Removal of Bisphenol S (BPS) by Adsorption on Activated Carbons Commercialized in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BPS and BPA Stock Solutions
Study Waters
2.3. Quantification of BPS and BPA
2.4. Characterization of Adsorbent Materials
2.5. Experimental Setup
- Ultrapure water + BPS + BETM.
- Ultrapure water + BPS + COCO.
- Ultrapure water + BPS + SIAL.
- Ultrapure water + BPA + BETM.
- Ultrapure water + BPA + COCO.
- Ultrapure water + BPA + SIAL.
2.6. Adsorption Kinetics Experiments
2.7. Adsorption Equilibrium Experiments
3. Results
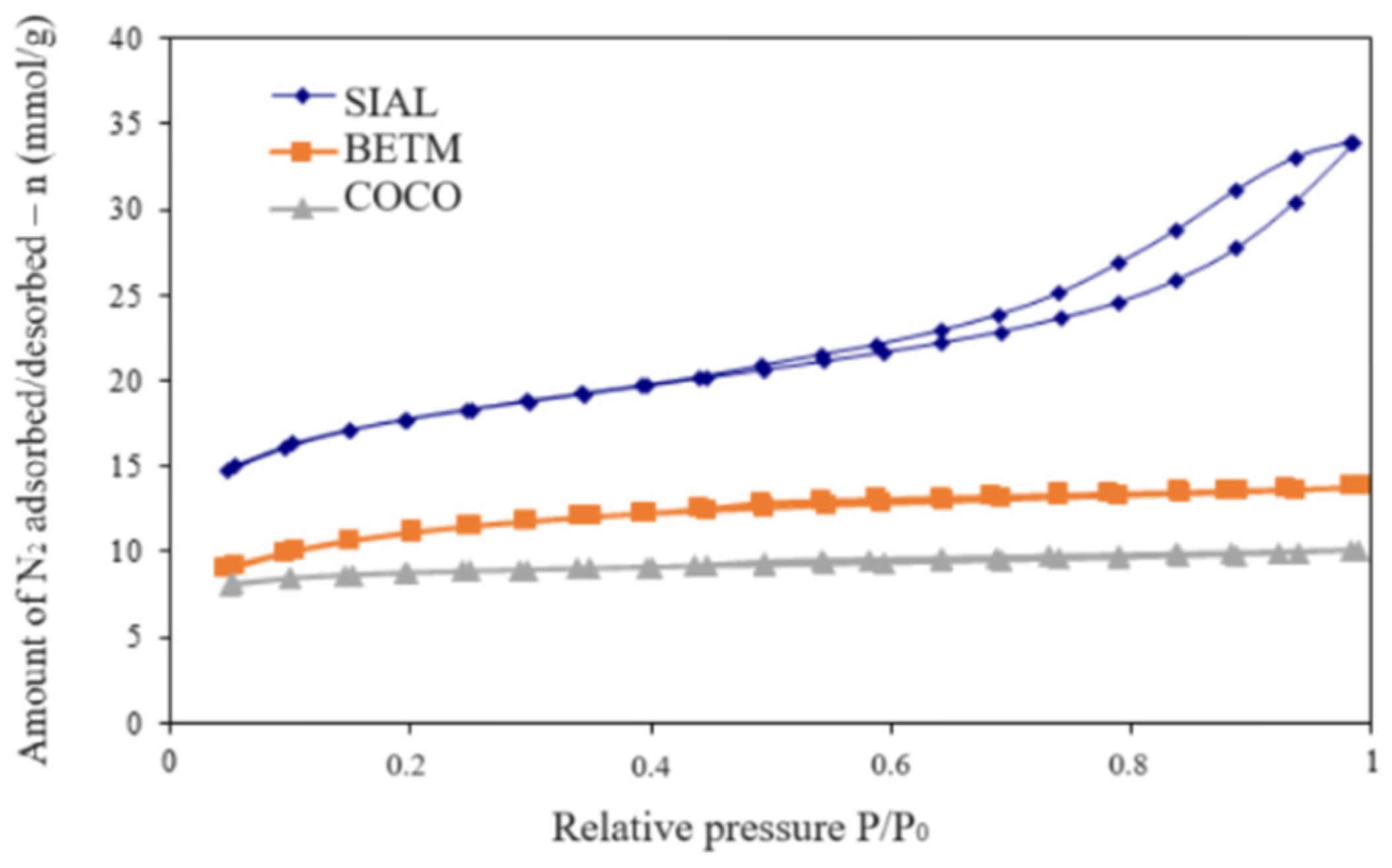
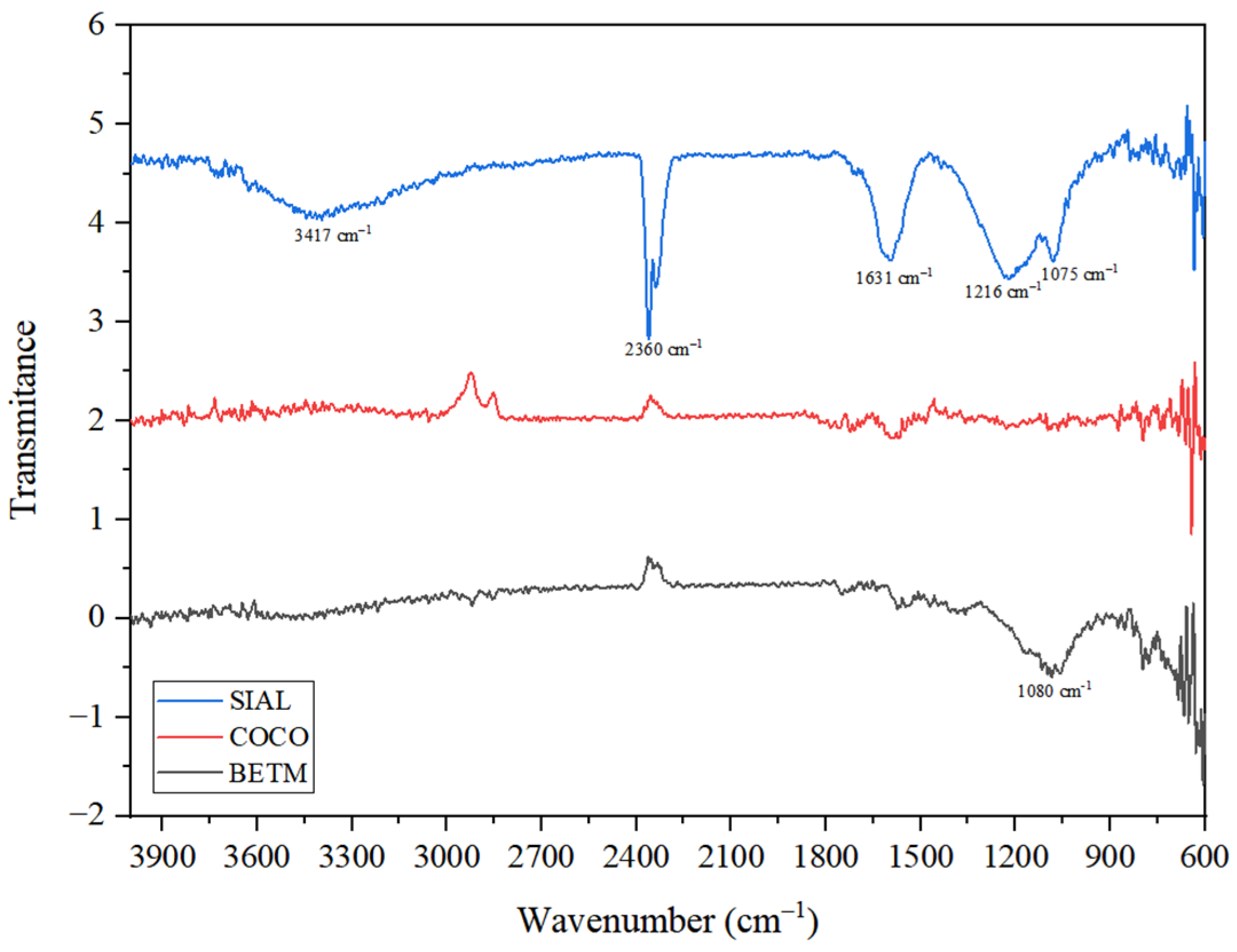
3.1. Characterization of the Adsorbent Materials
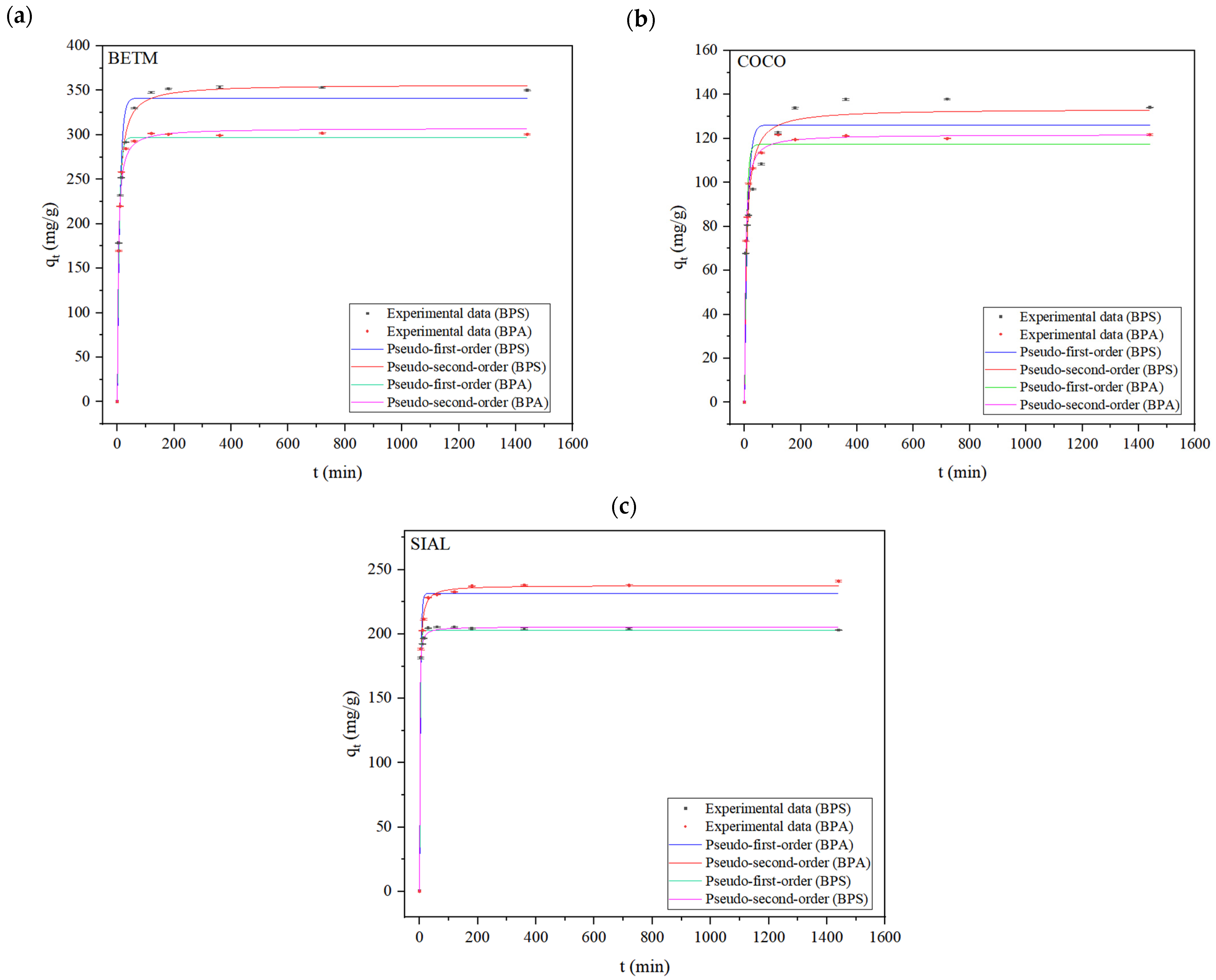
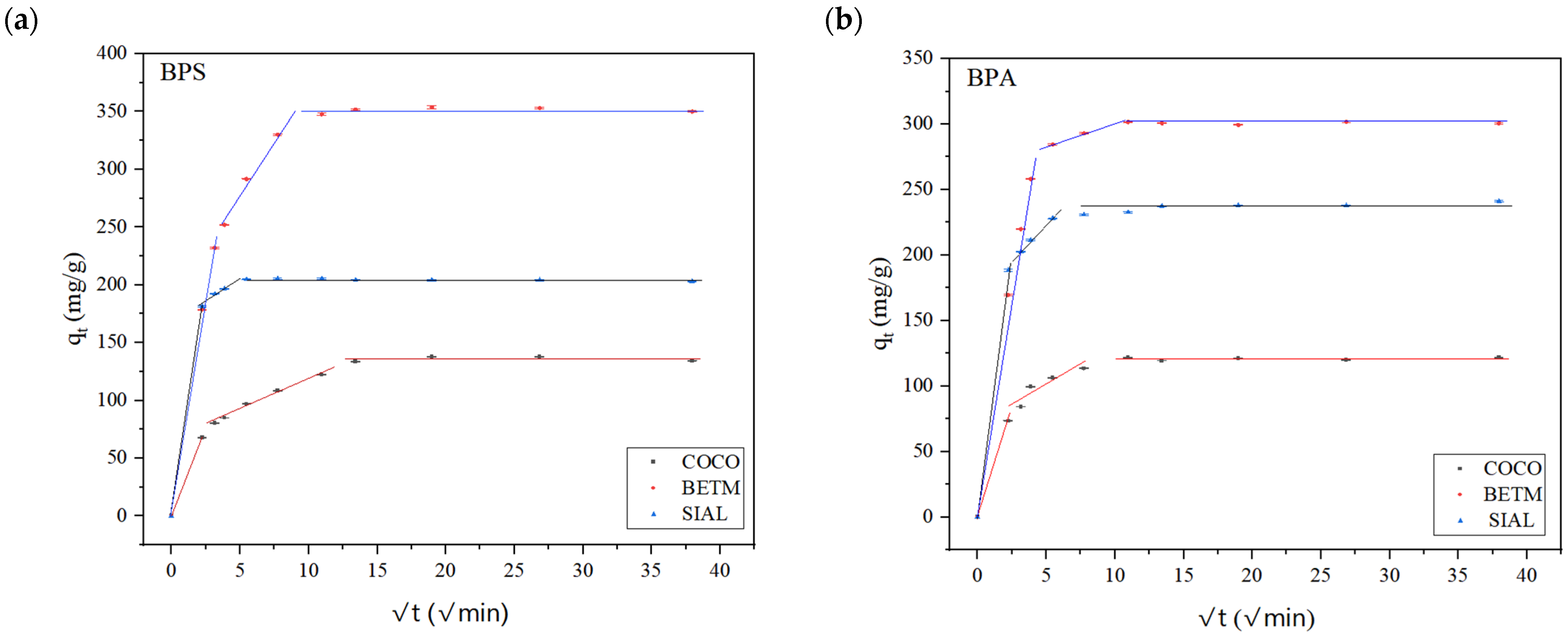
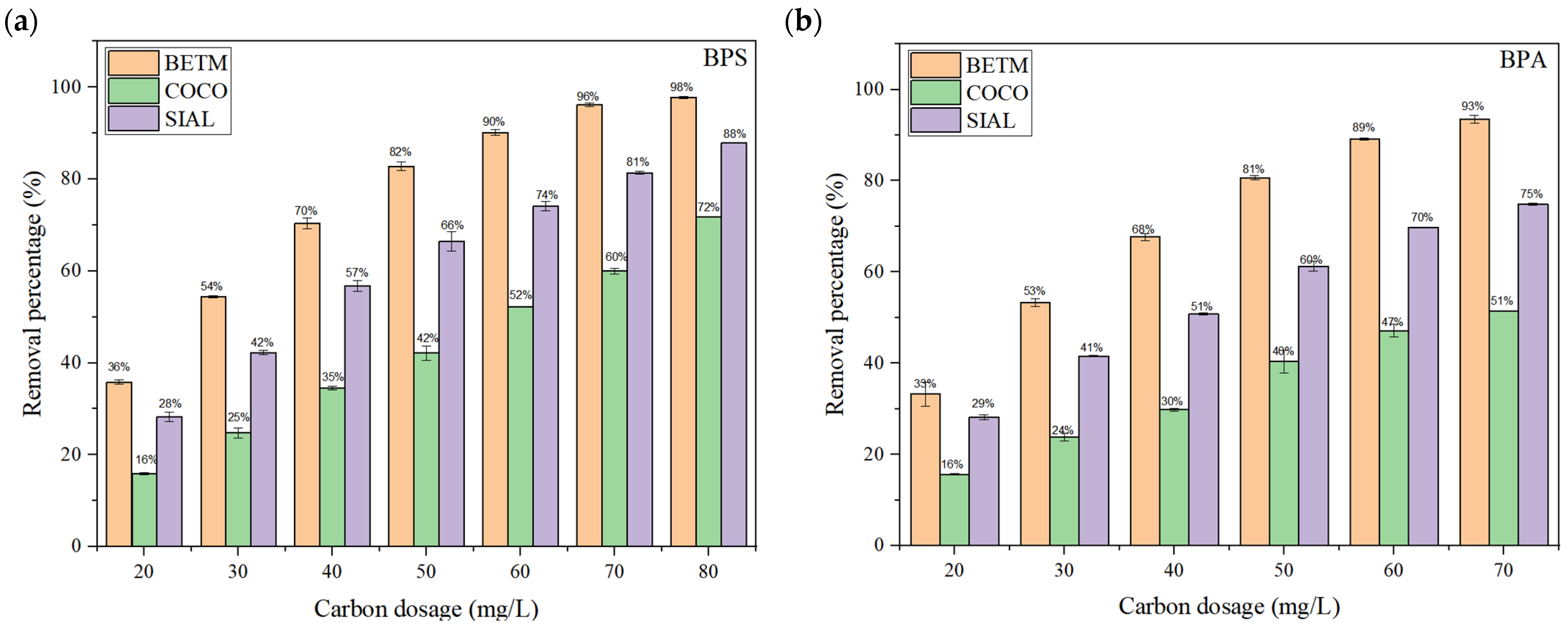
3.2. Adsorption Kinetics Experiments
3.3. Adsorption Equilibrium Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drinking Water. Available online: https://environment.ec.europa.eu/topics/water/drinking-water_en (accessed on 15 May 2024).
- Guo, Y.; Chen, L.; Wu, J.; Hua, J.; Yang, L.; Wang, Q.; Zhang, W.; Lee, J.; Zhou, B. Parental co-exposure to bisphenol A and nano-TiO2 causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring. Sci. Total Environ. 2019, 650, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Malaise, Y.; Lencina, C.; Cartier, C.; Olier, M.; Menard, S.; Guzylack-Piriou, L. Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice. Environ. Health 2020, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Luo, J.; Sun, J.; Ge, H.; Wang, Z. Associations of urinary bisphenol A and its alternatives bisphenol S and F concentrations with depressive symptoms among adults. Chemosphere 2021, 279, 130573. [Google Scholar] [CrossRef] [PubMed]
- Bisphenols. Available online: https://www.hbm4eu.eu/hbm4eu-substances/bisphenols/ (accessed on 10 December 2023).
- Narevski, A.C.; Novakovic, M.I.; Petrovic, M.Z.; Mihajlovic, I.J.; Maodu, N.B.; Vujic, G.V. Occurrence of bisphenol A and microplastics in landfill leachate: Lessons from South East Europe. Environ. Sci. Pollut. Res. 2021, 28, 42196–42203. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C. Microextração Líquido-Líquido Dispersiva na Determinação de Bisfenóis (A, S, F, AF) e de Benzofenona em Amostras Aquosas Reais e Complexas: Desenho Experimental (DoE) e Otimização de Processos. Master’s Thesis, Rio de Janeiro State University, Rio de Janeiro, Brazil, 2020. [Google Scholar]
- González, N.; Souza, M.C.O.; Cezarette, G.N.; Rocha, B.A.; Devoz, P.P.; Santos, L.C.; Barcelos, G.R.M.; Nadal, M.; Domingo, J.L.; Barbosa, F., Jr. Evaluation of exposure to multiple organic pollutants in riparian communities of the Brazilian Amazon: Screening levels and potential health risk. Sci. Total Environ. 2024, 908, 168294. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.S. Determinação de Contaminantes Emergentes em Mananciais de Água Bruta e na Água Para Consumo Humano do Distrito Federal. Master’s Thesis, University of Brasília, Brasília, Brazil, 2013. [Google Scholar]
- Costa, N.Y.M. Estudo Geoquímico e de Contaminantes Emergentes na Bacia do Lago Paranoá. Master’s Thesis, University of Brasília, Brasília, Brazil, 2014. [Google Scholar]
- Silva, S.R. Quantificação de Substâncias com Atividade Estrogênica e Caracterização da Toxicidade Embriolarval em Zebrafish (Danio Rerio) nos Efluentes das Estações de Tratamento de Esgotos Sul e Norte e no Lago Paranoá, Brasília-DF. Master’s Thesis, University of Brasília, Brasília, Brazil, 2015. [Google Scholar]
- Moura, H.S.R.P. Determinação de Micropoluentes de Preocupação Emergente em Amostras de Origem Biológica e Ambiental. Ph.D. Thesis, University of Brasília, Brasília, Brazil, 2022. [Google Scholar]
- European Comission. Directive (EU) 2020/2184 on the Quality of Water Intended for Human Consumption (Recast); European Commision: Brussels, Belgium, 2020. [Google Scholar]
- Global Bisphenol S Market Analysis: Plant Capacity, Location, Production, Operating Efficiency, Demand & Supply, End Use, Regional Demand, Company Share, Sales Channel, Manufacturing Process, Industry Market Size, 2015–2032. Available online: https://www.researchandmarkets.com/report/bisphenol?utm_source=GNE&utm_medium=PressRelease&utm_code=286v87&utm_campaign=1907371+-+Global+Bisphenol+S+Market+Analysis%3a+Growth%2c+Demand+%26+Supply+and+Industry+Trends+2015-2032&utm_exec=cari18prd (accessed on 5 January 2024).
- Martín-Lara, M.A.; Calero, M.; Ronda, A.; Iáñez-Rodríguez, I.; Escudero, C. Adsorptive Behavior of an Activated Carbon for Bisphenol A Removal in Single and Binary (Bisphenol A—Heavy Metal) Solutions. Water 2020, 12, 2150. [Google Scholar] [CrossRef]
- Duranceau, S.J.; Taylor, J.S.; Alexander, A. Membranes. In Water Quality & Treatment, 6th ed.; Edzwald, J.K., Ed.; American Water Works Association: Denver, CO, USA, 2011; pp. 11.1–11.106. [Google Scholar]
- Clifford, D.; Sorg, T.J.; Ghurye, G.L. Ion Exchange and Adsorption of Inorganic Contaminants. In Water Quality & Treatment, 6th ed.; Edzwald, J.K., Ed.; American Water Works Association: Denver, CO, USA, 2011; pp. 12.1–12.97. [Google Scholar]
- Summers, R.S.; Knappe, D.R.U.; Snoeyink, V.L. Adsorption of Organic Compounds by Activated Carbon. In Water Quality & Treatment, 6th ed.; Edzwald, J.K., Ed.; American Water Works Association: Denver, CO, USA, 2011; pp. 14.1–14.105. [Google Scholar]
- Linden, K.G.; Rosenfeldt, E.J. Ultraviolet Light Process. In Water Quality & Treatment, 6th ed.; Edzwald, J.K., Ed.; American Water Works Association: Denver, CO, USA, 2011; pp. 18.1–18.45. [Google Scholar]
- Phenol, 4,4′-sulfonylbis-(BPS). Available online: https://www.industrialchemicals.gov.au/sites/default/files/2022-06/EVA00053%20-%20Evaluation%20statement%20-%2030%20June%202022.pdf (accessed on 15 January 2024).
- Bisphenol A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol-A (accessed on 15 January 2024).
- Bisphenol S. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6626 (accessed on 15 January 2024).
- Zhang, X.; Han, X.; Chang, C.; Pan, L.; Li, H.; Xu, C.C. Bisphenol S adsorption with activated carbon prepared from corncob: Optimization using response surface methodology. Int. J. Chem. Eng. 2020, 18, 20190202. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Vogel, D.; Khan, S. Characterizing humic acid fouling of nanofiltration membranes using bisphenol A as a molecular indicator. Water Res. 2008, 42, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.A.F.O. Elaboração de Carvão Magnético Para Remoção de Bisfenol A em Águas Contaminadas. Master’s Thesis, University of Brasília, Brasília, Brazil, 2016. [Google Scholar]
- Cao, G.; Zhuang, Y.; Liu, B. Simultaneous Determination of Bisphenol A and Bisphenol S in Environmental Water using Ratio Derivative Ultraviolet Spectrometry. S. Afr. J. Chem. 2014, 67, 99–103. [Google Scholar]
- NBR 12073; Associação Brasileira de Normas Técnicas. Carvão Ativado Pulverizado—Determinação do Número de Iodo. ABNT: Rio de Janeiro, Brazil, 1991; 4p.
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; p. 536. [Google Scholar]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford Universty Press: New York, NY, USA, 2012; p. 1261. [Google Scholar]
- Moreno-Castilla, C.; Lopez-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L. Comparison between Linear and Non-Linear Forms of Pseudo-First-Order and Pseudo-Second-Order Adsorption Kinetic Models for the Removal of Methylene Blue by Activated Carbon. Front. Environ. Sci. Eng. 2009, 3, 320–324. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Hong, X.; Yang, H.; Fang, D.; Wang, Y.; Lei, M. Green Synthesis and Optimization of 3D Nitrogen-Doped Carbon Network via Biomass Waste for Highly Efficient Bisphenol S Adsorption. ChemistrySelect 2021, 6, 6348–6352. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Liu, L.; Ju, M. Study on the long-term effects of DOM on the adsorption of BPS by biochar. Chemosphere 2020, 242, 125165. [Google Scholar] [CrossRef]
- Shao, F.; Xu, J.; Jing, Y.; Zhao, C.; Zhu, X.; Lu, C.; Fu, Y.; Zhang, J.; Mu, R. Pyrolytic utilization of a typical halophyte: Suaeda glauca—The excellent adsorbent raw material for bisphenol S removal. Biomass Convers. Biorefin. 2022, 14, 8041–8055. [Google Scholar] [CrossRef]
- Aziz, A.; Yusop, M.F.M.; Ahmad, M.A. Removal of Bisphenol S from Aqueous Solution using Activated Carbon Derived from Rambutan Peel via Microwave Irradiation Technique. Sains Malays. 2022, 51, 3967–3980. [Google Scholar] [CrossRef]
- Liu, C.; Liang, L.; Han, X.; Chang, C.; Chen, J.; Song, J. Optimized preparation of activated carbon from furfural residue using response surface methodology and its application for bisphenol S adsorption. Water Sci. Technol. 2022, 85, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Zhao, R.; Jiang, J. Removal of bisphenol S from drinking water by adsorption using activated carbon and the mechanisms involved. Int. J. Environ. Sci. Technol. 2022, 19, 5289–5300. [Google Scholar] [CrossRef]
- Couto, J.M.S.; Souza, A.L.; Machado, C.R.A.; Almeida, R.; Salomão, A.L.S.; Campos, J.C. Adsorption of Bisphenol S from aqueous solution on powdered activated carbon and chronic toxicity evaluation with microcrustacean Ceriodaphnia dubia. J. Water Process Eng. 2020, 37, 101490. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Zheng, L.; Bao, S.; Liu, Y.; Fang, T.; Xing, B. Biomass-derived N/S dual-doped hierarchically porous carbon material as effective adsorbent for the removal of bisphenol F and bisphenol S. J. Hazard. Mater. 2021, 416, 126126. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Ongen, A.; Ozcan, H.K.; Aydin, S. Modelling of adsorption Kinetic Processes—Erros, Therory and Application. In Advanced Sorption Process Applications, 1st ed.; Edebali, S., Ed.; IntechOpen: London, UK, 2018; pp. 187–206. [Google Scholar]
- Cooney, D.O. Kinectics of Adsorption with Granular Adsorbets. In Adsorption Design for Wastewater Treatment, 1st ed.; Mogck, M., Ed.; CRC Press LLC: Boca Raton, FL, USA, 1999; pp. 65–89. [Google Scholar]
- Fierro, V.; Torné-Fernández, V.; Montané, D.; Celzard, A. Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Wirasnita, R.; Hadibarata, T.; Yusoff, A.R.M.; Yusop, Z. Removal of Bisphenol A from Aqueous Solution by Activated Carbon Derived from Oil Palm Empty Fruit Bunch. Water Air Soil Pollut. 2014, 225, 2148. [Google Scholar] [CrossRef]


| Molecular Formula | Molar Mass (g/mol) | Molecular Diameter (nm) | Log Kow | pKa | Water Solubility (mg/L) |
|---|---|---|---|---|---|
| C12H10O4S | 250.27 | 0.625 | 1.2 | 8 | 715 |
| C15H16O2 | 228.29 | 1.068 | 3.32 | 9.6 | 300 |
| Activated Carbon | SW1 (15 mg/L of BPS) | SW2 (15 mg/L of BPS) |
|---|---|---|
| BETM | 10; 20; 30; 40; 50; 70; and 80 | 5; 10; 20; 30; 40; 50; and 70 |
| COCO | 20; 30; 40; 50; 70; 80; 90; and 100 | 20; 30; 40; 50; 70; 80; 90; 100; and 110 |
| SIAL | 20; 30; 40; 50; 70; 80; and 90 | 10; 20; 30; 40; 50; 70; 80; 90; and 100 |
| Model | Name | Nonlinear Equation | Parameters |
|---|---|---|---|
| Isotherm | Langmuir | Ce: Concentration of bisphenol at equilibrium (mg/L) qe: Amount of adsorbate adsorbed per unit mass of adsorbent at equilibrium (mg/g) : Theoretical maximum adsorption capacity (mg/g) KL: Langmuir constant related to the rate of adsorption n and KF: Freundlich constants | |
| Freundlich | |||
| Kinetics | Pseudo-first-order | qt: Amount of adsorbate adsorbed per unit mass of adsorbent at time t constant (mg/g) k1: Pseudo-first-order rate constant k2: Second-order rate constant kp: Intraparticle diffusion rate constant C: Intraparticle diffusion rate constant F: Fraction between qt and qe Bt: Mathematical function of F | |
| Pseudo-second-order | |||
| Intraparticle diffusion | |||
| Boyd |
| Activated Carbon | Surface Area BET (m2/g) | V0.95 (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | pH | pHPZC |
|---|---|---|---|---|---|---|
| BETM | 880 | 0.473 | 0.397 | 0.076 | 9.60 | 9.06 |
| COCO | 673 | 0.345 | 0.314 | 0.031 | 10.00 | 9.97 |
| SIAL | 1397 | 1.054 | 0.632 | 0.423 | 3.67 | 3.08 |
| BPS | BPA | ||||||
|---|---|---|---|---|---|---|---|
| Kinetic Model | Parameter | BETM | COCO | SIAL | BETM | COCO | SIAL |
| Pseudo-first-order (PFO) | qe (exp) (mg/g) | 351.23 | 133.24 | 204.21 | 300.80 | 120.84 | 237.41 |
| qe (calc) (mg/g) | 341.42 ± 8.00 | 133.62 ± 5.01 | 202.80 ± 1.27 | 297.12 ± 2.87 | 117.34 ± 2.47 | 231.63 ± 3.54 | |
| k1 (min−1) | 0.1102 ± 1.25 × 10−2 | 0.1119 ± 2.04 × 10−2 | 0.4314 ± 3.30 × 10−2 | 0.1474 ± 7.6 × 10−3 | 0.1499 ± 1.6 × 10−4 | 0.2927 ± 3.65 × 10−2 | |
| R2 | 0.9685 | 0.9205 | 0.9967 | 0.9940 | 0.9713 | 0.9816 | |
| SQR | 37.71 | 14.85 | 1.24 | 5.15 | 3.82 | 8.97 | |
| Pseudo-second-order (PSO) | qe (calc) (mg/g) | 356.80 ± 2.93 | 140.70 ± 3.03 | 205.57 ± 0.71 | 307.51 ± 2.96 | 121.84 ± 1.09 | 237.84 ± 1.33 |
| k2 (mg/g/min) | 5.11 × 10−4 ± 3.00 × 10−5 | 1.24 × 10−3 ± 1.87 × 10−4 | 7.45 × 10−3 ± 6.42 × 10−4 | 8.86 × 10−4 ± 7.10 × 10−5 | 2.22 × 10−3 ± 1.65 × 10−4 | 2.84 × 10−3 ± 2.17 × 10−4 | |
| R2 | 0.9968 | 0.9785 | 0.9992 | 0.9951 | 0.9958 | 0.9981 | |
| SQR | 0.42 | 0.45 | 0.28 | 0.41 | 0.56 | 0.95 | |
| Intraparticle Diffusion Weber–Morris | C1 | 2.71 ± 11.32 | 2.00 ± 8.37 | 0.00 | 5.80 ± 11.17 | 4.42 ± 8.76 | 0.00 |
| 74.56 ± 5.06 | 26.35 ± 3.74 | 81.13 | 67.33 ± 4.08 | 25.83 ± 3.20 | 84.28 | ||
| R21 | 0.9954 | 0.9802 | 1 | 0.9927 | 0.9702 | 1 | |
| C2 | 197.39 ± 17.57 | 66.30 ± 1.99 | 177.71 ± 5.84 | 229.16 ± 20.92 | 88.60 ± 1.86 | 163.15 ± 3.34 | |
| 14.94 ± 2.56 | 5.14 ± 0.24 | 4.09 ± 1.19 | 8.65 ± 3.54 | 3.09 ± 0.25 | 12.08 ± 0.86 | ||
| R22 | 0.9189 | 0.9914 | 0.7971 | 0.8568 | 0.9873 | 0.9899 | |
| C3 | 350.56 | 136.36 ± 3.86 | 205.86 ± 0.35 | 296.78 ± 2.84 | 120.36 ± 1.28 | 229.02 ± 1.74 | |
| 0.03 ± 0.13 | −0.02 ± 0.15 | −0.08 ± 0.02 | 0.14 ± 0.13 | 0.02 ± 0.05 | 0.35 ± 0.09 | ||
| R23 | 0.0213 | 0.0075 | 0.8436 | 0.2259 | 0.0527 | 0.7752 | |
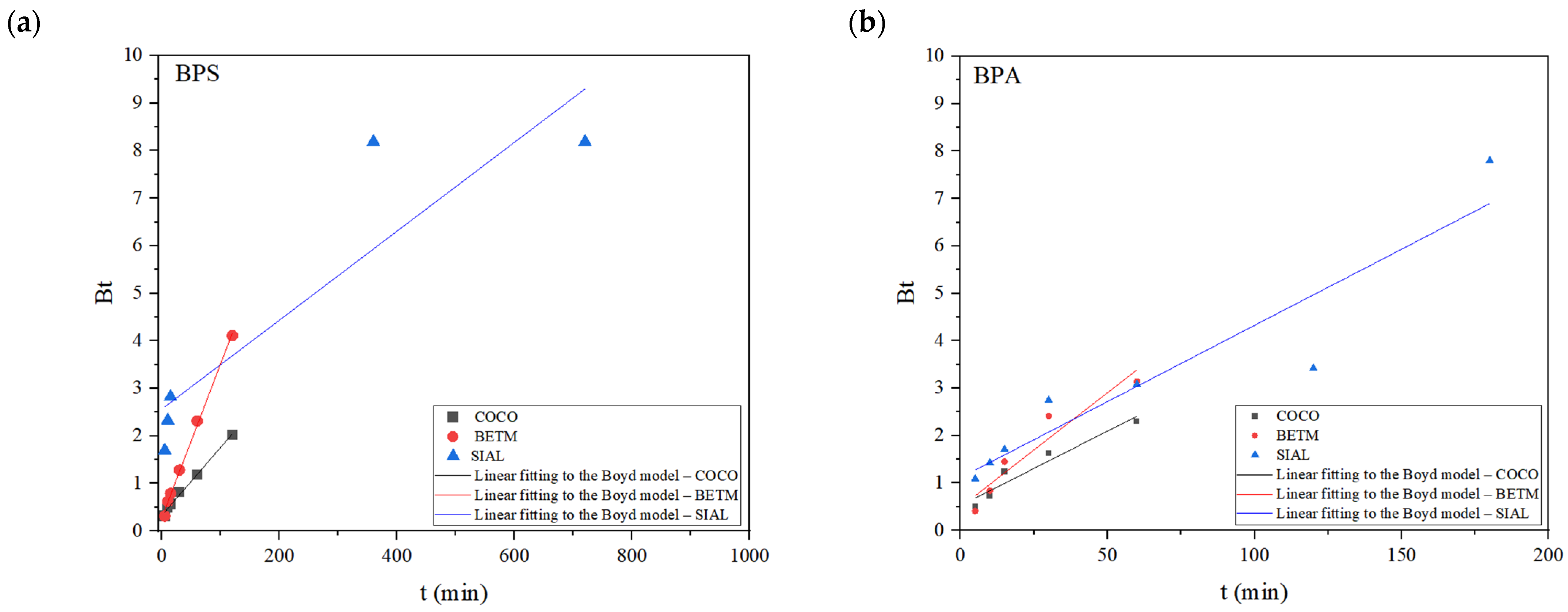
| Boyd | Angular coefficient (B) | 0.03 ± 9.0 × 10−4 | 0.01 ± 6.0 × 10−4 | 0.01 ± 2.5 × 10−3 | 0.05 ± 8.9 × 10−3 | 0.03 ± 4.9 × 10−3 | 0.03 ± 5.3 × 10−3 |
| Linear coefficient | 0.28 ± 4.9 × 10−2 | 0.33 ± 3.3 × 10−2 | 2.57 ± 0.89 | 0.49 ± 0.28 | 0.52 ± 0.15 | 1.11 ± 0.46 | |
| R2 | 0.9972 | 0.9936 | 0.8285 | 0.9075 | 0.9312 | 0.8805 | |
| Model | Adsorbent | Surface Area BET (m2/g) | [BPS]0 (mg/L) | Carbon Dosage (mg/L) | k2 (mg.g−1 min−1) | qe (exp) (mg.g−1) | qe (calc) (mg.g−1) | R2 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pseudo-second-order | CCAC | 722.22 | 10–90 | 200–600 | 3.74 × 10−3 | 181.98 | 182.13 | 0.9954 | [23] |
| PAC C | 726.68 | 20–60 | 2 | 1.3 × 10−6 | 52.2 | 55.00 | 0.9900 | [40] | |
| ACSG | 1339 | 20–60 | 1000 | 1.12 × 10−3 | 338 | 334.33 | 0.9996 | [36] | |
| BETM | 880 | 15 | 30 | 5.11 × 10−4 | 351.23 | 356.80 | 0.9968 | This study | |
| COCO | 673 | 15 | 50 | 1.24 × 10−3 | 133.24 | 140.70 | 0.9785 | This study | |
| SIAL | 1397 | 15 | 30 | 7.45 × 10−3 | 204.21 | 205.57 | 0.9992 | This study |
| BPS | BPA | ||||||
|---|---|---|---|---|---|---|---|
| Isotherm Models | Parameter | BETM | COCO | SIAL | BETM | COCO | SIAL |
| qmax (exp) (mg/g) | 265.83 | 129.31 | 231.12 | 264.73 | 122.49 | 215.94 | |
| Langmuir | Q0max (mg/g) | 260.62 ± 4.84 | 136.51 ± 3.46 | 248.25 ± 4.49 | 264.64 ± 4.19 | 150.03 ± 4.36 | 231.20 ± 3.44 |
| kL (L/mg) | 5.79 ± 0.89 | 0.92 ± 0.14 | 1.06 ± 0.09 | 2.01 ± 0.24 | 0.37 ± 0.04 | 0.87 ± 0.07 | |
| R2 | 0.9887 | 0.9906 | 0.9959 | 0.9940 | 0.9933 | 0.9958 | |
| SQR | 6.3 | 1.13 | 1.24 | 3.24 | 0.84 | 1.58 | |
| RL | 0.0110 | 0.0657 | 0.0574 | 0.0315 | 0.1503 | 0.0656 | |
| Freundlich | kF (mg/g)/(mg/L)n | 208.68 ± 2.99 | 83.17 ± 3.78 | 147.53 ± 3.22 | 188.61 ± 4.55 | 60.38 ± 4.05 | 132.27 ± 3.20 |
| n | 0.11 ± 8.3 × 10−3 | 0.17 ± 2.2 × 10−2 | 0.20 ± 1.3 × 10−2 | 0.13 ± 1.2 × 10−2 | 0.29 ± 3.2 × 10−2 | 0.20 ± 1.2 × 10−2 | |
| R2 | 0.9674 | 0.8998 | 0.9774 | 0.9515 | 0.9209 | 0.9706 | |
| SQR | 8.4 | 1.20 | 1.27 | 6.82 | 1.69 | 1.80 | |
| Bisphenol | Adsorbent | kL (L.mg−1) | Q0max (mg.g−1) | R2 | Reference |
|---|---|---|---|---|---|
| BPS | CCAC | 0.0086 | 617.29 | 0.9983 | [23] |
| BPS | CAP | 1.39 | 83.19 | 0.9820 | [39] |
| BPA | AC-40 | 0.64 | 91.90 | 0.9960 | [15] |
| BPA | EFB | 0.51 | 41.98 | 0.9985 | [47] |
| BPS | BETM | 5.79 | 260.62 | 0.9887 | This study |
| BPS | COCO | 0.92 | 136.51 | 0.9906 | This study |
| BPS | SIAL | 1.06 | 248.25 | 0.9959 | This study |
| BPA | BETM | 2.01 | 264.64 | 0.9940 | This study |
| BPA | COCO | 0.37 | 150.03 | 0.9933 | This study |
| BPA | SIAL | 0.87 | 231.20 | 0.9958 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, N.d.S.; Ginoris, Y.P.; Ratnaweera, H. Removal of Bisphenol S (BPS) by Adsorption on Activated Carbons Commercialized in Brazil. Int. J. Environ. Res. Public Health 2024, 21, 792. https://doi.org/10.3390/ijerph21060792
Oliveira NdS, Ginoris YP, Ratnaweera H. Removal of Bisphenol S (BPS) by Adsorption on Activated Carbons Commercialized in Brazil. International Journal of Environmental Research and Public Health. 2024; 21(6):792. https://doi.org/10.3390/ijerph21060792
Chicago/Turabian StyleOliveira, Nayara dos Santos, Yovanka Perez Ginoris, and Harsha Ratnaweera. 2024. "Removal of Bisphenol S (BPS) by Adsorption on Activated Carbons Commercialized in Brazil" International Journal of Environmental Research and Public Health 21, no. 6: 792. https://doi.org/10.3390/ijerph21060792
APA StyleOliveira, N. d. S., Ginoris, Y. P., & Ratnaweera, H. (2024). Removal of Bisphenol S (BPS) by Adsorption on Activated Carbons Commercialized in Brazil. International Journal of Environmental Research and Public Health, 21(6), 792. https://doi.org/10.3390/ijerph21060792







