Assessing the Impact of Non-Exhaust Emissions on the Asthmatic Airway (IONA) Protocol for a Randomised Three-Exposure Crossover Study
Abstract
:1. Introduction
2. Methods and Analysis
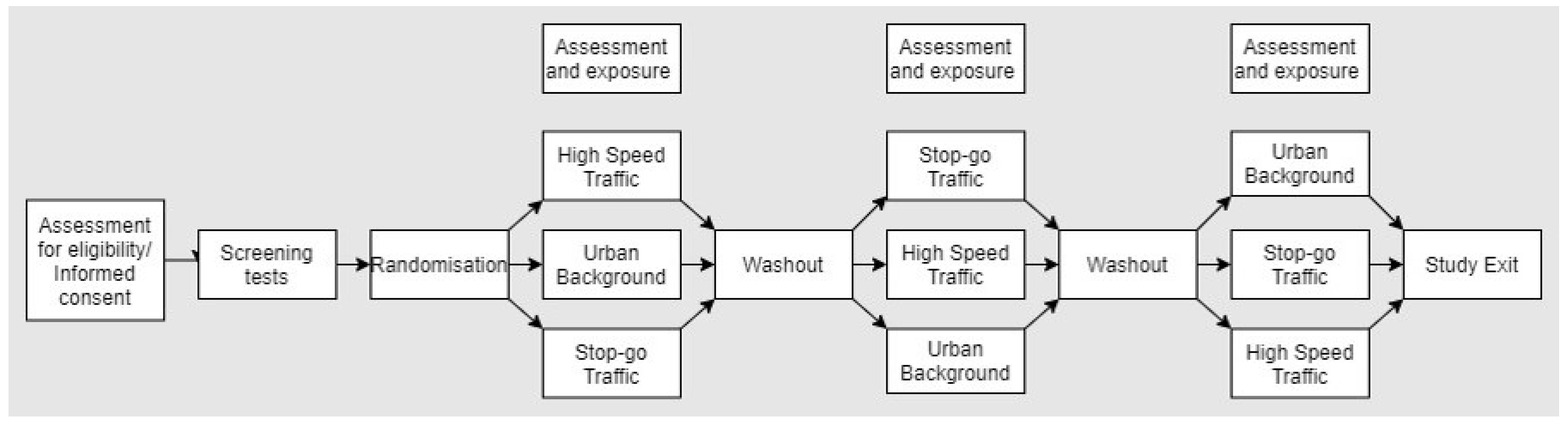
2.1. Study Design
2.2. Aims/Outcomes
2.2.1. Primary
- To compare the acute health effects (changes in lung function and airway inflammation) in mild/moderate asthmatics of exposure to PM2.5 and PM10 at three selected microenvironments, with contrasting contributions from brake wear and tyre and road wear (TRWP).
2.2.2. Secondary
- The provision of an air pollutant database (PM2.5 and PM10 mass and chemical composition, PNC, and NO2) and a time series of source-apportioned PM2.5 and PM10, covering all exposure days at the three selected sites.
- To examine the relationship between variations in daily non-exhaust source fractions derived from brake and TRWP, with the deposited dose, based on metal/metalloid concentrations determined in the nasal airways, relating these measures of biological doses to the physiologic and immunologic responses observed.
- To establish a biobank of plasma, nasal lavage, and urine samples for future work examining molecular signatures of exposure and responses to NEEs.
2.2.3. Outcome Measures
- Primary: Lung function as measured by the forced expiratory volume in one second (FEV1); this measure has been selected as the primary outcome based upon previous work showing the detrimental effects of the time spent in an urban atmosphere on lung function [22].
- Secondary: Spirometry (FVC, FVC/FEV1 ratio), Fractional Expired Nitric Oxide (FeNO), oscillometry (R5, R20, T5, T20, AX), asthma symptoms, nasal mucosal immune responses.
- Biobanks: urine and blood plasma samples will be collected for future analysis.
2.3. Study Interventions
- (1)
- A busy road junction characterised by stop–go traffic to enhance emissions from brake wear;
- (2)
- High speed continuous traffic to enhance TRWP emissions;
- (3)
- An urban background location away from nearby traffic sources.
2.3.1. Exposure Site Selection
2.3.2. Sample Size
2.3.3. Recruitment
Primary Care
Non-Primary Care
2.3.4. Patient and Public Involvement
2.4. Preliminary Assessment:
2.5. Estimated VO2max Fitness Assessment
2.6. Exposure
2.7. Data Collection before and after Exposure
2.8. Respiratory Function Assessment:
2.8.1. Spirometry
2.8.2. Fractional Exhaled Nitric Oxide
2.8.3. Oscillometry
2.9. Sample Collection
2.9.1. Venepuncture
2.9.2. Nasal Lavage
2.9.3. Urine Samples
2.10. Pre-Exposure Personal Air Pollution Monitoring:
2.11. Exercise Intensity
2.12. Common Allergy Assessment
2.13. Symptoms Surveys
2.14. Air Pollution Measurements and Characterisation of Non-Exhaust Emissions
2.15. Source Apportionment
3. Data Analysis
3.1. Statistical Methods
3.2. Ethics, Safety, and Dissemination
3.3. Governance
3.4. Consent
3.5. Data Management
3.6. Confidentiality
3.7. Record Retention and Archiving
3.8. Adverse Events Reporting
3.9. Dissemination Plan and Project Outputs
- (1)
- Webinars on websites of our institutions to provide more detailed summaries of results, with downloads of key documents.
- (2)
- Presentations, especially to London organisations including the GLA, councils, and health and wellbeing boards.
- (3)
- Peer-reviewed publications.
- (4)
- Presentations at national and international conferences.
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdollahi, M.; Abedi, P.; Abedi, A.; Abolhassani, H.; Aboyans, V.; et al. Five insights from the global burden of disease study 2019. Lancet 2020, 396, 1135–1159. [Google Scholar] [CrossRef]
- Su, J.G.; Barrett, M.A.; Combs, V.; Henderson, K.; Van Sickle, D.; Hogg, C.; Simrall, G.; Moyer, S.S.; Tarini, P.; Wojcik, O.; et al. Identifying impacts of air pollution on subacute asthma symptoms using digital medication sensors. Int. J. Epidemiol. 2022, 51, 213–224. [Google Scholar] [CrossRef]
- Delamater, P.L.; Finley, A.O.; Banerjee, S. An analysis of asthma hospitalizations, air pollution, and weather conditions in Los Angeles County, California. Sci. Total Environ. 2012, 425, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, M.; Analitis, A.; Whitney, D.; Samoli, E.; Zafeiratou, S.; Atkinson, R.; Dimakopoulou, K.; Beavers, S.; Schwartz, J.; Katsouyanni, K.; et al. STEAM project research group. Spatio-temporal associations of air pollutant concentrations, GP respiratory consultations and respiratory inhaler prescriptions: A 5-year study of primary care in the borough of Lambeth, South London. Environ. Health 2021, 20, 54. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Zhang, H.; Shi, C.; Li, G.; Peng, Z.; Ma, J.; Zhou, Y.; Zhang, L. Short-term exposure to ambient air pollution and asthma mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef]
- HEI Panel on the Health Effects of Long-Term Exposure to Traffic-Related Air Pollution. Systematic Review and Meta-Analysis of Selected Health Effects of Long-Term Exposure to Traffic-Related Air Pollution; Special Report 23; Health Effects Institute: Boston, MA, USA, 2022. [Google Scholar]
- Boogaard, H.; Patton, A.P.; Atkinson, R.W.; Brook, J.R.; Chang, H.H.; Crouse, D.L.; Fussell, J.C.; Hoek, G.; Hoffmann, B.; Kappeler, R.; et al. Long-term exposure to traffic-related air pollution and selected health outcomes: A systematic review and meta-analysis. Environ. Int. 2022, 164, 107262. [Google Scholar] [CrossRef]
- Janssen, N.A.; Hoek, G.; Simic-Lawson, M.; Fischer, P.; Van Bree, L.; Ten Brink, H.; Keuken, M.; Atkinson, R.W.; Anderson, H.R.; Brunekreef, B.; et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Fussell, J.C.; Franklin, M.; Green, D.C.; Gustafsson, M.; Harrison, R.M.; Hicks, W.; Kelly, F.J.; Kishta, F.; Miller, M.R.; Mudway, I.S.; et al. A Review of road traffic-derived non-exhaust particles: Emissions, physicochemical characteristics, health risks, and mitigation measures. Environ. Sci. Technol. 2022, 56, 6813–6835. [Google Scholar] [CrossRef] [PubMed]
- Piscitello, A.; Bianco, C.; Casasso, A.; Sethi, R. Non-exhaust traffic emissions: Sources, characterization, and mitigation measures. Sci. Total Environ. 2021, 766, 144440. [Google Scholar] [CrossRef]
- Air Quality Expert Group (AQEG). Non-Exhaust Emissions from Road Traffic. 2019. Available online: https://uk-air.defra.gov.uk/library/aqeg/publications (accessed on 1 March 2023).
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef]
- Orellano, P.; Quaranta, N.; Reynoso, J.; Balbi, B.; Vasquez, J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS ONE 2017, 12, e0174050. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Ding, H.; Jiang, L.N.; Chen, S.W.; Zheng, J.P.; Qiu, M.; Zhou, Y.X.; Chen, Q.; Guan, W.J. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef]
- Lippmann, M.; Chen, L.-C.; Gordon, T.; Ito, K.; Thurston, G.D. National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components; Research Report 177; Health Effects Institute: Boston, MA, USA, 2013. [Google Scholar]
- Steenhof, M.; Janssen, N.A.H.; Strak, M.; Hoek, G.; Gosens, I.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Pieters, R.H.H.; Cassee, F.R.; et al. Air pollution exposure affects circulating white blood cell counts in healthy subjects: The role of particle composition, oxidative potential and gaseous pollutants—The RAPTES project. Inhal. Toxicol. 2014, 26, 141–165. [Google Scholar] [CrossRef]
- Strak, M.; Hoek, G.; Steenhof, M.; Kilinc, E.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Van Oerle, R.; Spronk, H.M.H.; Cassee, F.R.; et al. Components of ambient air pollution affect thrombin generation in healthy humans: The RAPTES project. Occup. Environ. Med. 2013, 70, 332–340. [Google Scholar] [CrossRef]
- Steenhof, M.; Mudway, I.S.; Gosens, I.; Hoek, G.; Godri, K.J.; Kelly, F.J.; Harrison, R.M.; Pieters, R.H.H.; Cassee, F.R.; Lebret, E.; et al. Acute nasal pro-inflammatory response to air pollution depends on characteristics other than particle mass concentration or oxidative potential: The RAPTES project. Occup. Environ. Med. 2013, 70, 341–348. [Google Scholar] [CrossRef]
- Strak, M.; Hoek, G.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Van Oerle, R.; Spronk, H.M.H.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; et al. Composition of PM affects acute vascular inflammatory and coagulative markers—The RAPTES project. PLoS ONE 2013, 8, e58944. [Google Scholar] [CrossRef]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential--the RAPTES project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef]
- Strak, M.; Steenhof, M.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Cassee, F.R.; Lebret, E.; Brunekreef, B.; Kelly, F.J.; Harrison, R.M.; et al. Variation in characteristics of ambient particulate matter at eight locations in the Netherlands—The RAPTES project. Atmos. Environ. 2011, 45, 4442–4453. [Google Scholar] [CrossRef]
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.-K.; Ohman-Strickland, P.; et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Department for Transport. Department for Transport Road Traffic Statistics. 2022. Available online: https://roadtraffic.dft.gov.uk/#6/55.254/-6.053/basemap-regions-countpoints (accessed on 1 March 2023).
- USEPA. AP42 Compilation of Air Emissions Factors from Stationary Sources. In Vol Section 13.2.1; Agency USEP, Ed.; EPA: Washington, DC, USA, 2011. [Google Scholar]
- Williams, E.J. Experimental designs balanced for the estimation of residual effects of treatments. Aust. J. Chem. 1949, 2, 149–168. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Williams, L.; Wilkins, L. ACSM’s Guidelines For Exercise Testing and Prescription, 9th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2013; pp. 19–173. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, K.P.; Clayton, N.; Cliff, I.; Hepple, M.; Kendrick, A.; Kirkby, J.; Miller, M.; Moore, A.; Rafferty, G.F.; O’Reilly, L.; et al. ARTP statement on pulmonary function testing. BMJ Open Respir. Res. 2020, 7, e000575. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.B.; Iaccarino, J.M.; Barochia, A.; Soghier, I.; Akuthota, P.; Brady, A.; Covar, R.A.; Debley, J.S.; Diamant, Z.; Fitzpatrick, A.M.; et al. Use of fractional exhaled nitric oxide to guide the treatment of asthma: An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2021, 204, e97–e109. [Google Scholar] [CrossRef] [PubMed]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; De Melo, P.L.; Dellacà, R.L.; Farré, R.; Hall, G.L.; Ioan, I.; Irvin, C.G.; et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef] [PubMed]
- Mudway, I.S.; Blomberg, A.; Frew, A.J.; Holgate, S.T.; Sandström, T.; Kelly, F.J. Antioxidant consumption and repletion kinetics in nasal lavage fluid following exposure of healthy human volunteers to ozone. Eur. Respir. J. 1999, 13, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Khusial, R.J.; Honkoop, P.J.; Van der Meer, V.; Snoeck-Stroband, J.B.; Sont, J.K. Validation of online asthma control questionnaire and asthma quality of life questionnaire. ERJ Open Res. 2020, 6, 00289–2019. [Google Scholar] [CrossRef]
- Martineau, A.R.; Hanifa, Y.; Witt, K.D.; Barnes, N.C.; Hooper, R.L.; Patel, M.; Stevens, N.; Enayat, Z.; Balayah, Z.; Syed, A.; et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax 2015, 70, 953–960. [Google Scholar] [CrossRef]
- Tremper, A.H.; Font, A.; Priestman, M.; Hamad, S.H.; Chung, T.C.; Pribadi, A.; Brown, R.J.; Goddard, S.L.; Grassineau, N.; Petterson, K.; et al. Field and laboratory evaluation of a high time resolution x-ray fluorescence instrument for determining the elemental composition of ambient aerosols. Atmos. Meas. Tech. 2018, 11, 3541–3557. [Google Scholar] [CrossRef]
- Manousakas, M.; Furger, M.; Daellenbach, K.R.; Canonaco, F.; Chen, G.; Tobler, A.; Rai, P.; Qi, L.; Tremper, A.H.; Green, D.; et al. Source identification of the elemental fraction of particulate matter using size segregated, highly time-resolved data and an optimized source apportionment approach. Atmos. Environ. X 2022, 14, 100165. [Google Scholar] [CrossRef]
- Ng, N.L.; Herndon, S.C.; Trimborn, A.; Canagaratna, M.R.; Croteau, P.L.; Onasch, T.B.; Sueper, D.; Worsnop, D.R.; Zhang, Q.; Sun, Y.L.; et al. An Aerosol Chemical Speciation Monitor (ACSM) for routine monitoring of the composition and mass concentrations of ambient aerosol. Aerosol Sci. Technol. 2011, 45, 780–794. [Google Scholar] [CrossRef]
- Bressi, M.; Cavalli, F.; Putaud, J.P.; Fröhlich, R.; Petit, J.E.; Aas, W.; Äijälä, M.; Alastuey, A.; Allan, J.D.; Aurela, M.; et al. A European aerosol phenomenology-7: High-time resolution chemical characteristics of submicron particulate matter across Europe. Atmos. Environ. X 2021, 10, 100108. [Google Scholar] [CrossRef]
- Hicks, W.; Beevers, S.; Tremper, A.H.; Stewart, G.; Priestman, M.; Kelly, F.J.; Lanoisellé, M.; Lowry, D.; Green, D.C. Quantification of non-exhaust particulate matter traffic emissions and the impact of COVID-19 lockdown at London Marylebone road. Atmosphere 2021, 12, 190. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, L.; Zhou, M.; Zhu, S.; Griffith, S.; Li, L.; Yu, J.Z. Source apportionment of PM2.5 using hourly measurements of elemental tracers and major constituents in an urban environment: Investigation of time-resolution influence. J. Geophys. Res. Atmos. 2018, 123, 5284–5300. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Hopke, P.K.; Dai, Q.; Li, L.; Feng, Y. Global review of recent source apportionments for airborne particulate matter. Sci. Total Environ. 2020, 740, 140091. [Google Scholar] [CrossRef]
- Font, A.; Tremper, A.H.; Priestman, M.; Kelly, F.J.; Canonaco, F.; Prévôt, A.S.; Green, D.C. Source attribution and quantification of atmospheric nickel concentrations in an industrial area in the United Kingdom (UK). Environ. Pollut. 2022, 293, 118432. [Google Scholar] [CrossRef] [PubMed]
- Hasheminassab, S.; Sowlat, M.H.; Pakbin, P.; Katzenstein, A.; Low, J.; Polidori, A. High time-resolution and time-integrated measurements of particulate metals and elements in an environmental justice community within the Los Angeles Basin: Spatio-temporal trends and source apportionment. Atmos. Environ. X 2020, 7, 100089. [Google Scholar] [CrossRef]
- Rai, P.; Furger, M.; El Haddad, I.; Kumar, V.; Wang, L.; Singh, A.; Dixit, K.; Bhattu, D.; Petit, J.E.; Ganguly, D.; et al. Real-time measurement and source apportionment of elements in Delhi’s atmosphere. Sci. Total Environ. 2020, 742, 140332. [Google Scholar] [CrossRef]
- Rai, P.; Furger, M.; Slowik, J.G.; Zhong, H.; Tong, Y.; Wang, L.; Duan, J.; Gu, Y.; Qi, L.; Huang, R.J.; et al. Characteristics and sources of hourly elements in PM10 and PM2.5 during wintertime in Beijing. Environ. Pollut. 2021, 278, 116865. [Google Scholar] [CrossRef]
- Yu, Y.; He, S.; Wu, X.; Zhang, C.; Yao, Y.; Liao, H.; Wang, Q.G.; Xie, M. PM2.5 elements at an urban site in Yangtze River Delta, China: High time-resolved measurement and the application in source apportionment. Environ. Pollut. 2019, 253, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, R.; Crenn, V.; Setyan, A.; Belis, C.A.; Canonaco, F.; Favez, O.; Riffault, V.; Slowik, J.G.; Aas, W.; Aijälä, M.; et al. ACTRIS ACSM intercomparison–Part 2: Intercomparison of ME-2 organic source apportionment results from 15 individual, co-located aerosol mass spectrometers. Atmos. Meas. Tech. 2015, 8, 2555–2576. [Google Scholar] [CrossRef]
- Reyes-Villegas, E.; Green, D.C.; Priestman, M.; Canonaco, F.; Coe, H.; Prévôt, A.S.; Allan, J.D. Organic aerosol source apportionment in London 2013 with ME-2: Exploring the solution space with annual and seasonal analysis. Atmos. Chem. Phys. 2016, 16, 15545–15559. [Google Scholar] [CrossRef]
- Visser, S.; Slowik, J.G.; Furger, M.; Zotter, P.; Bukowiecki, N.; Canonaco, F.; Flechsig, U.; Appel, K.; Green, D.C.; Tremper, A.H.; et al. Advanced source apportionment of size-resolved trace elements at multiple sites in London during winter. Atmos. Chem. Phys. 2015, 15, 11291–11309. [Google Scholar] [CrossRef]
- Crippa, M.; Canonaco, F.; Lanz, V.A.; Äijälä, M.; Allan, J.D.; Carbone, S.; Capes, G.; Ceburnis, D.; Dall’Osto, M.; Day, D.A.; et al. Organic aerosol components derived from 25 AMS data sets across Europe using a consistent ME-2 based source apportionment approach. Atmos. Chem. Phys. 2014, 14, 6159–6176. [Google Scholar] [CrossRef]
- Chen, G.; Canonaco, F.; Tobler, A.; Aas, W.; Alastuey, A.; Allan, J.; Atabakhsh, S.; Aurela, M.; Baltensperger, U.; Bougiatioti, A.; et al. European aerosol phenomenology−8: Harmonised source apportionment of organic aerosol using 22 Year-long ACSM/AMS datasets. Environ. Int. 2022, 166, 107325. [Google Scholar] [CrossRef] [PubMed]
- Via, M.; Minguillón, M.C.; Reche, C.; Querol, X.; Alastuey, A. Increase in secondary organic aerosol in an urban environment. Atmos. Chem. Phys. 1234, 21, 8323–8339. [Google Scholar] [CrossRef]
- Chen, G.; Sosedova, Y.; Canonaco, F.; Fröhlich, R.; Tobler, A.; Vlachou, A.; Daellenbach, K.R.; Bozzetti, C.; Hueglin, C.; Graf, P.; et al. Time-dependent source apportionment of submicron organic aerosol for a rural site in an alpine valley using a rolling positive matrix factorisation (PMF) window. Atmos. Chem. Phys. 1234, 21, 15081–15101. [Google Scholar] [CrossRef]
- Zhang, Y.; Favez, O.; Petit, J.-E.; Canonaco, F.; Truong, F.; Bonnaire, N.; Crenn, V.; Amodeo, T.; Prévôt, A.S.H.; Sciare, J.; et al. Six-year source apportionment of submicron organic aerosols from near-continuous highly time-resolved measurements at SIRTA (Paris area, France). Atmos. Chem. Phys. 1234, 19, 14755–14776. [Google Scholar] [CrossRef]
- Canonaco, F.; Tobler, A.; Chen, G.; Sosedova, Y.; Slowik, J.G.; Bozzetti, C.; Daellenbach, K.R.; El Haddad, I.; Crippa, M.; Huang, R.-J.; et al. A new method for long-term source apportionment with time-dependent factor profiles and uncertainty assessment using SOFI Pro: Application to 1 year of Organic Aerosol Data. Atmos. Meas. Tech. 2021, 14, 923–943. [Google Scholar] [CrossRef]
- Via, M.; Chen, G.; Canonaco, F.; Daellenbach, K.R.; Chazeau, B.; Chebaicheb, H.; Jiang, J.; Keernik, H.; Lin, C.; Marchand, N.; et al. Rolling vs. seasonal PMF: Real-world multi-site and synthetic dataset comparison. Atmos. Meas. Tech. 2022, 15, 5479–5495. [Google Scholar] [CrossRef]
- Health Effects Institute; HEI Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects; HEI Special Report 17; Health Effects Institute: Boston, MA, USA, 2010. [Google Scholar]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Doctor-diagnosed asthma starting on or before age 16 years | 1. Current or previous tobacco smoking, or living with a smoker |
| 2. Prescribed regular inhaled corticosteroid medication | 2. BMI > 30 |
| 3. Able to use a static exercise bicycle for the study duration | 3. Asthma hospitalisation within 12 months |
| 4. Three or more asthma episodes requiring oral corticosteroid medication within 12 months | |
| 5. Other major lung disease | |
| 6. Chest surgery within 6 months | |
| 7. Unable to give informed consent | |
| 8. Occupational exposure to PM or high levels of air pollution a | |
| 9. Under the age of 18 | |
| 10. Individuals at any stage of pregnancy | |
| 11. Currently breast feeding |
| IONA: Schedule of Assessment and Exposure | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Study | Initial Assessment | 3-Day Pre | Pre | Exposure | Post | 24 h Post | 3-Day Post | |
| Clinic Room | Self-Assess | Field Lab | Field Lab | Field Lab | Clinic Room | Self-Assess | ||
| Consent | x | |||||||
| Exercise test | x | |||||||
| FeNO | x | x | x | x | x | |||
| Oscillometry | x | x | x | x | x | |||
| Spirometry | x | x | x | x | x | |||
| Asthma control test | x | |||||||
| Asthma quality of life test | x | |||||||
| Asthma symptoms | x | x | x | |||||
| Venepuncture | x | x | ||||||
| Nasal lavage | x | x | x | |||||
| Urine Sample | x | x | ||||||
| Heart rate | x | |||||||
| Measurement | High-Speed Continuous Traffic | Stop–Go Traffic | Urban Background |
|---|---|---|---|
| White City | Marylebone Road | Honor Oak Park | |
| PM10 and PM2.5 Mass Concentration | Optical Particle Counter, Fidas 200E, Palas, Karlsruhe, Germany | ||
| NO2 | T200 Chemiluminescence NO/NOx, or N500 Cavity Attenuated Phase Shift, Teledyne, Thousand Oaks, CA, USA | ||
| O3 | T400, UV Absorption, Teledyne, USA | ||
| 40 elements using XRF (Al, Si, P, S, Cl, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Sr, Y, Zr, Mo, Pd, Ag, Cd, In, Sn, Sb, Te, Ba, La, Ce, Pt, Hg, Tl, Pb, Bi) in PM2.5 and PM10 | Xact 625i, Cooper Environmental Services, Edgartown, MA, USA | ||
| 23 elements using XRF (Si, S, Cl, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Sr, Mo, Cd, Sb, Ba, Ce, Pt, Pb) in PM2.5 and PM10 | Xact 625, Cooper Environmental Services, USA | ||
| Organic Mass, NO3, SO4, NH4 in PM2.5 or PM1 | Aerosol Chemical Speciation Monitor (ACSM), Aerodyne Research Inc., Billerica, MA, USA | ||
| Black Carbon in PM2.5 | Aethalometer AE33, Magee Scientific, Berkeley, CA, USA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scales, J.; Hajmohammadi, H.; Priestman, M.; McIlvenna, L.C.; de Boer, I.E.; Hassan, H.; Tremper, A.H.; Chen, G.; Wood, H.E.; Green, D.C.; et al. Assessing the Impact of Non-Exhaust Emissions on the Asthmatic Airway (IONA) Protocol for a Randomised Three-Exposure Crossover Study. Int. J. Environ. Res. Public Health 2024, 21, 895. https://doi.org/10.3390/ijerph21070895
Scales J, Hajmohammadi H, Priestman M, McIlvenna LC, de Boer IE, Hassan H, Tremper AH, Chen G, Wood HE, Green DC, et al. Assessing the Impact of Non-Exhaust Emissions on the Asthmatic Airway (IONA) Protocol for a Randomised Three-Exposure Crossover Study. International Journal of Environmental Research and Public Health. 2024; 21(7):895. https://doi.org/10.3390/ijerph21070895
Chicago/Turabian StyleScales, James, Hajar Hajmohammadi, Max Priestman, Luke C. McIlvenna, Ingrid E. de Boer, Haneen Hassan, Anja H. Tremper, Gang Chen, Helen E. Wood, David C. Green, and et al. 2024. "Assessing the Impact of Non-Exhaust Emissions on the Asthmatic Airway (IONA) Protocol for a Randomised Three-Exposure Crossover Study" International Journal of Environmental Research and Public Health 21, no. 7: 895. https://doi.org/10.3390/ijerph21070895
APA StyleScales, J., Hajmohammadi, H., Priestman, M., McIlvenna, L. C., de Boer, I. E., Hassan, H., Tremper, A. H., Chen, G., Wood, H. E., Green, D. C., Katsouyanni, K., Mudway, I. S., & Griffiths, C. (2024). Assessing the Impact of Non-Exhaust Emissions on the Asthmatic Airway (IONA) Protocol for a Randomised Three-Exposure Crossover Study. International Journal of Environmental Research and Public Health, 21(7), 895. https://doi.org/10.3390/ijerph21070895







