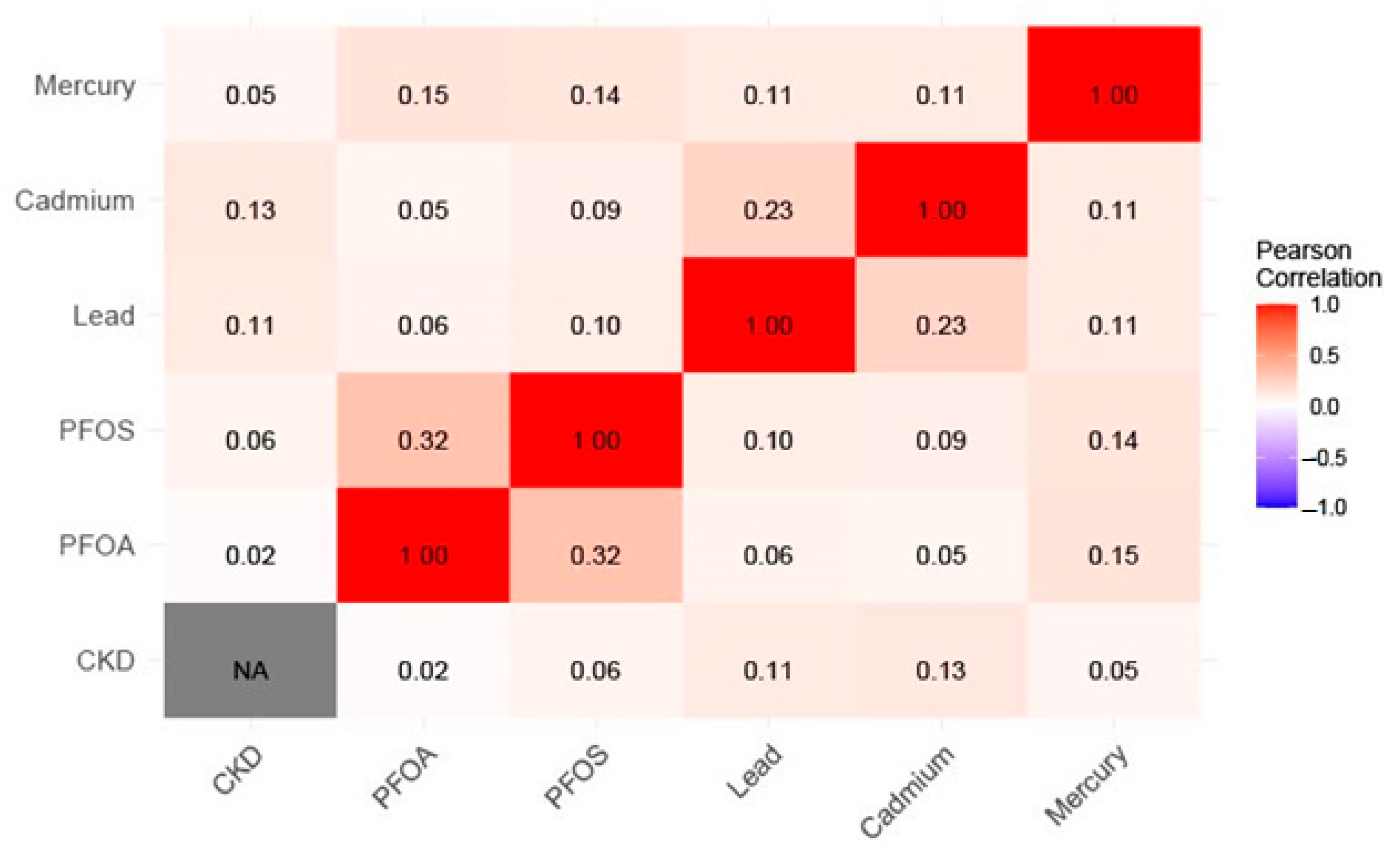
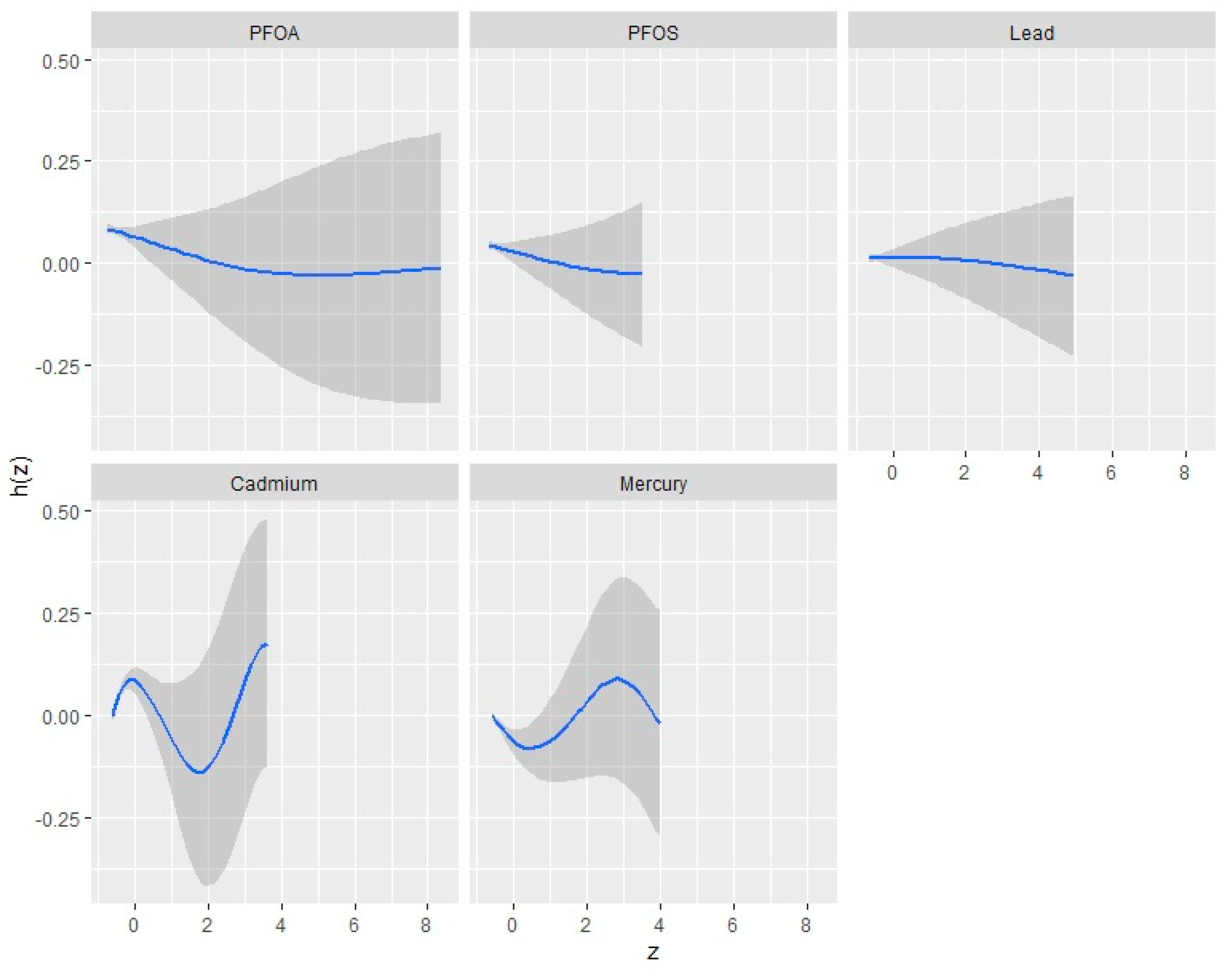
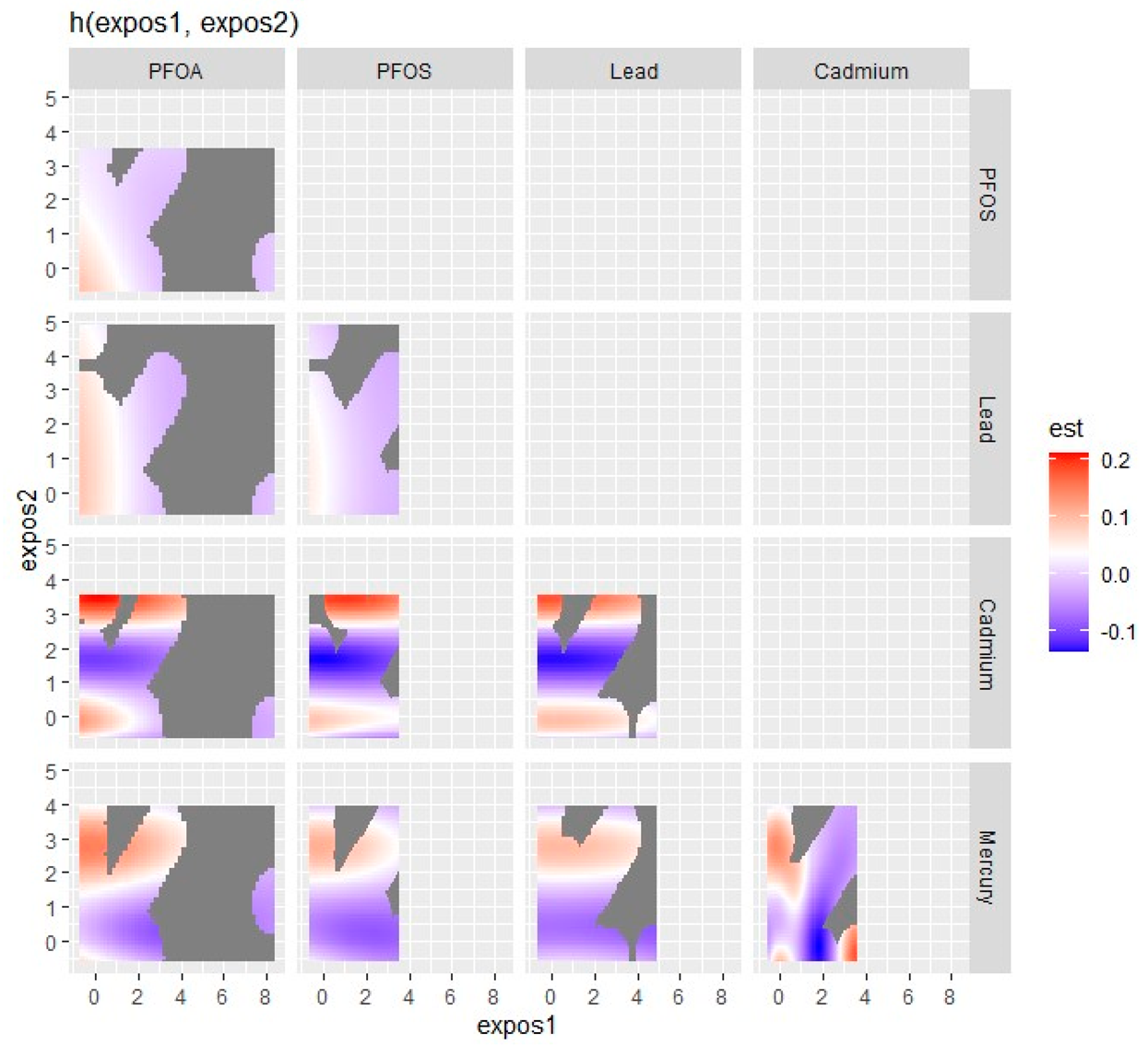
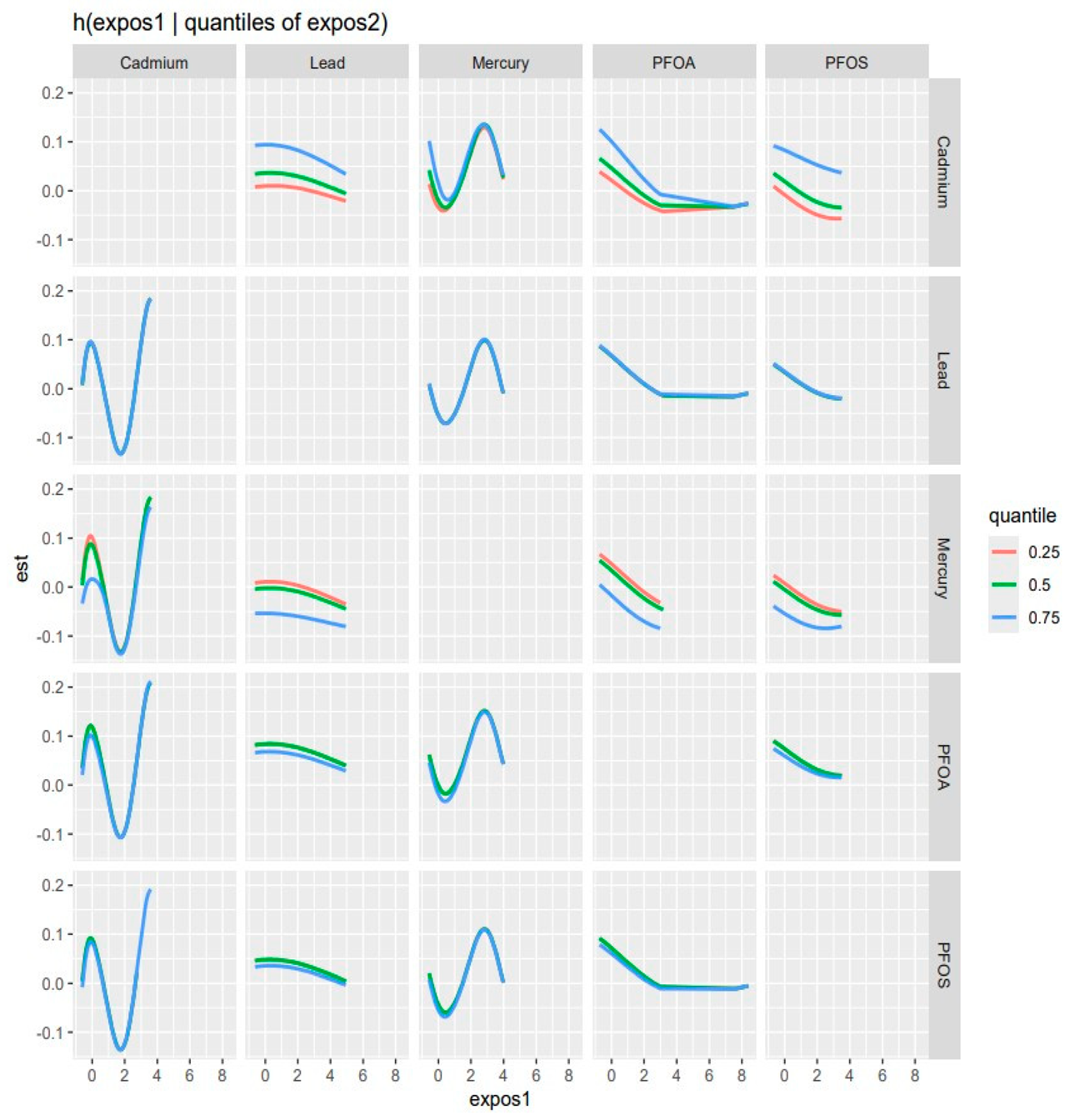

| Variable | Mean (SD) | Minimum | 25th Percentile | Median (50th) | 75th Percentile | Maximum |
|---|---|---|---|---|---|---|
| PFOS | 6.51 (7.74) | 0.14 | 2.40 | 4.20 | 7.80 | 104.9 |
| PFOA | 1.71 (1.82) | 0.14 | 0.87 | 1.37 | 2.07 | 52.87 |
| Pb | 1.08 (1.29) | 0.05 | 0.46 | 0.76 | 1.30 | 42.48 |
| Hg | 1.14 (2.27) | 0.20 | 0.20 | 0.51 | 1.12 | 63.64 |
| Cd | 0.37 (0.50) | 0.07 | 0.12 | 0.22 | 0.42 | 13.03 |
| Age | 34.33 (25.50) | 0 | 11 | 31 | 58 | 80 |
| BMI | 26.58 (8.26) | 12.30 | 20.40 | 25.80 | 31.30 | 86.20 |
| Kidney Biomarker | ||||||
| Serum creatinine | 0.88 (0.45) | 0.25 | 0.68 | 0.82 | 0.98 | 12.74 |
| Albumin creatinine ratio | 42.80 (299.69) | 0.27 | 5.23 | 8.28 | 15.88 | 11,676.92 |
| eGFR | 98.40 (36.41) | 3.44 | 76.32 | 93.58 | 114.31 | 523.29 |
| Variable | Description | Frequency (n) | percentage (%) | |||
| Gender | 9254 | |||||
| Male | 4557 | 49.24 | ||||
| Female | 4697 | 50.76 | ||||
| Race/ethnicity | Mexican American | 1367 | 14.77 | |||
| Other Hispanic | 820 | 8.86 | ||||
| Non-Hispanic White | 3150 | 34.04 | ||||
| Non-Hispanic Black | 2115 | 22.85 | ||||
| Non-Hispanic Asian | 1168 | 12.62 | ||||
| Non-Hispanic Multiracial | 634 | 6.85 | ||||
| Alcohol use | 5130 | |||||
| Yes | 4545 | 88.6 | ||||
| No | 585 | 11.4 | ||||
| Smoking | 5856 | |||||
| Yes | 2359 | 40.28 | ||||
| No | 3497 | 59.72 | ||||
| Hypertension | 1942 | |||||
| Yes | 1650 | 84.96 | ||||
| No | 292 | 15.04 | ||||
| Having diabetes | 8897 | |||||
| Yes | 893 | 10.04 | ||||
| No | 7816 | 87.85 | ||||
| Borderline | 184 | 2.07 | ||||
| Don’t know | 4 | 0.04 | ||||
| Having weak/failing kidneys | 5569 | |||||
| Yes | 223 | 4.0 | ||||
| No | 5337 | 95.83 | ||||
| Don’t know | 9 | 0.16 | ||||
| Variable | Description | Frequency (n) | Percentage (%) | |||
| Albuminuria | Negative | 6614 | 86.66 | |||
| Microalbuminuria | 864 | 11.32 | ||||
| Macroalbuminuria | 154 | 2.02 | ||||
| 5903 | ||||||
| eGFR stages | Stage 1 | 3249 | 55.04 | |||
| Stage 2 | 2142 | 36.29 | ||||
| Stage 3 | 462 | 7.83 | ||||
| Stage 4 | 34 | 0.58 | ||||
| Stage 5 | 16 | 0.27 | ||||
| CKD | 5800 | |||||
| Negative | 4729 | 81.53 | ||||
| positive | 1071 | 18.47 | ||||
| CKD Levels | Negative | 4729 | 81.53 | |||
| Mild | 664 | 11.45 | ||||
| Moderate-to-severe | 512 | 8.83 | ||||
| CKD Stages | Negative | 4729 | 80.08 | |||
| Stage 1 | 383 | 6.49 | ||||
| Stage 2 | 281 | 4.76 | ||||
| Stage 3 | 462 | 7.82 | ||||
| Stage 4 | 34 | 0.58 | ||||
| Stage 5 | 16 | 0.27 |
| Variable | Mean (SD) n = 9254 | CDK (+) n = 1071 | No CDK (−) n = 4729 | p-Value |
|---|---|---|---|---|
| PFOS | 6.51 (7.74) | 7.07 (8.93) | 6.24 (7.44) | 0.0009 * |
| PFOA | 1.71 (1.82) | 1.77 (1.24) | 1.70 (1.93) | 0.2848 |
| Pb | 1.08 (1.29) | 1.35 (1.17) | 1.09 (1.36) | <0.0001 * |
| Hg | 1.14 (2.27) | 1.13 (2.72) | 1.32 (2.45) | 0.5546 |
| Cd | 0.37 (0.50) | 0.50 (0.62) | 0.42 (0.52) | <0.0001 * |
| Variable | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| PFOS | 0.91 (0.79–1.01) | 0.03 * |
| PFOA | 1.68 (1.08–2.62) | 0.20 |
| Pb | 1.53 (0.68–3.42) | 0.28 |
| Hg | 0.69 (0.43–1.10) | 0.11 |
| Cd | 1.71 (0.49–5.98) | 0.38 |
| Variable | PIP |
|---|---|
| PFOA | 0.7880 |
| PFOS | 0.7604 |
| Lead | 0.6940 |
| Cadmium | 1.0000 |
| Mercury | 0.9984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haruna, I.; Obeng-Gyasi, E. Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2024, 21, 468. https://doi.org/10.3390/ijerph21040468
Haruna I, Obeng-Gyasi E. Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Chronic Kidney Disease. International Journal of Environmental Research and Public Health. 2024; 21(4):468. https://doi.org/10.3390/ijerph21040468
Chicago/Turabian StyleHaruna, Issah, and Emmanuel Obeng-Gyasi. 2024. "Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Chronic Kidney Disease" International Journal of Environmental Research and Public Health 21, no. 4: 468. https://doi.org/10.3390/ijerph21040468





