The Risk Factors Associated with the Prevalence of Multimorbidity of Anaemia, Malaria, and Malnutrition among Children Aged 6–59 Months in Nigeria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Data
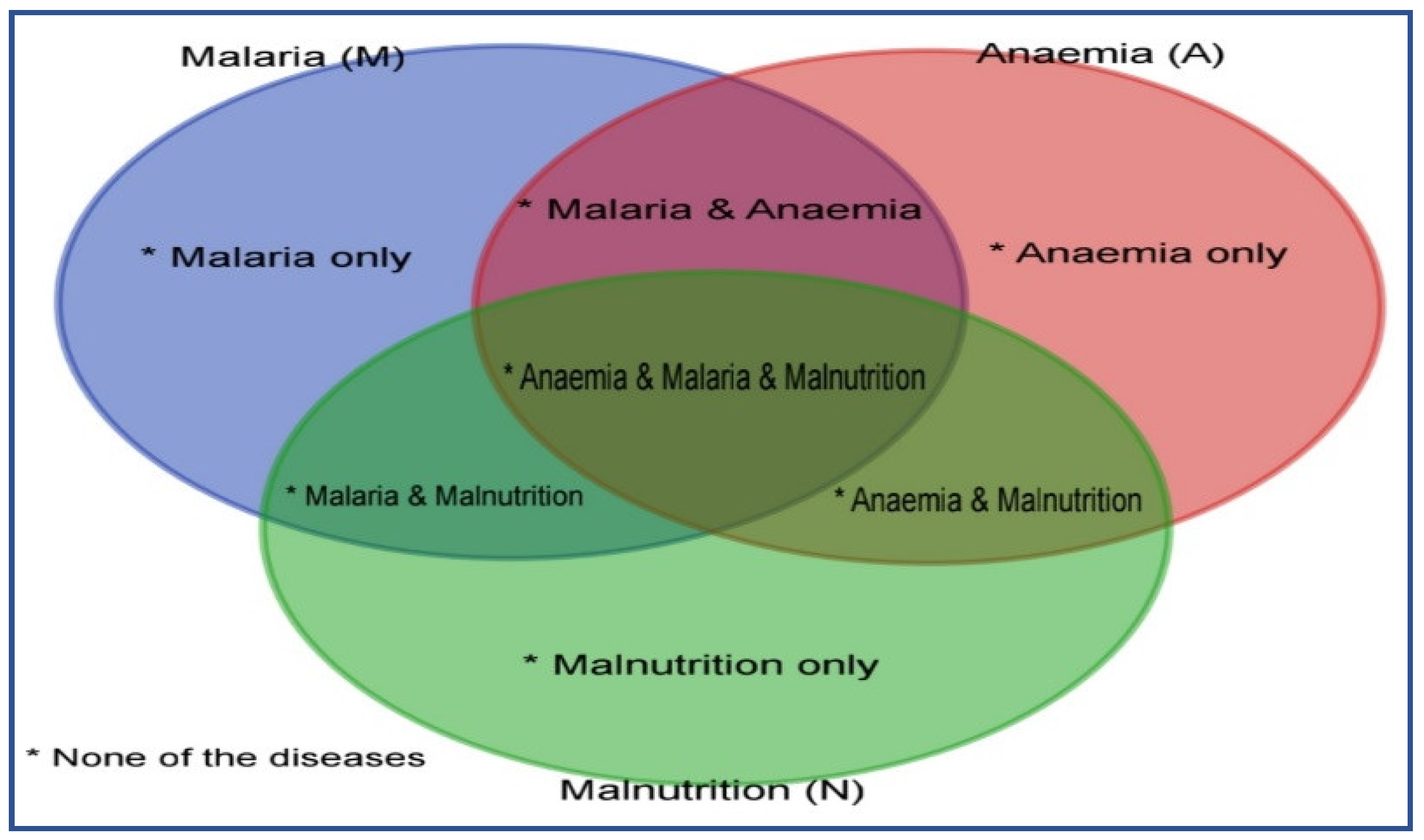
2.2. The Outcome Variables
2.3. Risk Factors (Predictor Variables)
2.4. Analyses Techniques
Levels of Statistical Analyses
- Based on the full model (considering all the independent variables), the Brant test was used after performing ordinal logistic regression to identify which variables were proportional to their coefficients or not. Then, the individual predicted probabilities and the mean were computed.
- The Brant test was also performed based on the partial model (after removing those variables that violated the proportionality assumption in step 1) and found no more violation of the proportionality assumption. Then, individual predicted probabilities and the mean were computed.
- The test of difference in the two predicted means was performed and no significant difference at the 5% level (both at two- and at one-tailed test) was found.
3. Results
3.1. Characteristics Associated with the Prevalence of Multimorbidity
3.1.1. Spatial Proportions of the Multimorbidity of Two or More Diseases by State and Region
3.1.2. Multivariate Multilevel Analysis of Multimorbidity Status
3.1.3. Multilevel Mixed-Effect Ordinal Logistic Regression Models
3.2. Model Building
3.3. Fixed and Variance Effects of Multimorbidity
4. Discussion
5. Strengths and Limitations of the Study
6. Policy Implications
- These spatial map descriptions of the prevalence of MAMM could be used by decision-makers to quickly target development efforts for health care, food security and poverty alleviation interventions in areas with a high prevalence of MAMM. Hence, success-driven efforts to end the long-lasting security issues in these regions are required so that people already displaced can return to their homes to farm again.
- Addressing the disparities between genders in childhood MAMM in Nigerian community, mobilisation based on gender-based prejudice and gender-sensitive policies is needed.
- Public health initiatives to lower childhood MAMM should pay more attention to reducing poor nutrition and other infections among pregnant, nursing mothers and children still breastfeeding. For instance, between 2013 and 2025, the Nigerian National Policy on Food and Nutrition has as one of its goals the reduction in maternal anaemia during pregnancy by 27% [18,56]. Therefore, there should be political commitment to make this happen. However, the high rate of poverty among families in Nigeria should be addressed. This provision will enable families to afford good nutrition sources. To address this situation, social security that will take care of the immediate necessities of life (food, shelter, and health) for poor households in Nigeria should be in place.
- Also, public health measures to lower childhood MAMM should pay more attention to increasing access to health centres through expanding the primary health care system.
- Finally, public health approaches should include making health care facilities closer to people in rural areas. More importantly, there should be an assurance that health workers posted to these rural areas are well remunerated and monitored in order to dwell among these people.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Child Mortality and Causes of Death. The Global Health Observatory. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/child-mortality-and-causes-of-death (accessed on 9 July 2022).
- De Benoist, B.; Cogswell, M.; Egli, I.; McLean, E. Worldwide Prevalence of Anaemia 1993–2005 of: WHO Global Database of Anaemia; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Nikoi, E.; Anthamatten, P. Childhood anaemia in Ghana: An examination of associated socioeconomic and health factors. Afr. Geogr. Rev. 2013, 33, 19–35. [Google Scholar] [CrossRef]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. A Scoping Review of the Risk Factors Associated with Anaemia among Children Under Five Years in Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2020, 17, 8829. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Prevalence of Anaemia in Children under 5 Years. Available online: https://www.who.int/data/maternal-newborn-child-adolescent/monitor (accessed on 23 July 2020).
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2021, 18, 2119. [Google Scholar] [CrossRef]
- Aychiluhm, S.B.; Gelaye, K.A.; Angaw, D.A.; Dagne, G.A.; Tadesse, A.W.; Abera, A.; Dillu, D. Determinants of malaria among under-five children in Ethiopia: Bayesian multilevel analysis. BMC Public Health 2020, 20, 1468. [Google Scholar] [CrossRef]
- Bennett, A.; Bisanzio, D.; O Yukich, J.; Mappin, B.; A Fergus, C.; Lynch, M.; E Cibulskis, R.; Bhatt, S.; Weiss, D.J.; Cameron, E.; et al. Population coverage of artemisinin-based combination treatment in children younger than 5 years with fever and Plasmodium falciparum infection in Africa, 2003–2015: A modelling study using data from national surveys. Lancet Glob. Health 2017, 5, e418–e427. [Google Scholar] [CrossRef]
- Ugwu, C.L.J.; Zewotir, T. Evaluating the Effects of Climate and Environmental Factors on Under-5 Children Malaria Spatial Distribution Using Generalized Additive Models (GAMs). J. Epidemiol. Glob. Health 2020, 10, 304–314. [Google Scholar] [CrossRef]
- Global Nutrition Report. The Burden of Malnutrition. Available online: https://globalnutritionreport.org/reports/global-nutrition-report-2018/burden-malnutrition/ (accessed on 25 June 2020).
- UNICEF/WHO/World Bank Group. Levels and Trends in Child Malnutrition: Key Findings of the 2019 Edition. Available online: https://iris.who.int/handle/10665/331097 (accessed on 8 March 2020).
- Ehrhardt, S.; Burchard, G.D.; Mantel, C.; Cramer, J.P.; Kaiser, S.; Kubo, M.; Mockenhaupt, F.P. Malaria, anemia, and malnutrition in African children—Defining intervention priorities. J. Infect. Dis. 2006, 194, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sumbele, I.U.N.; Kimbi, H.K.; Ndamukong-Nyanga, J.L.; Nweboh, M.; Anchang-Kimbi, J.K.; Lum, E.; Nana, Y.; Ndamukong, K.K.J.; Lehman, L.G. Malarial anaemia and anaemia severity in apparently healthy primary school children in urban and rural settings in the mount cameroon area: Cross sectional survey. PLoS ONE 2015, 10, e0123549. [Google Scholar] [CrossRef]
- Wanzira, H.; Katamba, H.; Okullo, A.E.; Agaba, B.; Kasule, M.; Rubahika, D. Factors associated with malaria parasitaemia among children under 5 years in Uganda: A secondary data analysis of the 2014 Malaria Indicator Survey dataset. Malar. J. 2017, 16, 191. [Google Scholar] [CrossRef]
- Sakwe, N.; Bigoga, J.; Ngondi, J.; Njeambosay, B.; Esemu, L.; Kouambeng, C.; Nyonglema, P.; Seumen, C.; Gouado, I.; Oben, J. Relationship between malaria, anaemia, nutritional and socio-economic status amongst under-ten children, in the North Region of Cameroon: A cross-sectional assessment. PLoS ONE 2019, 14, e0218442. [Google Scholar] [CrossRef] [PubMed]
- Teh, R.N.; Sumbele, I.U.N.; Meduke, D.N.; Ojong, S.T.; Kimbi, H.K. Malaria parasitaemia, anaemia and malnutrition in children less than 15 years residing in different altitudes along the slope of Mount Cameroon: Prevalence, intensity and risk factors. Malar. J. 2018, 17, 336. [Google Scholar] [CrossRef]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. Individual and Contextual Factors Associated with Malaria among Children 6–59 Months in Nigeria: A Multilevel Mixed Effect Logistic Model Approach. Int. J. Environ. Res. Public Health 2021, 18, 11234. [Google Scholar] [CrossRef] [PubMed]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. Individual, household and area predictors of anaemia among children aged 6–59 months in Nigeria. Public Health Pract. 2022, 3, 100229. [Google Scholar] [CrossRef] [PubMed]
- Obasohan, P.E. Investigating Multiple Overlaps in the Determinants of Risk Factors of Anaemia, Malaria, and Malnutrition, and Their Multimorbidity, among Children aged 6 to 59 months in Nigeria. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2022. Available online: https://etheses.whiterose.ac.uk/32621/ (accessed on 16 May 2023).
- Obasohan, P.E. The Impact of Food Insecurity, Household Socioeconomic Status, and Maternal Educational Attainment on the Optimal Number of Clusters of Malaria, Anaemia, and Malnutrition among Children Aged 6–23 Months in Nigeria: A mediation analysis. Young Statisticians’ Meetings (16–17 August); University of Leicester: Leicester, UK, 2023. [Google Scholar]
- Atsu, B.K.; Guure, C.; Laar, A.K. Determinants of overweight with concurrent stunting among Ghanaian children. BMC Pediatr. 2017, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Keino, S.; Plasqui, G.; Ettyang, G.; Borne, B.v.D. Determinants of Stunting and Overweight among Young Children and Adolescents in Sub-Saharan Africa. Food Nutr. Bull. 2014, 35, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.M.; Nour, T.Y.; Endris, B.S.; Gebreyesus, S.H. Concurrence of stunting and overweight/obesity among children: Evidence from Ethiopia. PLoS ONE 2021, 16, e0245456. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Richards, M.K.; Montiero, C.A. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J. Nutr. 1996, 126, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Bamiwuye, S.O.; De Wet, N.; Adedini, S.A. Linkages between autonomy, poverty and contraceptive use in two sub-Saharan African countries. Afr. Popul. Stud. 2013, 27, 164–173. [Google Scholar] [CrossRef]
- Nandy, S.; Daoud, A.; Gordon, D. Examining the changing profile of undernutrition in the context of food price rises and greater inequality. Soc. Sci. Med. 2016, 149, 153–163. [Google Scholar] [CrossRef]
- Nandy, S.; Miranda, J.J. Overlooking undernutrition? Using a composite index of anthropometric failure to assess how underweight misses and misleads the assessment of undernutrition in young children. Soc. Sci. Med. 2008, 66, 1963–1966. [Google Scholar] [CrossRef]
- The Stata Forums. Meologit and Parallel Odds Assumption—Statalist. Available online: https://www.statalist.org/forums/forum/general-stata-discussion/general/1372125-meologit-and-parallel-odds-assumption (accessed on 31 July 2021).
- Park, B.; Lee, H.A.; Park, H. Use of latent class analysis to identify multimorbidity patterns and associated factors in Korean adults aged 50 years and older. PLoS ONE 2019, 14, e0216259. [Google Scholar] [CrossRef]
- Duah, H.O.; Amankwa, C.E.; Adomako, I.; Owusu, B.; Agbadi, P. Comorbid patterns of anaemia and diarrhoea among children aged under 5 years in Ghana: A multivariate complex sample logistic regression analysis and spatial mapping visualisation. Int. Health 2020, 13, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Geda, N.R.; Feng, C.X.; Henry, C.J.; Lepnurm, R.; Janzen, B.; Whiting, S.J. Multiple anthropometric and nutritional deficiencies in young children in Ethiopia: A multi-level analysis based on a nationally representative data. BMC Pediatr. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Biggs, B.-A.; Holton, S.; Nguyen, H.T.M.; Hanieh, S.; Fisher, J. Co-morbid anaemia and stunting among children of pre-school age in low- and middle-income countries: A syndemic. Public Health Nutr 2019, 22, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, D.A.; Petrucka, P.M.; Isaac, E.W.; Muhajarine, N. Changing patterns of gender inequities in childhood mortalities during the Sustainable Development Goals era in Nigeria: Findings from an artificial neural network analysis. BMJ Open 2021, 11, e040302. [Google Scholar] [CrossRef]
- Khatab, K.; Kandala, N.-B. Latent variable modelling of risk factors associated with childhood diseases: Case study for Nigeria. Asian Pac. J. Trop. Dis. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Adedokun, S.T. Correlates of childhood morbidity in Nigeria: Evidence from ordinal analysis of cross-sectional data. PLoS ONE 2020, 15, e0233259. [Google Scholar] [CrossRef] [PubMed]
- Mulatya, D.M.; Mutuku, F.W. Assessing Comorbidity of Diarrhea and Acute Respiratory Infections in Children Under 5 Years: Evidence From Kenya’s Demographic Health Survey 2014. J. Prim. Care Community Health 2020, 11, 2150132720925190. [Google Scholar] [CrossRef] [PubMed]
- Philadelphia TCH of. Small for Gestational Age. Available online: https://www.chop.edu/conditions-diseases/small-gestational-age (accessed on 19 August 2022).
- March of Dimes. Low Birthweight. Available online: https://www.marchofdimes.org/complications/low-birthweight.aspx (accessed on 19 August 2022).
- Kandala, N.B.; Ji, C.; Stallard, N.; Stranges, S.; Cappuccio, F. Spatial Analysis of Risk Factors for Childhood Morbidity in Nigeria. Am. J. Trop. Med. Hyg. 2007, 77, 770–779. [Google Scholar] [CrossRef]
- Ntenda, P.A.M.; Nkoka, O.; Bass, P.; Senghore, T. Maternal anemia is a potential risk factor for anemia in children aged 6–59 months in Southern Africa: A multilevel analysis. BMC Public Health 2018, 18, 650. [Google Scholar] [CrossRef]
- Rahman, M.; Abe, S.K.; Rahman, S.; Kanda, M.; Narita, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 495–504. [Google Scholar] [CrossRef]
- Kazembe, L.N.; Namangale, J.J. A Bayesian multinomial model to analyse spatial patterns of childhood co-morbidity in Malawi. Eur. J. Epidemiol. 2007, 22, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, A.S.; Fagbamigbe, A.F.; Akinyemi, J.O.; Olowolafe, T.; Onwusaka, O.; Adewole, D.; Sadiku, S.; Palamuleni, M. Dynamics of poverty-related dissimilarities in fertility in Nigeria: 2003-2018. Sci. Afr. 2020, 9, e00468. [Google Scholar] [CrossRef]
- Gupta, R.P.-S.; de Wit, M.L.; McKeown, D. The impact of poverty on the current and future health status of children. Paediatr. Child Health 2007, 12, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, C.E.; Sie, A.; Ouattara, M.; Bountogo, M.; Boudo, V.; Kouanda, I.; Lebas, E.; Brogdon, J.M.; Lin, Y.; Nyatigo, F.; et al. Distance to primary care facilities and healthcare utilization for preschool children in rural northwestern Burkina Faso: Results from a surveillance cohort. BMC Health Serv. Res. 2021, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Moïsi, J.C.; Gatakaa, H.; Noor, A.M.; Williams, T.N.; Bauni, E.; Tsofa, B.; Levine, O.S.; Scott, J.A.G. Geographic access to care is not a determinant of child mortality in a rural Kenyan setting with high health facility density. BMC Public Health 2010, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.E.; Dockerty, J.D.; Jasseh, M.; Howie, S.R.; Herbison, P.; Jeffries, D.J.; Hill, P.C. Access to health care and mortality of children under 5 years of age in the Gambia: A case–control study. Bull World Health Organ 2009, 87, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.-Y.; Hu, Q.-D.; Wang, M.; Zhao, X.-Y.; Wu, W.-T.; Huang, J.-M.; Liang, T.-B. Impact of national Human Development Index on liver cancer outcomes: Transition from 2008 to 2018. World J. Gastroenterol. 2019, 25, 4749–4763. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Arah, O.A. The impact of human development on individual health: A causal mediation analysis examining pathways through education and body mass index. PeerJ 2017, 5, e3053. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, S.J. Intersections of Gender, Ethnicity, and Socioeconomic Position in Health in England: A Mixed Methods Study. White Rose eTheses. Available online: https://etheses.whiterose.ac.uk/cgi/search/simple?q=ScHARR&_action_search=&_order=bytitle&basic_srchtype=ALL&_satisfyall=ALL (accessed on 20 August 2022).
- Kandala, N.B.M. Socio-Demographic Determinants of Anaemia and Nutritional Status in the Democratic Republic of Congo, Uganda and Malawi. Ph.D. Thesis, University of Southampton, Southampton, UK, 2013. Available online: https://eprints.soton.ac.uk/354347/ (accessed on 25 July 2022).
- NorthWindProject.com. Proposed Health Sector Reform Aimed at Improving Availability, Accessibility, Quality, Affordability of Health Services-Okoh. Bureau of Public Enterprises—BPE. Available online: https://www.bpe.gov.ng/proposed-health-sector-reform-aimed-at-improving-availability-accessibility-quality-affordability-of-health-services-okoh/ (accessed on 20 August 2022).
- Oni, G.; Samuel, G. Effect of Birth Spacing on Under-five Mortality in Nigeria: A Proximate Determinant Approach (Birth Spacing and Under-five Mortality). In 3rd International Conference on African Development Issues (CU-ICADI 2016); Covenant University Press: Ota, Nigeria, 2016. [Google Scholar]
- Pondei, K.; Kunle-Olowu, O.E.; Peterside, O. The aetiology of non-malarial febrile illness in children in the malaria-endemic Niger Delta Region of Nigeria. Asian Pac. J. Trop. Dis. 2013, 3, 56–60. [Google Scholar] [CrossRef]
- Orimadegun, A.E.; Dada-Adegbola, H.O.; Michael, O.S.; Adepoju, A.A.; Funwei, R.E.; Olusola, F.I.; Ajayi, I.O.; Ogunkunle, O.O.; Ademowo, O.G.; Jegede, A.S.; et al. Non-Malaria Causes of Fever among under-5 Children with Negative Results for Malaria Rapid Diagnostic Test in South-Western Nigeria. J. Trop. Pediatr. 2022, 68, fmac061. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Budget and National Planning. National Policy on Food and Nutrition in Nigeria. Available online: https://nigeria.savethechildren.net/sites/nigeria.savethechildren.net/files/library/NPFN%20manual%20design%20%20v13.pdf (accessed on 20 August 2022).
- Whitty, C.J.M.; MacEwen, C.; Goddard, A.; Alderson, D.; Marshall, M.; Calderwood, C.; Atherton, F.; McBride, M.; Atherton, J.; Stokes-Lampard, H.; et al. Rising to the challenge of multimorbidity. BMJ 2020, 368, l6964. [Google Scholar] [CrossRef] [PubMed]


| Multimorbidity Status | ||||
|---|---|---|---|---|
| Variables | Total | None of the Diseases | One Disease Only | Two or More of the Diseases |
| N (%) | N (%) | N (%) | N (%) | |
| Prevalence rate | 1767 (17.3) | 3499 (34.4) | 4918 (48.3) | |
| Child-related characteristics | ||||
| Child’s sex | χ2 (2) = 25.03, p = 0.0002 | |||
| Male | 5217 (51.23) | 841 (16.13) | 1734 (33.25) | 2641 (50.62) |
| Female | 4967 (48.77) | 926 (18.64) | 1764 (35.52) | 2277 (45.84) |
| Child’s age in group | χ2 (8) = 205.55, p < 0.0001 | |||
| 6–11 months | 1232 (12.1) | 165 (13.35) | 566 (45.91) | 502 (40.74) |
| 12–23 months | 2422 (23.78) | 289 (11.95) | 888 (36.66) | 1245 (51.39) |
| 24–35 months | 2160 (21.21) | 363 (16.82) | 694 (32.13) | 1102 (51.05) |
| 36–47 months | 2227 (21.87) | 452 (20.32) | 664 (29.83) | 1110 (49.85) |
| 48–59 months | 2143 (21.04) | 498 (23.23) | 687 (32.06) | 958 (44.71) |
| Child’s birth size | χ2 (4) = 38.28, p < 0.0001 | |||
| Large | 923 (9.18) | 190 (20.63) | 343 (37.13) | 390 (42.23) |
| Average | 7914 (78.68) | 1387 (17.53) | 2741 (34.64) | 3785 (47.83) |
| Small | 1222 (12.15) | 177 (14.52) | 371 (30.37) | 673 (55.11) |
| Preceding birth interval | χ2 (8) = 135.23, p < 0.0001 | |||
| None | 1944 (19.13) | 449 (23.09) | 689 (35.42) | 807 (41.49) |
| 8–24 months | 2191 (21.55) | 319 (14.56) | 736 (33.58) | 1136 (51.87) |
| 25–35 months | 2884 (28.38) | 435 (15.07) | 924 (32.05) | 1525 (52.88) |
| 36–59 months | 2351 (23.13) | 383 (16.28) | 827 (35.16) | 1141 (48.55) |
| 60+ months | 795 (7.82) | 177 (22.23) | 317 (39.88) | 301 (37.89) |
| Took Iron supplements | χ2 (2) = 67.80, p < 0.0001 | |||
| No | 8224 (81.02) | 1347 (16.38) | 2748 (33.42) | 4128 (50.2) |
| Yes | 1927 (18.98) | 416 (21.62) | 737 (38.25) | 773 (40.13) |
| Duration of breastfeeding | χ2 (4) = 119.28, p < 0.0001 | |||
| Ever breastfed, not currently breastfeeding | 7440 (73.06) | 1474 (19.82) | 2448 (32.9) | 3518 (47.29) |
| Never breastfed | 171 (1.68) | 19 (11) | 68 (39.89) | 84 (49.11) |
| Still breastfeeding | 2572 (25.26) | 274 (10.65) | 983 (38.22) | 1315 (51.13) |
| Child took deworming drug in the last 6 months | χ2 (2) = 348.84, p < 0.0001 | |||
| No | 7235 (71.41) | 1033 (14.28) | 2302 (31.82) | 3899 (53.89) |
| Yes | 2897 (28.59) | 729 (25.16) | 1173 (40.5) | 995 (34.33) |
| Total | 10,132 (100) | 1762 (17.39) | 3476 (34.31) | 4894 (48.3) |
| Child had a fever in last 2 weeks before the survey | χ2 (2) = 281.15, p < 0.0001 | |||
| No | 7485 (73.52) | 1473 (19.68) | 2764 (36.93) | 3248 (43.39) |
| Yes | 2696 (26.48) | 294 (10.9) | 735 (27.25) | 1667 (61.85) |
| Place of child’s delivery | χ2 (6) = 794.52, p < 0.0001 | |||
| Home | 5348 (52.51) | 572 (10.69) | 1543 (28.86) | 3233 (60.45) |
| Public facility | 2975 (29.22) | 672 (22.58) | 1163 (39.08) | 1141 (38.34) |
| Private facility | 1660 (16.3) | 490 (29.52) | 701 (42.25) | 469 (28.23) |
| Elsewhere | 200 (1.97) | 34 (16.83) | 91 (45.59) | 75 (37.59) |
| Parental-related characteristics | ||||
| Maternal/caregiver’s highest educational level | χ2 (6) = 1417.75, p < 0.0001 | |||
| No education | 3970 (38.98) | 332 (8.37) | 951 (23.95) | 2687 (67.68) |
| Primary | 1643 (16.14) | 221 (13.45) | 581 (35.35) | 841 (51.2) |
| Secondary | 3597 (35.32) | 832 (23.13) | 1542 (42.88) | 1223 (34) |
| Higher | 974 (9.56) | 382 (39.23) | 425 (43.64) | 167 (17.13) |
| Mother is currently residing with husband/partner | χ2 (2) = 20.09, p = 0.0022 | |||
| Living with her partner | 8861 (91.05) | 1479 (16.69) | 3039 (34.3) | 4343 (49.02) |
| Staying elsewhere | 871 (8.95) | 195 (22.41) | 298 (34.22) | 378 (43.37) |
| Mother’s religious status | χ2 (6) = 595.20, p < 0.0001 | |||
| Catholic | 1027 (10.09) | 241 (23.43) | 442 (43.03) | 344 (33.54) |
| Other Christian | 3438 (33.76) | 835 (24.28) | 1378 (40.09) | 1225 (35.63) |
| Islam | 5654 (55.52) | 682 (12.07) | 1651 (29.2) | 3321 (58.73) |
| Traditionalist and others | 64 (0.63) | 9 (14.57) | 28 (42.81) | 27 (42.62) |
| Maternal ethnicity | χ2 (6) = 737.24, p < 0.0001 | |||
| Hausa/Fulani/Kanuri/Seribiri | 4067 (39.94) | 407 (10) | 1070 (26.3) | 2591 (63.69) |
| Ibo | 1650 (16.2) | 404 (24.49) | 710 (43.04) | 536 (32.47) |
| Yoruba | 1488 (14.62) | 392 (26.34) | 562 (37.73) | 535 (35.93) |
| Others | 2978 (29.24) | 564 (18.94) | 1157 (38.86) | 1256 (42.19) |
| Mother’s anaemia status | χ2 (2) = 269.34, p < 0.0001 | |||
| Not anaemic | 4206 (41.84) | 997 (23.7) | 1534 (36.47) | 1675 (39.83) |
| Anaemic | 5847 (58.16) | 761 (13.02) | 1930 (33.01) | 3156 (53.97) |
| Maternal body weight status | χ2 (6) = 518.14, p < 0.0001 | |||
| Normal | 5311 (60.82) | 776 (14.62) | 1757 (33.09) | 2777 (52.29) |
| Underweight | 885 (10.13) | 84 (9.48) | 233 (26.35) | 568 (64.17) |
| Overweight | 1668 (19.1) | 429 (25.72) | 689 (41.3) | 550 (32.98) |
| Obese | 869 (9.95) | 255 (29.39) | 405 (46.62) | 208 (23.99) |
| Paternal work status | χ2 (2) = 6.04, p = 0.2041 | |||
| No | 304 (2.98) | 37 (12.09) | 112 (36.88) | 155 (51.02) |
| Yes | 9880 (97.01) | 1731 (17.52) | 3387 (34.28) | 4763 (48.21) |
| Partner education status | χ2 (6) = 969.76, p < 0.0001 | |||
| No education | 2872 (29.91) | 231 (8.04) | 651 (22.66) | 1990 (69.3) |
| Primary education | 1423 (14.82) | 183 (12.84) | 485 (34.1) | 755 (53.06) |
| Secondary education | 3741 (38.95) | 775 (20.72) | 1514 (40.47) | 1452 (38.81) |
| Tertiary education | 1566 (16.31) | 471 (30.1) | 651 (41.55) | 444 (28.35) |
| Household-related characteristics | ||||
| Household wealth index | χ2 (8) = 1635.53, p < 0.0001 | |||
| Poorest | 1893 (18.59) | 109 (5.73) | 392 (20.7) | 1393 (73.57) |
| Poorer | 1989 (19.53) | 166 (8.33) | 555 (27.9) | 1268 (63.77) |
| Middle | 2139 (21) | 328 (15.35) | 753 (35.19) | 1058 (49.46) |
| Richer | 2144 (21.05) | 445 (20.75) | 876 (40.85) | 823 (38.4) |
| Richest | 2019 (19.83) | 720 (35.66) | 924 (45.75) | 376 (18.6) |
| Children under 5 slept under a mosquito bed net last night | χ2 (6) = 176.44, p < 0.0001 | |||
| No child | 1316 (13.02) | 250 (19) | 451 (34.24) | 615 (46.75) |
| All children | 4715 (46.64) | 744 (15.78) | 1549 (32.86) | 2422 (51.37) |
| Some children | 996 (9.85) | 114 (11.42) | 269 (26.97) | 613 (61.61) |
| No net in household | 3083 (30.5) | 635 (20.6) | 1211 (39.27) | 1238 (40.13) |
| Sex of household head | χ2 (2) = 20.61, p = 0.0007 | |||
| Male | 9096 (89.32) | 1528 (16.8) | 3124 (34.34) | 4444 (48.85) |
| Female | 1087 (10.67) | 239 (21.98) | 375 (34.46) | 473 (43.56) |
| Number of people in household | χ2 (6) = 247.76, p < 0.0001 | |||
| 0–3 | 979 (9.61) | 195 (19.95) | 354 (36.18) | 429 (43.86) |
| 4–6 | 4836 (47.48) | 985 (20.37) | 1802 (37.27) | 2048 (42.36) |
| 7–9 | 2462 (24.17) | 372 (15.1) | 842 (34.21) | 1248 (50.69) |
| 10+ | 1907 (18.73) | 215 (11.28) | 500 (26.22) | 1192 (62.5) |
| Community-related characteristics | ||||
| Proportion of community wealth level | χ2 (2) = 1183.30, p < 0.0001 | |||
| Low | 4647 (45.63) | 387 (8.34) | 1174 (25.26) | 3086 (66.4) |
| High | 5536 (54.36) | 1380 (24.92) | 2325 (41.99) | 1832 (33.08) |
| Community distance to health facility is not problematic | χ2 (2) = 245.38, p < 0.0001 | |||
| Low | 4702 (46.17) | 629 (13.38) | 1417 (30.14) | 2656 (56.48) |
| High | 5481 (53.82) | 1138 (20.76) | 2082 (37.98) | 2262 (41.26) |
| Proportion of community maternal education level | χ2 (2) = 972.85, p < 0.0001 | |||
| Low | 5025 (49.34) | 494 (9.83) | 1337 (26.6) | 3194 (63.56) |
| High | 5158 (50.65) | 1273 (24.68) | 2162 (41.91) | 1723 (33.41) |
| Area-related characteristics | ||||
| Multidimensional Poverty Index by State (MPI) | χ2 (8) = 913.28, p < 0.0001 | |||
| Highly Deprived | 847 (8.32) | 57 (6.7) | 208 (24.54) | 582 (68.75) |
| Above averagely deprived | 3093 (30.37) | 309 (9.98) | 791 (25.59) | 1992 (64.43) |
| Averagely Deprived | 2319 (22.77) | 402 (17.36) | 884 (38.14) | 1032 (44.51) |
| Mildly Deprived | 1939 (19.04) | 487 (25.11) | 720 (37.16) | 732 (37.73) |
| Lowest Deprived | 1987 (19.51) | 512 (25.8) | 895 (45.04) | 579 (29.16) |
| Human Development Index by State (HDI) | χ2 (8) = 860.46, p < 0.0001 | |||
| Lowest HDI | 2150 (21.11) | 201 (9.35) | 552 (25.68) | 1397 (64.97) |
| Low HDI | 2416 (23.73) | 267 (11.06) | 690 (28.56) | 1459 (60.39) |
| Average HDI | 2223 (21.83) | 442 (19.9) | 846 (38.08) | 934 (42.02) |
| High HDI | 2680 (26.31) | 600 (22.37) | 1096 (40.92) | 984 (36.71) |
| Highest HDI | 715 (7.02) | 257 (35.97) | 314 (43.88) | 144 (20.15) |
| Gender Inequality Index by State (GII) | χ2 (8) = 551.09, p < 0.0001 | |||
| Lowest GII | 2726 (26.77) | 660 (24.23) | 1129 (41.42) | 936 (34.35) |
| Low GII | 1171 (11.5) | 266 (22.71) | 507 (43.26) | 398 (34.03) |
| Average GII | 977 (9.59) | 165 (16.91) | 301 (30.79) | 511 (52.3) |
| High GII | 4054 (39.81) | 557 (13.74) | 1222 (30.13) | 2275 (56.13) |
| Highest GII | 1256 (12.33) | 119 (9.46) | 341 (27.13) | 796 (63.41) |
| Region of residence | χ2 (10) = 761.25, p < 0.0001 | |||
| North-central | 1436 (14.1) | 277 (19.29) | 523 (36.43) | 636 (44.28) |
| North-east | 1573 (15.44) | 204 (13) | 433 (27.5) | 936 (59.49) |
| North-west | 2967 (29.13) | 286 (9.65) | 781 (26.31) | 1900 (64.04) |
| South-east | 1328 (13.04) | 292 (22.01) | 584 (43.97) | 452 (34.02) |
| South-south | 1086 (10.66) | 224 (20.61) | 480 (44.21) | 382 (35.19) |
| South-west | 1793 (17.61) | 483 (26.95) | 698 (38.93) | 612 (34.12) |
| Type of place of residence | χ2 (2) = 682.03, p < 0.0001 | |||
| Urban | 4483 (44.02) | 1117 (24.92) | 1828 (40.77) | 1538 (34.31) |
| Rural | 5700 (55.97) | 650 (11.4) | 1671 (29.31) | 3379 (59.28) |
| Model 0 (N = 10,451) No Covariates | Model 1 (N = 10,451) (Level 1 Covariates Only) | Model 2 (N = 10,451) (Level 1 and 2 Covariates Only) | Model 3 (N = 10,451) (Level 1, 2, and 3 Covariates) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aOR | p | 95% CI | aOR | p | 95% CI | aOR | p | 95% CI | ||
| Child’s sex | ||||||||||
| Male | ||||||||||
| Female | 0.76 | <0.001 | 0.70–0.83 | 0.76 | <0.001 | 0.70–0.83 | 0.76 | <0.001 | 0.70–0.83 | |
| Child’s age in group | ||||||||||
| 6–11 months | ||||||||||
| 12–23 months | 1.44 | <0.001 | 1.23–1.68 | 1.43 | <0.001 | 1.23–1.67 | 1.44 | <0.001 | 1.23–1.68 | |
| 24–35 months | 1.34 | <0.001 | 1.10–1.64 | 1.33 | 0.01 | 1.09–1.63 | 1.34 | <0.001 | 1.10–1.63 | |
| 36–47 months | 1.11 | 0.31 | 0.91–1.36 | 1.11 | 0.33 | 0.90–1.35 | 1.11 | 0.30 | 0.91–1.36 | |
| 48–59 months | 0.89 | 0.27 | 0.73–1.09 | 0.89 | 0.24 | 0.72–1.08 | 0.89 | 0.25 | 0.72–1.09 | |
| Child’s birth size | ||||||||||
| Large | ||||||||||
| Average | 1.09 | 0.22 | 0.95–1.26 | 1.10 | 0.19 | 0.95–1.27 | 1.09 | 0.23 | 0.95–1.26 | |
| Small | 1.37 | <0.001 | 1.14–1.65 | 1.38 | <0.001 | 1.14–1.66 | 1.38 | <0.001 | 1.14–1.66 | |
| Preceding birth interval | ||||||||||
| None | ||||||||||
| 8–24 months | 1.31 | <0.001 | 1.14–1.50 | 1.31 | <0.001 | 1.15–1.51 | 1.31 | <0.001 | 1.15–1.50 | |
| 25–35 months | 1.11 | 0.13 | 0.95–1.26 | 1.11 | 0.11 | 0.98–1.27 | 1.12 | 0.10 | 0.98–1.27 | |
| 36–59 months | 1.01 | 0.90 | 0.88–1.15 | 1.02 | 0.82 | 0.89–1.16 | 1.02 | 0.79 | 0.89–1.17 | |
| 60+ months | 0.89 | 0.21 | 0.75–1.07 | 0.90 | 0.24 | 0.75–1.07 | 0.90 | 0.22 | 0.75–1.07 | |
| Child took Iron supplements | ||||||||||
| No | ||||||||||
| Yes | 1.06 | 0.30 | 0.95–1.19 | 1.07 | 0.28 | 0.95–1.20 | 1.06 | 0.32 | 0.94–1.19 | |
| Duration of breastfeeding | ||||||||||
| Ever breastfed, not currently breastfeeding | ||||||||||
| Never breastfed | 1.30 | 0.12 | 0.94–1.81 | 1.28 | 0.14 | 0.93–1.78 | 1.26 | 0.16 | 0.91–1.75 | |
| Still breastfeeding | 0.97 | 0.70 | 0.83–1.13 | 0.96 | 0.64 | 0.83–1.12 | 0.97 | 0.66 | 0.83–1.13 | |
| Child was dewormed in last 6 months before the survey | ||||||||||
| No | ||||||||||
| Yes | 0.80 | <0.001 | 0.72–0.89 | 0.80 | <0.001 | 0.72–0.89 | 0.81 | <0.001 | 0.72–0.90 | |
| Child had fever in last 2 weeks before the survey | ||||||||||
| No | ||||||||||
| Yes | 1.59 | <0.001 | 1.43–1.75 | 1.58 | <0.001 | 1.43–1.75 | 1.60 | <0.001 | 1.44–1.76 | |
| Place of child’s delivery | ||||||||||
| Home | ||||||||||
| Public facility | 0.88 | 0.03 | 0.79–0.99 | 0.90 | 0.07 | 0.80–1.01 | 0.91 | 0.10 | 0.81–1.02 | |
| Private facility | 0.83 | 0.02 | 0.72–0.97 | 0.84 | 0.02 | 0.73–0.98 | 0.84 | 0.02 | 0.72–0.98 | |
| Elsewhere | 1.04 | 0.82 | 0.76–1.41 | 1.03 | 0.83 | 0.76–1.41 | 1.03 | 0.85 | 0.76–1.40 | |
| Maternal/caregiver highest educational level | ||||||||||
| No education | ||||||||||
| Primary | 0.83 | 0.02 | 0.72–0.97 | 0.85 | 0.03 | 0.73–0.99 | 0.85 | 0.03 | 0.73–0.99 | |
| Secondary | 0.67 | <0.001 | 0.57–0.77 | 0.67 | <0.001 | 0.57–0.79 | 0.67 | <0.001 | 0.57–0.79 | |
| Higher | 0.44 | <0.001 | 0.36–0.55 | 0.45 | <0.001 | 0.36–0.56 | 0.46 | <0.001 | 0.36–0.57 | |
| Mother staying with a partner | ||||||||||
| Staying with partner | ||||||||||
| Staying elsewhere | 0.96 | 0.59 | 0.82–1.12 | 0.96 | 0.64 | 0.82–1.13 | 0.96 | 0.57 | 0.81–1.12 | |
| Mother’s religious status | ||||||||||
| Catholic | ||||||||||
| Other Christian | 1.00 | 0.97 | 0.85–1.17 | 1.01 | 0.89 | 0.86–1.19 | 1.03 | 0.69 | 0.88–1.22 | |
| Islam | 1.15 | 0.15 | 0.95–1.40 | 1.19 | 0.09 | 0.98–1.45 | 1.28 | 0.02 | 1.04–1.58 | |
| Traditionalist and others | 0.94 | 0.79 | 0.58–1.51 | 0.93 | 0.77 | 0.58–1.50 | 0.95 | 0.83 | 0.59–1.53 | |
| Mother’s anaemia status | ||||||||||
| Not anaemic | ||||||||||
| Anaemic | 1.58 | <0.001 | 1.45–1.72 | 1.58 | <0.001 | 1.45–1.72 | 1.58 | <0.001 | 1.45–1.72 | |
| Maternal body mass index | ||||||||||
| Normal | ||||||||||
| Underweight | 1.07 | 0.26 | 0.95–1.21 | 1.08 | 0.23 | 0.96–1.21 | 1.08 | 0.19 | 0.96–1.22 | |
| Overweight | 0.76 | <0.001 | 0.68–0.85 | 0.77 | <0.001 | 0.68–0.86 | 0.77 | <0.001 | 0.68–0.86 | |
| Obese | 0.67 | <0.001 | 0.57–0.78 | 0.68 | <0.001 | 0.58–0.79 | 0.68 | <0.001 | 0.58–0.79 | |
| Paternal work status | ||||||||||
| Not working | ||||||||||
| Working | 1.21 | 0.14 | 0.94–1.57 | 1.21 | 0.15 | 0.93–1.56 | 1.19 | 0.19 | 0.92–1.54 | |
| Partner education status | ||||||||||
| No education | ||||||||||
| Primary education | 0.90 | 0.21 | 0.77–1.06 | 0.92 | 0.30 | 0.78–1.08 | 0.91 | 0.27 | 0.78–1.07 | |
| Secondary education | 0.82 | 0.01 | 0.70–0.95 | 0.83 | 0.02 | 0.72–0.97 | 0.84 | 0.02 | 0.72–0.98 | |
| Tertiary education | 0.72 | <0.001 | 0.60–0.86 | 0.73 | <0.001 | 0.61–0.88 | 0.74 | <0.001 | 0.61–0.89 | |
| Household wealth index | ||||||||||
| Poorest | ||||||||||
| Poorer | 0.83 | 0.02 | 0.71–0.97 | 0.87 | 0.09 | 0.74–1.02 | 0.87 | 0.08 | 0.74–1.02 | |
| Middle | 0.60 | <0.001 | 0.51–0.71 | 0.71 | <0.001 | 0.59–0.85 | 0.72 | <0.001 | 0.60–0.86 | |
| Richer | 0.47 | <0.001 | 0.39–0.56 | 0.59 | <0.001 | 0.48–0.73 | 0.61 | <0.001 | 0.50–0.76 | |
| Richest | 0.32 | <0.001 | 0.26–0.39 | 0.41 | <0.001 | 0.33–0.53 | 0.43 | <0.001 | 0.34–0.55 | |
| Children under 5 slept under a bed net | ||||||||||
| No child | ||||||||||
| All children | 0.90 | 0.13 | 0.79–1.03 | 0.90 | 0.13 | 0.78–1.03 | 0.89 | 0.10 | 0.78–1.02 | |
| Some children | 1.10 | 0.30 | 0.92–1.32 | 1.10 | 0.31 | 0.92–1.32 | 1.10 | 0.31 | 0.92–1.32 | |
| No net in household | 0.89 | 0.11 | 0.78–1.02 | 0.90 | 0.12 | 0.78–1.03 | 0.90 | 0.13 | 0.78–1.03 | |
| Sex of household head | ||||||||||
| Male | ||||||||||
| Female | 0.93 | 0.34 | 0.80–1.08 | 0.93 | 0.37 | 0.81–1.08 | 0.94 | 0.38 | 0.81–1.08 | |
| Number of people in household | ||||||||||
| 2–3 | ||||||||||
| 4–6 | 0.94 | 0.42 | 0.80–1.10 | 0.94 | 0.42 | 0.80–1.10 | 0.94 | 0.40 | 0.80–1.09 | |
| 7–9 | 1.00 | 0.99 | 0.84–1.19 | 1.00 | 0.96 | 0.83–1.19 | 0.99 | 0.92 | 0.83–1.18 | |
| 10+ | 1.18 | 0.10 | 0.97–1.43 | 1.17 | 0.12 | 0.96–1.42 | 1.17 | 0.12 | 0.96–1.42 | |
| Median community wealth level | ||||||||||
| Low | ||||||||||
| High | 0.73 | <0.001 | 0.62–0.87 | 0.79 | 0.01 | 0.67–0.94 | ||||
| Median proportion of community distance to health facility is no big problem | ||||||||||
| Low | ||||||||||
| High | 0.84 | 0.01 | 0.75–0.95 | 0.85 | 0.01 | 0.76–0.96 | ||||
| Media proportion of community maternal education level | ||||||||||
| Low | ||||||||||
| High | 1.05 | 0.56 | 0.89–1.25 | 1.06 | 0.50 | 0.89–1.26 | ||||
| State multidimensional poverty index (SMPI) | ||||||||||
| Highly deprived | ||||||||||
| Above averagely deprived | 1.35 | 0.05 | 1.01–1.83 | |||||||
| Averagely deprived | 0.75 | 0.16 | 0.50–1.12 | |||||||
| Mildly deprived | 0.72 | 0.14 | 0.47–1.11 | |||||||
| Least deprived | 0.74 | 0.23 | 0.44–1.22 | |||||||
| State human development index (HDI) | ||||||||||
| Lowest HDI | ||||||||||
| Low HDI | 1.30 | 0.06 | 0.99–1.72 | |||||||
| Average HDI | 1.31 | 0.12 | 0.93–1.86 | |||||||
| High HDI | 1.47 | 0.07 | 0.97–2.22 | |||||||
| Highest HDI | 1.18 | 0.48 | 0.75–1.87 | |||||||
| Gender inequality index by state (GII) | ||||||||||
| Lowest GII | ||||||||||
| Low GII | 0.78 | 0.08 | 0.60–1.02 | |||||||
| Average GII | 1.32 | 0.06 | 0.99–1.77 | |||||||
| High GII | 1.01 | 0.96 | 0.78–1.29 | |||||||
| Highest GII | 1.30 | 0.11 | 0.94–1.79 | |||||||
| Region of residence | ||||||||||
| North-central | ||||||||||
| North-east | 0.68 | 0.03 | 0.49–0.96 | |||||||
| North-west | 1.19 | 0.36 | 0.82–1.74 | |||||||
| South-east | 1.60 | <0.001 | 1.17–2.18 | |||||||
| South-south | 1.63 | <0.001 | 1.17–2.25 | |||||||
| South-west | 1.66 | <0.001 | 1.19–2.30 | |||||||
| Type of place of residence | ||||||||||
| Urban | ||||||||||
| Rural | 1.29 | <0.001 | 1.13–1.46 | |||||||
| Variance components | ||||||||||
| Community level variance | 0.81 | 0.31 | 0.24–0.40 | 0.31 | 0.25–0.40 | 0.30 | 0.24–0.39 | |||
| State level variance | 0.47 | 0.08 | 0.04–0.14 | 0.09 | 0.05–0.16 | 0.02 | 0.01–0.05 | |||
| ICC at the community level | 0.28 | 0.11 | 0.09–0.13 | 0.11 | 0.09–0.13 | 0.09 | 0.07–0.11 | |||
| ICC at the state level | 0.10 | 0.02 | 0.01–0.04 | 0.02 | 0.1–0.04 | 0.004 | 0.00–0.01 | |||
| VPC at the community level | 0.18 | 0.08 | 0.08 | 0.08 | ||||||
| VPC at the state level | 0.10 | 0.02 | 0.02 | 0.004 | ||||||
| MOR at the community level | 2.36 | 1.70 | 1.60–1.83 | 1.70 | 1.61–1.83 | 1.69 | 1.60–1.81 | |||
| MOR at the state level | 1.92 | 1.31 | 1.21–1.43 | 1.33 | 1.24–1.46 | 1.14 | 1.10–1.24 | |||
| AIC | 19,609 | 18,553 | 18,535 | 18,519 | ||||||
| BIC | 19,638 | 18,909 | 18,912 | 19,027 | ||||||
| Log-likelihood | −9800 | −9227 | 9215 | −9189 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obasohan, P.E.; Walters, S.J.; Jacques, R.M.; Khatab, K. The Risk Factors Associated with the Prevalence of Multimorbidity of Anaemia, Malaria, and Malnutrition among Children Aged 6–59 Months in Nigeria. Int. J. Environ. Res. Public Health 2024, 21, 765. https://doi.org/10.3390/ijerph21060765
Obasohan PE, Walters SJ, Jacques RM, Khatab K. The Risk Factors Associated with the Prevalence of Multimorbidity of Anaemia, Malaria, and Malnutrition among Children Aged 6–59 Months in Nigeria. International Journal of Environmental Research and Public Health. 2024; 21(6):765. https://doi.org/10.3390/ijerph21060765
Chicago/Turabian StyleObasohan, Phillips Edomwonyi, Stephen J. Walters, Richard M. Jacques, and Khaled Khatab. 2024. "The Risk Factors Associated with the Prevalence of Multimorbidity of Anaemia, Malaria, and Malnutrition among Children Aged 6–59 Months in Nigeria" International Journal of Environmental Research and Public Health 21, no. 6: 765. https://doi.org/10.3390/ijerph21060765






