Psychological Therapies Used for the Reduction of Habitual Cigarette Smoking Cigarette Consumption: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Review questions
- Inclusion criteria
- Exclusion criteria
- Information sources
- Search strategy
- Data extraction process
- Characteristics
- Risk of bias assessment of individual studies
- Selection and analysis
- Assessment of publication bias
3. Results
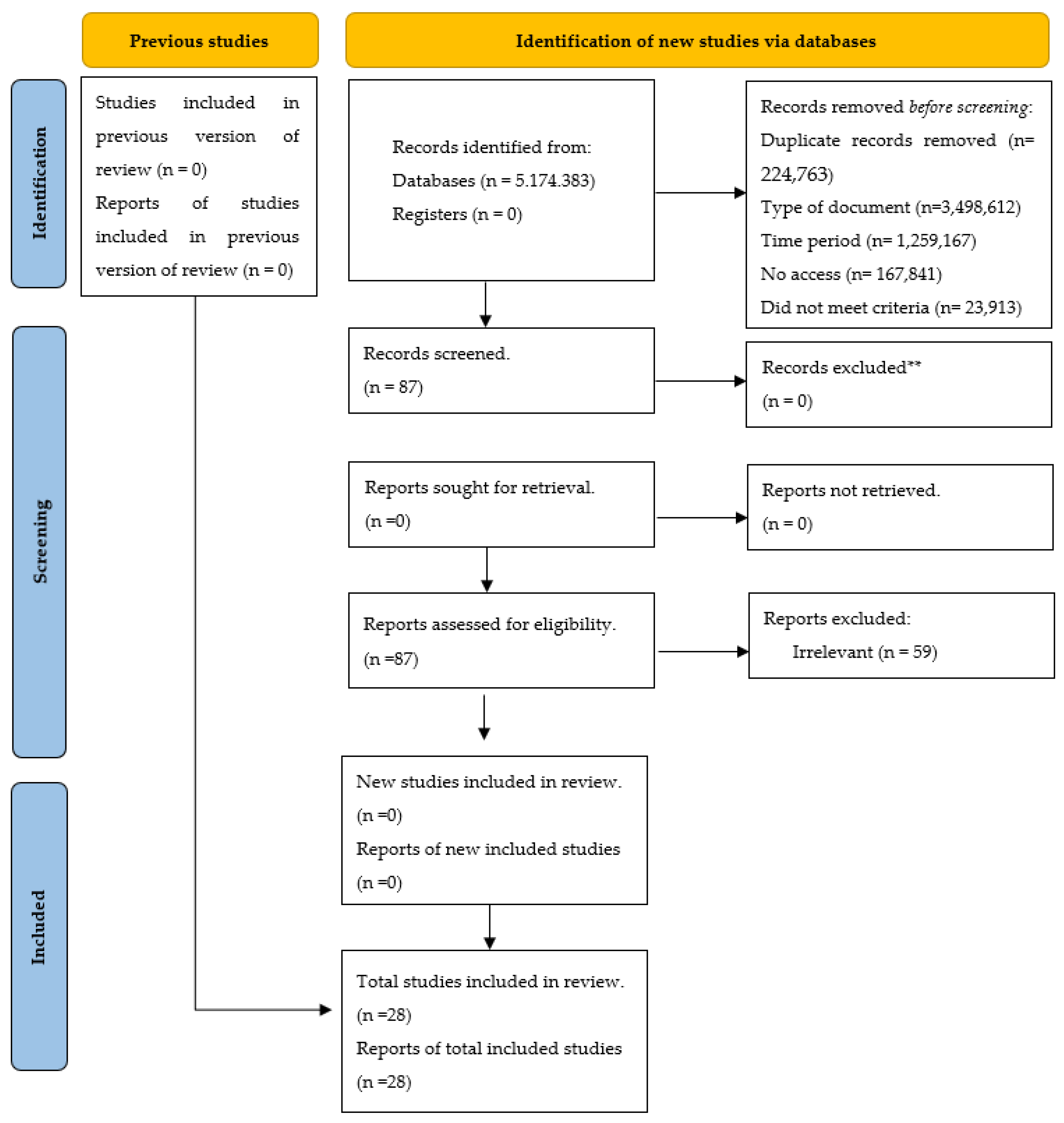
3.1. Selection of Studies
3.2. Characteristics of the Studies
3.3. Risk of Bias of Individual Studies
3.4. Analysis of the Treatments
3.5. Techniques Used in the Studies
3.6. Characteristics of the Sessions
3.7. Evaluators of the Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Allen Carr’s Easy way |
| CAD | Coronary artery disease |
| CBT | Cognitive behavioral therapy |
| COPD | Chronic obstructive pulmonary disease |
| CS | Cognitive strategy |
| EC | Enhanced care. |
| EST | Enhanced standard treatment. |
| IC | Intensive care |
| MBAT | Mindfulness-based addiction treatment |
| MESH | Medical Subject Headings |
| NRT | Nicotine replacement therapy |
| NT | No treatment |
| SC | Standard Care |
| TAU | Treatment as usual |
| UC | Usual care |
| PHS | Public Health Service |
| MB | Mindfulness-based yogic breathing |
| CBT | Cognitive behavioral therapy |
| PP | Point-prevalence |
| DSC | Dual-smoker couples |
| N-O-T | Not-on-Tobacco |
| PA | Physical activity |
| N-O-T+FIT | Module |
| BI | Brief Intervention |
| CI | Intervention Condition |
References
- Inoue-Choi, M.; Shiels, M.S.; McNeel, T.S.; Graubard, B.I.; Hatsukami, D.; Freedman, N.D. Contemporary Associations of Exclusive Cigarette, Cigar, Pipe, and Smokeless Tobacco Use With Overall and Cause-Specific Mortality in the United States. JNCI Cancer Spectr. 2019, 3, pkz036. [Google Scholar] [CrossRef] [PubMed]
- Méndez, D.; Alshanqeety, O.; Warner, K.E. The potential impact of smoking control policies on future global smoking trends. Tob. Control 2013, 22, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud. Cigarrillo. Available online: https://www.who.int/es/newsroom/fact-sheets/detail/tobacco (accessed on 23 August 2021).
- Organización Mundial de la Salud. Declaración de la OMS: Consumo de Cigarrillo y COVID-19. 2020. Available online: https://www.paho.org/es/noticias/11-5-2020-declaracion-oms-consumo-cigarrillo-covid-19 (accessed on 20 September 2021).
- Mahendiran, T.; Hoepli, A.; Foster-Witassek, F.; Rickli, H.; Roffi, M.; Eberli, F.; Pedrazzini, G.; Jeger, R.; Radovanovic, D.; Fournier, S.; et al. Twenty-year trends in the prevalence of modifiable cardiovascular risk factors in young acute coronary syndrome patients hospitalized in Switzerland. Eur. J. Prev. Cardiol. 2023, 30, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.A.; Lowe, K.E.; Make, B.J.; Lynch, D.A.; Kinney, G.L.; Budoff, M.J.; Mao, S.S.; Dyer, D.; Curtis, J.L.; Bowler, R.P.; et al. Identifying smoking-related disease on lung cancer screening CT scans: Increasing the value. Chronic Obstr. Pulm. Dis. J. COPD Found. 2019, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, J. Effects of Radiation on the Environment. In Radiation Effects in Polymeric Materials; Springer: Cham, Switzerland, 2019; pp. 1–34. [Google Scholar]
- Kubalek, D.; Serša, G.; Štrok, M.; Benedik, L.; Jeran, Z. Radioactivity of cigarettes and the importance of 210Po and thorium isotopes for radiation dose assessment due to smoking. J. Environ. Radioact. 2016, 155, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.M.; Dybing, E.; Gray, N.; Hecht, S.; Anderson, C.; Sanner, T.; Straif, K. Reducción obligatoria de sustancias tóxicas en el humo del cigarrillo: Una descripción de la propuesta TobReg de la Organización Mundial de la Salud. Control del Tabaco 2008, 17, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gülsen, A.; Yigitbas, B.A.; Uslu, B.; Drömann, D.; Kilinc, O. The effect of smoking on COVID-19 symptom severity: Systematic review and meta-analysis. Pulm. Med. 2020, 2020, 7590207. [Google Scholar] [CrossRef] [PubMed]
- Jebet, A.; Kibet, J.; Ombaka, L.; Kinyanjui, T. Surface bound radicals, char yield and particulate size from the burning of tobacco cigarette. Chem. Cent. J. 2017, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Hertz-Schünemann, R.; Ehlert, S.; Liu, C.; McAdam, K.; Baker, R.; Streibel, T. Highly time-resolved imaging of combustion and pyrolysis product concentrations in solid fuel combustion: NO formation in a burning cigarette. Anal. Chem. 2015, 87, 1711–1717. [Google Scholar] [CrossRef]
- Hertz-Schünemann, R.; Ehlert, S.; Streibel, T.; Liu, C.; McAdam, K.; Baker, R.R.; Zimmermann, R. High-resolution time and spatial imaging of tobacco and its pyrolysis products during a cigarette puff by microprobe sampling photoionisation mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2293–2299. [Google Scholar] [CrossRef]
- Hong, S.W.; Teesdale-Spittle, P.; Page, R.; Ellenbroek, B.; Truman, P. Biologically Active Compounds Present in Tobacco Smoke: Potential Interactions Between Smoking and Mental Health. Front. Neurosci. 2022, 16, 885489. [Google Scholar] [CrossRef] [PubMed]
- Zagà, V.; Gattavecchia, E. Poloniul: Ucigaşul radioactiv din fumul de tutun [Polonium: The radioactive killer from tobacco smoke]. Pneumologia 2008, 57, 249–254. [Google Scholar] [PubMed]
- American Cancer Society. Sustancias Químicas Nocivas en los Productos de Cigarrillo. Available online: https://www.cancer.org/es/cancer/causas-del-cancer/cigarrillo-y-cancer/agentescancerigenos-en-los-productos-de-cigarrillo.html (accessed on 25 September 2021).
- Roh, S. Scientific Evidence for the Addictiveness of Tobacco and Smoking Cessation in Tobacco Litigation. J. Prev. Med. Public Health=Yebang Uihakhoe Chi 2018, 51, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chaiton, M.; Diemert, L.M.; Bondy, S.J.; Cohen, J.E.; Fung, M.D.; Zhang, B.R.; Ferrence, R.G. Real-world effectiveness of pharmaceutical smoking cessation aids: Time-varying effects. Nicotine Tob. Res. 2020, 22, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud. Informe de la OMS Sobre la Epidemia Mundial de Tabaquismo. 2019. Available online: https://apps.who.int/iris/handle/10665/326072 (accessed on 1 March 2023).
- Fernández, M.R.V. Efectividad de una Intervención Enfermera Sobre Abordaje al Tabaquismo en Diferentes Servicios de la Red de Salud Mental. Ph.D. Dissertation, Universidad de Murcia, Murcia, Spain, 2018. [Google Scholar]
- McRobbie, H.; Hajek, P. Effects of rapid smoking on post-cessation urges to smoke. Addiction 2007, 102, 483–489. [Google Scholar] [CrossRef] [PubMed]
- McRobbie, H.; Lee, M.; Juniper, Z. Non-nicotine pharmacotherapies for smoking cessation. Respir. Med. 2005, 99, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Kader, J.; Airagnes, G.; D’almeida, S.; Limosin, F.; Le Faou, A.L. Les outils du sevrage tabagique en 2018 [Interventions for smoking cessation in 2018]. Rev. Pneumol. Clin. 2018, 74, 160–169. [Google Scholar] [CrossRef]
- Kawai, A.; Kano, M.; Sato, T. Motivational interviewing and cognitive behavior therapy for smoking cessation. Nihon Rinsho. Jpn. J. Clin. Med. 2013, 71, 493–498. [Google Scholar]
- Borgne, A.; Aubin, H.J.; Berlin, I. Les stratégies thérapeutiques actuelles du sevrage tabagique [Current therapeutic strategies in smoking cessation]. Rev. Prat. 2004, 54, 1883–1893. [Google Scholar]
- Carim-Todd, L.; Mitchell, S.H.; Oken, B.S. Mind-body practices: An alternative, drug-free treatment for smoking cessation? A systematic review of the literature. Drug Alcohol Depend. 2013, 132, 399–410. [Google Scholar] [CrossRef]
- Farooq, M.U.; Puranik, M.P.; Uma, S.R. Effectiveness of cognitive-behavioral therapy compared with basic health education for tobacco cessation among smokers: A randomized controlled trial. J. Indian Assoc. Public Health Dent. 2020, 18, 25–30. [Google Scholar] [CrossRef]
- Brett, E.I.; Chavarria, J.; Liu, M.; Hedeker, D.; King, A.C. Effects of a brief motivational smoking intervention in non-treatment seeking disadvantaged Black smokers. J. Consult. Clin. Psychol. 2021, 89, 241. [Google Scholar] [CrossRef] [PubMed]
- Lindson-Hawley, N.; Thompson, T.P.; Begh, R. Motivational interviewing for smoking cessation. Cochrane Database Syst. Rev. 2015, 3, CD006936. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.T.; Cahill, K.; Qin, Y.; Tang, J.L. Motivational interviewing for smoking cessation. Cochrane Database Syst. Rev. 2010, 1, CD006936. [Google Scholar]
- Lotfalian, S.; Spears, C.A.; Juliano, L.M. The effects of mindfulness-based yogic breathing on craving, affect, and smoking behavior. Psychol. Addict. Behav. 2020, 34, 351. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Chen, T.A.; Martinez Leal, I.; Correa-Fernández, V.; Obasi, E.M.; Kyburz, B.; Williams, T.; Casey, K.; Brown, H.A.; O’connor, D.P.; et al. Organizational-level moderators impacting tobacco-related knowledge change after tobacco education training in substance use treatment centers. Int. J. Environ. Res. Public Health 2021, 18, 7597. [Google Scholar] [CrossRef] [PubMed]
- Tucker, I. Temporalities of peer support: The role of digital platforms in the ‘living presents’ of mental ill-health. Health Sociol. Rev. 2024, 33, 59–72. [Google Scholar] [CrossRef]
- PRISMA. PRISMA Flow Diagram. 2020. Available online: https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on 14 October 2021).
- Cañón, M.; Buitrago-Gómez, Q. The research question in clinical practice: A guideline for its formulation. Rev. Colomb. Psiquiatr. Engl. Ed. 2018, 47, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Joanna Briggs Institute. Critical Appraisal Tools. 2020. Available online: https://jbi.global/critical-appraisal-tools (accessed on 15 January 2022).
- Mundt, M.P.; Bak, T.B.; Piper, M.E.; Smith, S.S.; Fraser, D.L.; Fiore, M.C. Financial incentives to Medicaid smokers for engaging tobacco quit line treatment: Maximising return on investment. Tob. Control 2020, 29, 320–325. [Google Scholar] [CrossRef]
- Daly, A.T.; Deshmukh, A.A.; Vidrine, D.J.; Prokhorov, A.V.; Frank, S.G.; Tahay, P.D.; Houchen, M.E.; Cantor, S.B. Cost-effectiveness analysis of smoking cessation interventions using cell phones in a low-income population. Tob. Control 2019, 28, 88–94. [Google Scholar] [CrossRef]
- Martínez-Vispo, C.; López-Durán, A.; Rodríguez-Cano, R.; Senra, C.; Becoña, E. Treatment completion and anxiety sensitivity effects on smoking cessation outcomes. Addict. Behav. 2021, 117, 106856. [Google Scholar] [CrossRef] [PubMed]
- Peckham, E.; Arundel, C.; Bailey, D.; Brownings, S.; Fairhurst, C.; Heron, P.; Li, J.; Parrott, S.; Gilbody, S. Smoking Cessation Intervention for Severe Mental Ill Health Trial (SCIMITAR+): Study protocol for a randomised controlled trial. Trials 2017, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Frings, D.; Albery, I.; Moss, A.; Brunger, H.; Burghelea, M.; White, S.; Wood, K. Comparison of Allen Carr’s Easyway programme with NHS-standard specialist cessation support: A randomised controlled trial. Addiction 2020, 115, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.L.; Rosen, R.L.; Versella, M.V.; Borges, A.; Leyro, T.M. A pilot randomized clinical trial of brief interventions to encourage quit attempts in smokers from socioeconomic disadvantage. Nicotine Tob. Res. 2020, 22, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Siman, N.; Cleland, C.M.; Van Devanter, N.; Nguyen, T.; Nguyen, N.; Shelley, D. Effectiveness of village health worker–delivered smoking cessation counseling in Vietnam. Nicotine Tob. Res. 2019, 21, 1524–1530. [Google Scholar] [CrossRef]
- Bendtsen, M. Heterogeneous treatment effects of a text messaging smoking cessation intervention among university students. PLoS ONE 2020, 15, e0229637. [Google Scholar] [CrossRef] [PubMed]
- Spears, C.A.; Hedeker, D.; Li, L.; Wu, C.; Anderson, N.K.; Houchins, S.C.; Vinci, C.; Hoover, D.S.; Vidrine, J.I.; Cinciripini, P.M.; et al. Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. J. Consult. Clin. Psychol. 2017, 85, 1029. [Google Scholar] [CrossRef] [PubMed]
- Laude, J.R.; Bailey, S.R.; Crew, E.; Varady, A.; Lembke, A.; McFall, D.; Jeon, A.; Killen, D.; Killen, J.D.; David, S.P. Extended treatment for cigarette smoking cessation: A randomized control trial. Addiction 2017, 112, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Haskins, L.B.; Payne, C.A.; Schiavone, W.M.; Beach, S.R.; MacKillop, J.; VanDellen, M.R. Feasibility, tolerability, and potential advantages of a dyadic financial incentive treatment for smoking cessation among dual-smoker couples: A pilot study. Exp. Clin. Psychopharmacol. 2022, 30, 1001. [Google Scholar] [CrossRef]
- Minami, H.; Nahvi, S.; Arnsten, J.H.; Brinkman, H.R.; Rivera-Mindt, M.; Wetter, D.W.; Bloom, E.L.; Price, L.H.; Richman, E.K.; Betzler, T.F.; et al. A pilot randomized controlled trial of smartphone-assisted mindfulness-based intervention with contingency management for smokers with mood disorders. Exp. Clin. Psychopharmacol. 2022, 30, 653. [Google Scholar] [CrossRef]
- Scholten, H.; Luijten, M.; Poppelaars, A.; Johnson-Glenberg, M.C.; Granic, I. Mechanisms of change in a go/no-go training game for young adult smokers. Health Psychol. 2021, 40, 998. [Google Scholar] [CrossRef] [PubMed]
- Bloom, E.L.; Japuntich, S.J.; Pierro, A.; Dallery, J.; Leahey, T.M.; Rosen, J. Pilot trial of QuitBet: A digital social game that pays you to stop smoking. Exp. Clin. Psychopharmacol. 2022, 30, 642. [Google Scholar] [CrossRef] [PubMed]
- Zale, E.L.; Maisto, S.A.; De Vita, M.J.; Hooten, W.M.; Ditre, J.W. Increasing cessation motivation and treatment engagement among smokers in pain: A pilot randomized controlled trial. Exp. Clin. Psychopharmacol. 2021, 29, 593. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Zou, G.; Shi, J.; Chen, W.; Gong, X.; Wei, X.; Ling, L. Evaluation of the effectiveness of a WHO-5A model based comprehensive tobacco control program among migrant workers in Guangdong, China: A pilot study. BMC Public Health 2018, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Hausherr, Y.; Quinto, C.; Grize, L.; Schindler, C.; Probst-Hensch, N. Smoking cessation in workplace setting: Quit rates and determinants in a group behaviour therapy programme. Swiss Med. Wkly. 2017, 147, w14500. [Google Scholar] [CrossRef]
- Blank, M.D.; Ferris, K.A.; Metzger, A.; Gentzler, A.; Duncan, C.; Jarrett, T.; Dino, G. Physical activity and quit motivation moderators of adolescent smoking reduction. Am. J. Health Behav. 2017, 41, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.S.; Bell, M.L.; Armin, J.S.; Giacobbi, P.R.; Nair, U.S. A telephone-based guided imagery tobacco cessation intervention: Results of a randomized feasibility trial. Transl. Behav. Med. 2021, 11, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Larisa, A.Z.; Ibatov, A. Effectiveness of pharmacological and nonpharmacological treatment of tobacco dependence among health professionals. Hyg. Sanit. 2020, 99, 390–393. [Google Scholar]
- Correa-Fernández, V.; Díaz-Toro, E.C.; Reitzel, L.R.; Guo, L.; Chen, M.; Li, Y.; Calo, W.A.; Shih, Y.C.; Wetter, D.W. Combined treatment for at-risk drinking and smoking cessation among Puerto Ricans: A randomized clinical trial. Addict. Behav. 2017, 65, 185–192. [Google Scholar] [CrossRef]
- Jhanjee, S.; Lal, R.; Mishra, A.; Yadav, D. A randomized pilot study of brief intervention versus simple advice for women tobacco users in an urban community in India. Indian J. Psychol. Med. 2017, 39, 131–136. [Google Scholar] [CrossRef]
- Brown, R.A.; Palm Reed, K.M.; Bloom, E.L.; Minami, H.; Strong, D.R.; Lejuez, C.W.; Zvolensky, M.J.; Hayes, S.C. A randomized controlled trial of distress tolerance treatment for smoking cessation. Psychol. Addict. Behav. 2018, 32, 389. [Google Scholar] [CrossRef] [PubMed]
- Japuntich, S.J.; Lee, L.O.; Pineles, S.L.; Gregor, K.; Joos, C.M.; Patton, S.C.; Krishnan-Sarin, S.; Rasmusson, A.M. Contingency management and cognitive behavioral therapy for trauma-exposed smokers with and without posttraumatic stress disorder. Addict. Behav. 2019, 90, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.; Staiger, P.K.; Hayden, M.J.; Hughes, L.K.; Youssef, G.; Lawrence, N.S. A randomized controlled trial of inhibitory control training for smoking cessation and reduction. J. Consult. Clin. Psychol. 2019, 87, 831. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Zorick, T.; Hubert, R.; Hellemann, G.S.; Balali, S.; Kawasaki, S.S.; Garcia, L.Y.; Enoki, R.; Abraham, P.; Young, P.; et al. Combination extended smoking cessation treatment plus home visits for smokers with schizophrenia: A randomized controlled trial. Nicotine Tob. Res. 2016, 19, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Boffo, M.; Zerhouni, O.; Gronau, Q.F.; van Beek, R.J.; Nikolaou, K.; Marsman, M.; Wiers, R.W. Cognitive bias modification for behavior change in alcohol and smoking addiction: Bayesian meta-analysis of individual participant data. Neuropsychol. Rev. 2019, 29, 52–78. [Google Scholar] [CrossRef] [PubMed]
- Briere, J.B.; Bowrin, K.; Taieb, V.; Millier, A.; Toumi, M.; Coleman, C. Meta-analyses using real-world data to generate clinical and epidemiological evidence: A systematic literature review of existing recommendations. Curr. Med. Res. Opin. 2018, 34, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Song, F.; Gu, H.; Wang, J.; Jia, G.; Lu, M.; Qian, J.; Wang, L.; Shen, J.; Ren, Z. An assessment of factors associated with quality of randomized controlled trials for smoking cessation. Oncotarget 2016, 16, 53762–53771. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, S.; He, Y.; Zeng, J. Effect of Smoking Reduction Therapy on Smoking Cessation for Smokers without an Intention to Quit: An Updated Systematic Review and Meta-Analysis of Randomized Controlled. Int. J. Environ. Res. Public Health 2015, 12, 10235–10253. [Google Scholar] [CrossRef] [PubMed]
- Delle, S.; Kraus, L.; Maspero, S.; Pogarell, O.; Hoch, E.; Lochbühler, K. Effectiveness of the national German quitline for smoking cessation: Study protocol of a randomized controlled trial. BMC Public Health 2022, 22, 1386. [Google Scholar] [CrossRef]
- Le Foll, B.; Piper, M.E.; Fowler, C.D.; Tonstad, S.; Bierut, L.; Lu, L.; Jha, P.; Hall, W.D. Tobacco and nicotine use. Nature reviews. Dis. Primers 2022, 8, 19. [Google Scholar] [CrossRef]
| Source | Keywords | Related Terms |
|---|---|---|
| DECS | Cigarette | Cigar, cigarette, cigarettes, cigars. |
| DECS | Smoking | Smokers, cigarette smoker, cigarette smokers. |
| DECS | Therapy | Therapeutic, therapy(s), treatment(s). |
| MESH | Nicotine Dependence | No records found. |
| MESH | Psychological Intervention | Psychological intervention, psychological interventions. |
| MESH | Smoking | No records found. |
| MESH | Psychological therapy | No records found. |
| Database | Search Algorithm |
|---|---|
| COCHRANE | (“Psychotherapy”) OR (“Psychological Treatment”) AND (“Tobacco”) OR (“Smoker”) OR (“Nicotine”) |
| PROQUEST | (“Psychotherapy”) OR (“Psychological Treatment”) AND (“Tobacco”) OR (“Smoker”) OR (“Smokers”) OR (“Nicotine”) |
| PSYCNET | (“Psychotherapy”) OR (“Psychological Treatment”) AND (“Tobacco”) OR (“Smoker”) OR (“Nicotine”) |
| PUBMED | (“Psychotherapy”) OR (“Psychological Treatment”) OR (“Intervention”) AND (“Tobacco”) OR (“Smoking”) OR (“Cigarette”) OR (“Cigar”) OR (“Smoker”) OR (“Smokers”) OR (“Nicotine”) AND (“Dependence”) |
| SCIENCE DIRECT | (“Psychotherapy”) OR (“Clinical Psychology”) OR (“Psychological Therapies”) AND (“Smoking”) OR (“Tobacco”) OR (“Smoker”) OR (“Cigar”) OR (“Cigarette”) OR (“Nicotine”) |
| SCOPUS | (“Psychotherapy”) OR (“Clinical Psychology”) OR (“Psychological Therapies”) AND (“Smoking”) OR (“Tobacco”) OR (“Smoker”) OR (“Cigar”) OR (“Cigarette”) OR (“Nicotine”) |
| Database | Total Number of Studies Found | Type of Document | Period | Incomplete/ Duplicated Texts | No Access | Noncompliance with Criteria | Total Number of Selected Studies |
|---|---|---|---|---|---|---|---|
| PROQ UEST | 3,158,562 | 2,429,719 | 466,408 | 42,598 | 122,704 | 10,062 | 2 |
| SCIEN CE DIREC T | 1,333,687 | 563,134 | 586,014 | 142,449 | 38,747 | 3380 | 1 |
| PUBM ED | 425,914 | 409,030 | 13,610 | 1187 | 0 | 2084 | 8 |
| COCH RANE | 105,049 | 12,323 | 57,973 | 27,878 | 6387 | 834 | 0 |
| SCOP US | 25,276 | 4712 | 12,478 | 3047 | 3 | 4990 | 11 |
| PSYC NET | 125,895 | 79,694 | 59,116 | 7607 | 0 | 2577 | 6 |
| TOTAL | 5,174,383 | 3,498,612 | 1,195,599 | 224,766 | 167,841 | 23,927 | 28 |
| Study | Database | Title | Author(s) | Year |
|---|---|---|---|---|
| 1 | PROQUEST | Financial incentives to Medicaid smokers for engaging tobacco quit line treatment: maximizing return on investment | Mundt, Baker, Piper, Smith, Fraser, and Fiore [37]. | 2020 |
| 2 | PROQUEST | Cost-effectiveness analysis of smoking cessation interventions using cell phones in a low-income Population | Daly, Deshmukh, Vidrine, Prokhorov, Tahaky, Houchen, and Cantor [38]. | 2019 |
| 3 | SCIENCE DIRECT | Treatment completion and anxiety sensitivity effects on smoking cessation outcomes | Martínez, López, Rodríguez, Senra, and Becoña [39]. | 2021 |
| 4 | PUBMED | Smoking cessation intervention for severe mental ill health trial (SCIMITAR+): study protocol for a randomized controlled trial | Peckham, Arundel, Bai ley, Brownings, Fairhurst, Heron, Li, Parrott, and Gilbody [40]. | 2017 |
| 5 | PUBMED | Comparison of Allen Carr’s Easy way programmed with a specialist behavioral and pharmacological smoking cessation support service: a randomized controlled trial | Frings, Albery, Moss, Brunger, Burghelea, White, and Wood [41]. | 2020 |
| 6 | PUBMED | A pilot randomized clinical trial of brief interventions to encourage quit attempts in smokers from socioeconomic disadvantage | Steinberg, Rosen, Versella, Borges, and Leyro [42]. | 2020 |
| 7 | PUBMED | Effectiveness of village health worker-delivered smoking cessation counseling in Vietnam | Jiang Siman, Cleland, Van, Nguyen, Nguyen, and Shelley [43]. | 2019 |
| 8 | PUBMED | The effects of mindfulness- based yogic breathing on craving, affect and smoking behavior | Lotfalian, Spears, and Juliano [31]. | 2020 |
| 9 | PUBMED | Heterogeneous treatment effects of a text messaging smoking cessation intervention among university students | Bendtsen [44]. | 2020 |
| 10 | PUBMED | Mechanisms underlying mindfulness- based addiction treatment versus cognitive behavioral therapy and usual care for smoking Cessation | Spears, Hedeker, Li, Wu, Anderson, Houchins, Vinci, Hoover, Vidrine, Cinciripini, Waters, and Wetter [45]. | 2017 |
| 11 | PUBMED | Extended treatment for cigarette smoking cessation: a randomized control trial | Laude, Bailey, Crew, Varady, Lembke, McFall, Jeon, Killen, Killen, and David [46]. | 2017 |
| 12 | PSYCNET | Feasibility, tolerability, and potential advantages of a dyadic financial incentive treatment for smoking cessation among dual-Smoker couples: a pilot study | Haskins, Payne, Schiavone, Beach, MacKillop, and VanDellen [47]. | 2021 |
| 13 | PSYCNET | A pilot randomized controlled trial of smartphone- assisted mindfulness- based intervention with contingency management for smokers with mood disorders | Minami, Nahvi, Arnsten, Brinkman, Rivera, Wetter, Bloom, Price, Richman, Betzler, Stockmal, Donnelly, McClain, Kennedy, Vieira, Fine, McCarthy, Thomas, Hecht, and Brown [48]. | 2021 |
| 14 | PSYCNET | Mechanisms of change in a Go/No-Go training game for young adult smokers | Scholten, Hanneke Luijten, Maartje Poppelaars, Anouk Johnson-Glenberg, Granic, Isabela [49]. | 2021 |
| 15 | PSYCNET | Pilot trial of Quit Bet: A digital social game that pays you to stop smoking | Bloom, Japuntich, Pierro, Dallery, Leahey, and Rosen [50]. | 2021 |
| 16 | PSYCNET | Effects of a brief motivational smoking intervention in non-treatment seeking disadvantaged Black smokers | Brett, Chavarria, Liu, Hedeker, and King [28]. | 2021 |
| 17 | PSYCNET | Increasing cessation motivation and treatment engagement among smokers in pain: A pilot randomized controlled trial | Zale, Maisto, De Vita, Hooten, and Ditre [51]. | 2021 |
| 18 | SCOPUS | Evaluation of the effectiveness of a WHO5A model based comprehensive tobacco control program among migrant workers in Guangdong China: a pilot study | Chai, Zou, Shi, Chen, Gong, Wei, and Ling [52]. | 2018 |
| 19 | SCOPUS | Smoking cessation in workplace settings: quit rates and determinants in a group behavior therapy programmed | Hausherr, Quinto, Grize, Schindler, and Probst [53]. | 2017 |
| 20 | SCOPUS | Physical activity and quit motivation moderators of adolescent smoking reduction | Blank, Ferris, Metzger, Gentzler, Duncan, Jarrett, and Dino [54]. | 2017 |
| 21 | SCOPUS | A telephone- based guided imagery tobacco cessation intervention: results of a randomized feasibility trial | Gordon, Bell, Armin, Giacobbi, and Nair [55]. | 2021 |
| 22 | SCOPUS | Effectiveness of drug and non- drug treatment of tobacco dependence among medical Workers | Zakharova and Ibatov [56]. | 2021 |
| 23 | SCOPUS | Combined treatment for at- risk drinking and smoking cessation among Puerto Ricans: A randomized clinical trial | Correa, Díaz, Reitzel, Guo, Chen, Li, Calo, Shih YT, and Wetter DW [57]. | 2017 |
| 24 | SCOPUS | A randomized pilot study of brief intervention versus simple advice for women tobacco users in an urban community in India | Jhanjee, Lal, Mishra, and Yadav [58]. | 2017 |
| 25 | SCOPUS | A randomized controlled trial of distress tolerance treatment for smoking cessation | Brown, Palm, Bloom, Minami, Strong, Lejuez, Zvolensky, and Hayes [59]. | 2018 |
| 26 | SCOPUS | Contingency management and cognitive behavioral therapy for trauma-exposed smokers with and without posttraumatic stress disorder | Japuntich, Lee, Pineles, Gregor, Joos, Patton, Krishnan, and Rasmusson [60]. | 2019 |
| 27 | SCOPUS | A randomized controlled trial of inhibitory control training for smoking cessation and reduction | Bos, Staiger Hayden, Hughes, Youssef, and Lawrence [61]. | 2019 |
| 28 | SCOPUS | Combination extended smoking cessation treatment plus home visits for smokers with schizophrenia: A randomized controlled trial | Brody, Zorick, Hubert, Hellemann, Balali, Kawasaki, Garcia, Enoki, Abraham, Young, and McCreary [62]. | 2017 |
| No. | Study | Authors | Year | Treatment | Measurements Made in the Study | |
|---|---|---|---|---|---|---|
| Initial | Finale | |||||
| 1 | Financial incentives to Medicaid smokers for engaging tobacco quit line treatment: maximizing return on investment [37]. | Mundt, Baker, Piper, Smith, Fraser, and Fiore | 2020 | Incentive (USD 30) Wisconsin tobacco Quitline (WTQL) | Biochemically confirmed 7-day point abstinence at the 6-month follow-up visit. | 21.6% of the participants in the incentive group were biochemically confirmed as abstinent at 6-month follow-up vs. 13.7% in the control group. |
| 2 | Cost-effectiveness analysis of smoking cessation interventions using cell phones in a low-income population [38]. | Daly, Deshmukh, Prokhorov, Houchen, and Cantor | 2019 | No report | 11 telephone counseling sessions were scheduled during the 12-week treatment period. The first session took place one day before the quit date, the next four sessions were scheduled during the first week after quitting, and the remaining six sessions were scheduled every two weeks until the end of treatment. | All active participants were followed for 6 months after enrollment and were asked how their smoking habits had changed by cell phone assessments. |
| 3 | Effects of treatment completion and anxiety sensitivity on smoking cessation outcomes [39]. | Martínez-Vispo, López-Duran, Rodríguez-Cano, Senra and Becoña | 2021 | Standard cognitive-behavioral smoking cessation treatment (SCBSCT). Standard cognitive-behavioral smoking cessation treatment with behavioral activation components (SCBSCT-BA). (c) Waiting list control group. | No report | Participants were considered abstainers if they reported abstinence, not even a puff, for ≥30 days at 3-, 6-, and 12-month follow-up, and had an expired carbon monoxide (CO) reading of <6 parts per million. |
| 4 | Smoking cessation intervention for severe mental health trial (SCIMITAR +): study protocol for a randomized controlled trial [40]. | Peckham, Arundel, Bailey, Brownings, Fairhurst, Heron, Li, Parrott, and Gilbody | 2017 | Hypnotic suggestion condition Stroop test | No report | The primary outcome will be self-reported smoking cessation at 12 months, verified by measurement of expired carbon monoxide (CO). |
| 5 | Comparison of Allen Carr’s Easy way program with a specialized pharmacological and behavioral support service for smoking cessation: a randomized controlled trial [41]. | Frings, Albery, Moss, Brunger, Burghelea, White and Wood | 2020 | Allen Carr’s Easy way (ACE) Specialized behavioral and pharmacological support | No report | The primary outcome was self-reported continuous abstinence for 26 weeks from the quit/smoking cessation reset date verified by a measurement of exhaled breath carbon monoxide <10 parts per million (ppm). The primary analysis was by intention-to-treat. Secondary outcomes were use of pharmacotherapy, adverse events, and continued abstinence up to 4 and 12 weeks. |
| 6 | A pilot randomized clinical trial of brief interventions to encourage quit attempts among socioeconomically disadvantaged smokers [42]. | Steinberg, Rosen, Versella, Borges and Leyro | 2020 | Nicotine replacement therapy. Motivational interviewing | No report | Follow-up was completed approximately 1 month after the intervention. |
| 7 | Effectiveness of smoking cessation counseling delivered by village health workers in Vietnam [43]. | Jiang, Siman, Cleland, Van Devanter, Nguyen, Nguyen, and Shelley | 2019 | Counseling and assistance from the health care provider (ARM 1). ARM 1 plus advice from village health workers (VHWS) (ARM 2). | The main outcome of this study was the point prevalence of 7 days | At 6-month follow-up, abstinence rates in ARM 2 were significantly higher than those in ARM 1 (25.7% vs. 10.5%; p < 0.001). |
| 8 | The effects of mindfulness-based yogic breathing on craving, affect, and smoking [31]. | Lotfalian, Spears and Juliano | 2020 | Yogic breathing intervention (MB). Active treatment (cognitive strategy [CS]). No treatment (NT) | No report | No report |
| 9 | Heterogeneous treatment effects of a text-messaging smoking cessation intervention among college students [44]. | Bendtsen | 2020 | Smoking cessation interventions via text messaging. | No report | At 3 months after randomization, follow-up, data were collected from 1502 students (94.5%, 1502/1590). The primary outcome measure in the next trial was subjective reporting of prolonged abstinence, following Russel’s standard definition [20], as not having smoked more than 5 cigarettes in the past 8 weeks. |
| 10 | Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation [44]. | Spears, Hedeker, Li, Wu, Anderson, Houchins, Vinci, Hoover, Vidrine, Cinciripini, Waters and Wetter | 2017 | Mindfulness-based addiction treatment (MBAT). Cognitive behavioral therapy (CBT). Habitual Care (UC) for smoking cessation (all participants received self-help materials based on the Clinical Practice Guideline for the Treatment of Tobacco Use and Dependence (Fiore et al., 2008), psychoeducation on tobacco dependence/relapse/relapse and nicotine patch therapy). | Biochemically confirmed 7-day abstinence from smoking | 4 and 26 weeks after quitting smoking. |
| 11 | Extended treatment for cigarette smoking cessation: a randomized control trial [46]. | Laude, Bailey, Crew, Varady, Lembke, McFall, Jeon, Killen, Killen, and David | 2017 | Prolonged cognitive behavioral therapy. Nonprolonged cognitive behavioral therapy. | The primary outcome was the 7-day point prevalence (PP) confirmed by expired CO | PP abstinence rates at 52-week follow-up were comparable between the nonextended CBT (40%) and CBT-E (39%) groups [odds ratio (OR) = 0.99; 95% confidence interval (CI) = 0.55, 1.78]. A similar pattern was observed in the nonextended CBT (39%) and CBT-E (33%) groups at 104-week follow-up (OR = 0.79; 95% CI = 0.44, 1.40). |
| 12 | Feasibility, tolerability, and potential advantages of a dyadic financial incentive treatment for smoking cessation among dual-smoking couples [47]. | Haskins, Payne, Schiavone, Beach, MacKillop and VanDellen | 2021 | Financial incentive treatments | Among participants who completed the follow-up session, cravings for smoking and the severity of tobacco withdrawal symptoms were reduced during the study period. | No report |
| 13 | A pilot randomized controlled trial of smartphone-assisted mindfulness-based intervention with contingency management for smokers with mood disorders [48]. | Minami, Navhi, Arnesten, Brikman, Rivera-Mindt, Wetter, Bloom, Price, Richman, Betzler, Stockmal, Donnelley, McClain, Kennedy, Viera, Fine, McCarthy, Thomas, Hecht, and Brown | 2021 | Mindfulness intervention | Biochemically verified 7-day point prevalence abstinence | 2, 4 y 13 weeks |
| 14 | Mechanisms of change in a Go/No-Go training game for young adult smokers [49]. | Scholten, Luijten, Poppelaars, Johnson-Glenberg and Granic | 2021 | Hitnrun | No report | No report |
| 15 | Quit Bet pilot test: a digital social game that pays you to quit smoking [50]. | Bloom, Japuntich, Pierro, Dallery, Leahey and Rosen | 2021 | Didactical quit bet | After a week to prepare for quitting, quit day was day 8. Between day 9 and day 28 (a 20-day period), participants recouped USD 1 of their USD 30 wager for each day of verified abstinence with carbon monoxide (co) (≤6 ppm). The remaining stake money was combined into a “grand prize” pot. Participants who were abstinent on at least 19 out of the 20 days | No report |
| 16 | Effects of a brief motivational intervention on smoking in disadvantaged black smokers who do not seek treatment [28]. | Brett, Chavarria, Liu, Hedeker and King | 2021 | Motivational intervention | No report | No report |
| 17 | Increased motivation to quit smoking and commitment to treatment among smokers with pain: a randomized controlled pilot trial [51]. | Zale, Maisto, De Vita, Hooten and Ditre | 2021 | Motivational intervention psychoeducation about smoking | No report | At 1-month follow-up, advances in the knowledge of the interrelations between pain and smoking were maintained (p = 0.009). |
| 18 | Evaluating the effectiveness of a comprehensive tobacco control program based on the WHO-5A model among migrant workers in Guangdong, China: a pilot study [52]. | Chai, Zou, Shi, Chen, Gong, Wei, and Ling | 2020 | Model WHO-5A (OMS 5A) | No report | The primary outcome was the change in smoking rate according to salivary cotinine concentration at 3-month follow-up compared to the control arm. |
| 19 | Smoking cessation in the workplace: quit rates and determinants in a group behavioral therapy program [53]. | Hausherr, Quinto, Grize, Schindler and Probst | 2018 | Cognitive behavioral therapy. Cognitive preparation, motivation, psychoeducation. Motivation, reinforcement of ambivalence, self-control. Motivation, psychoeducation, coping skills, behavioral alternatives to smoking. Coping skills, self-management. Proactive telephone counseling | The evaluation consisted of three anonymized questionnaires (pre- and post-intervention and 12-month follow-up). | The evaluation consisted of three anonymized questionnaires (pre- and post-intervention and 12-month follow-up). |
| 20 | Moderators of physical activity and motivation to quit smoking in reducing adolescent smoking [54]. | Blank, Ferris, Metzger, Gentzler, Duncan, Jarrett, and Dino | 2017 | Brief motivational intervention with promotion of healthy habits emphasizing physical activity. | They were significantly correlated at both baseline and 3-month follow-up. | Were significantly correlated at both baseline and correlated at both baseline and 3-month follow-up. |
| 21 | A telephone smoking cessation intervention with guided imagery: results of a randomized controlled trial [55]. | Gordon, Bell, Armin, Giacobbi, and Nair | 2017 | Intervention with guided imagery and active behavioral control | Not specified, however it can be interpreted that measurement was generated at the end of treatment. | Ee evaluate 6-month dropout rates |
| 22 | The efficacy of pharmacological and nonpharmacological treatment of tobacco dependence among health professionals [56]. | Zakharova and Ibatov | 2020 | Cognitive behavioral therapy, psychosocial support using cognitive aspects; brief psychotherapy; breathing exercises; acupuncture and increased physical activity. | After treatment in the second group, 195 (64%) people out of 305 people stopped smoking completely, in the first group of medical workers who received nondrug therapy, 177 (56%) people out of 316 people stopped smoking completely. | 6 months after the end of the treatment program, 26.7% (84 medical workers) returned to smoking in group 1 and 10.2% (31 medical workers) in group 2 (or 3.02, 95% ci 2, 05–5.02; p < 0.00001). |
| 23 | Combination treatment for smoking cessation and at-risk alcohol use among Puerto Ricans: a randomized clinical trial [57]. | Correa, Díaz, Reitzel, Guo, Chen, Li, Calo, Shih and Wetter | 2020 | Behavioral intervention, motivation, and problem solving. | Blinded follow-up evaluations were performed by telephone at weeks 12, 26, and 52. | Blinded follow-up evaluations were performed by telephone at weeks 12, 26, and 52. |
| 24 | A Randomized Pilot Study of Brief Intervention versus Counseling of Brief Intervention versus Simple Counseling for Women Tobacco Users in an Urban Indian Community. urban community in India [58]. | Jhanjee, Lal, Mishra, and Yadav | 2017 | Brief psychoeducation and/or counseling intervention | All participants were tracked and evaluated at one week. | 3 months after surgery |
| 25 | A randomized controlled trial of stress tolerance treatment for smoking cessation [59]. | Brown, Palm, Bloom, Minami, Strong, Lejuez, Zvolensky and Hayes | 2017 | Standard behavioral protocol (Brown, 2003). Distress tolerance treatment. | There was no significant difference between conditions in the primary outcome of biochemically verified 7-day point prevalence smoking abstinence after 7 days. | No report |
| 26 | Contingency management and cognitive behavioral therapy for trauma-exposed smokers with and without posttraumatic stress disorder [60]. | Japuntich, Lee, Pineles, Gregor, Joos, Patton, Krishnan and Rasmusson | 2018 | Cognitive behavioral therapy | Seven-day post-quit abstinence rates for participants with and without PTSD, respectively, were similar: 39% vs. 38% (1-week), 33% vs. 28% (2-week). | Abstinence rates, 22% vs. 19% (3 weeks) and 22% vs. 13% (4 weeks). |
| 27 | A randomized controlled trial of inhibitory control training for smoking cessation and reduction [61]. | Bos, Staiger Hayden, Hughes, Youssef, and Lawrence | 2019 | Inhibitory control training. Go/no-go training. | No report | No report |
| 28 | Prolonged combined smoking cessation treatment plus home visits for smokers with schizophrenia: randomized controlled trial [62]. | Brody, Zorick, Hubert, Hellemann, Balali, Kawasaki, Garcia, Enoki, Abraham, Young and McCreary | 2017 | Cognitive behavioral therapy | 7-day point prevalence abstinence rates for the three groups were 45%, 20%, and 8%. | No report |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Sierra, S.-M.; Cárdenas-Cáceres, L.; Cadrazco-Urquijo, J.A.; Salazar-Gómez, A.N.; Rivera-Porras, D.; Bermúdez, V. Psychological Therapies Used for the Reduction of Habitual Cigarette Smoking Cigarette Consumption: A Systematic Review. Int. J. Environ. Res. Public Health 2024, 21, 753. https://doi.org/10.3390/ijerph21060753
Carrillo-Sierra S-M, Cárdenas-Cáceres L, Cadrazco-Urquijo JA, Salazar-Gómez AN, Rivera-Porras D, Bermúdez V. Psychological Therapies Used for the Reduction of Habitual Cigarette Smoking Cigarette Consumption: A Systematic Review. International Journal of Environmental Research and Public Health. 2024; 21(6):753. https://doi.org/10.3390/ijerph21060753
Chicago/Turabian StyleCarrillo-Sierra, Sandra-Milena, Lorena Cárdenas-Cáceres, John Anderson Cadrazco-Urquijo, Angie Natalia Salazar-Gómez, Diego Rivera-Porras, and Valmore Bermúdez. 2024. "Psychological Therapies Used for the Reduction of Habitual Cigarette Smoking Cigarette Consumption: A Systematic Review" International Journal of Environmental Research and Public Health 21, no. 6: 753. https://doi.org/10.3390/ijerph21060753
APA StyleCarrillo-Sierra, S.-M., Cárdenas-Cáceres, L., Cadrazco-Urquijo, J. A., Salazar-Gómez, A. N., Rivera-Porras, D., & Bermúdez, V. (2024). Psychological Therapies Used for the Reduction of Habitual Cigarette Smoking Cigarette Consumption: A Systematic Review. International Journal of Environmental Research and Public Health, 21(6), 753. https://doi.org/10.3390/ijerph21060753







