A Systematic Review on the In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Co-Carcinogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: rodents of both sexes, spanning all age groups and species, and encompassing various genetic backgrounds (including wild-type, transgenic, and tumor-prone animal models).
- Exposure: exposure to an electromagnetic field within the frequency range from 100 kHz to 300 GHz, with a precise characterization through dose assessment [8,9]. For frequencies up to 10 GHz, the assessment of the Specific Absorption Rate (SAR) was required. Any type of animal treatment with physical and chemical agents as tumor co-promoter was included.
- Comparison: the “sham” sample, i.e., animals treated with well-known carcinogens and kept under the same conditions as those used for irradiated animals but without RF–EMF exposure.
- Outcome: the onset of neoplasms in treated laboratory animals assessed in terms of tumor incidence (i.e., the number of animals developing cancer), latency (the time elapsed between treatment and the onset of neoplasms) and survival (number of live animals at the end of the experimental period).
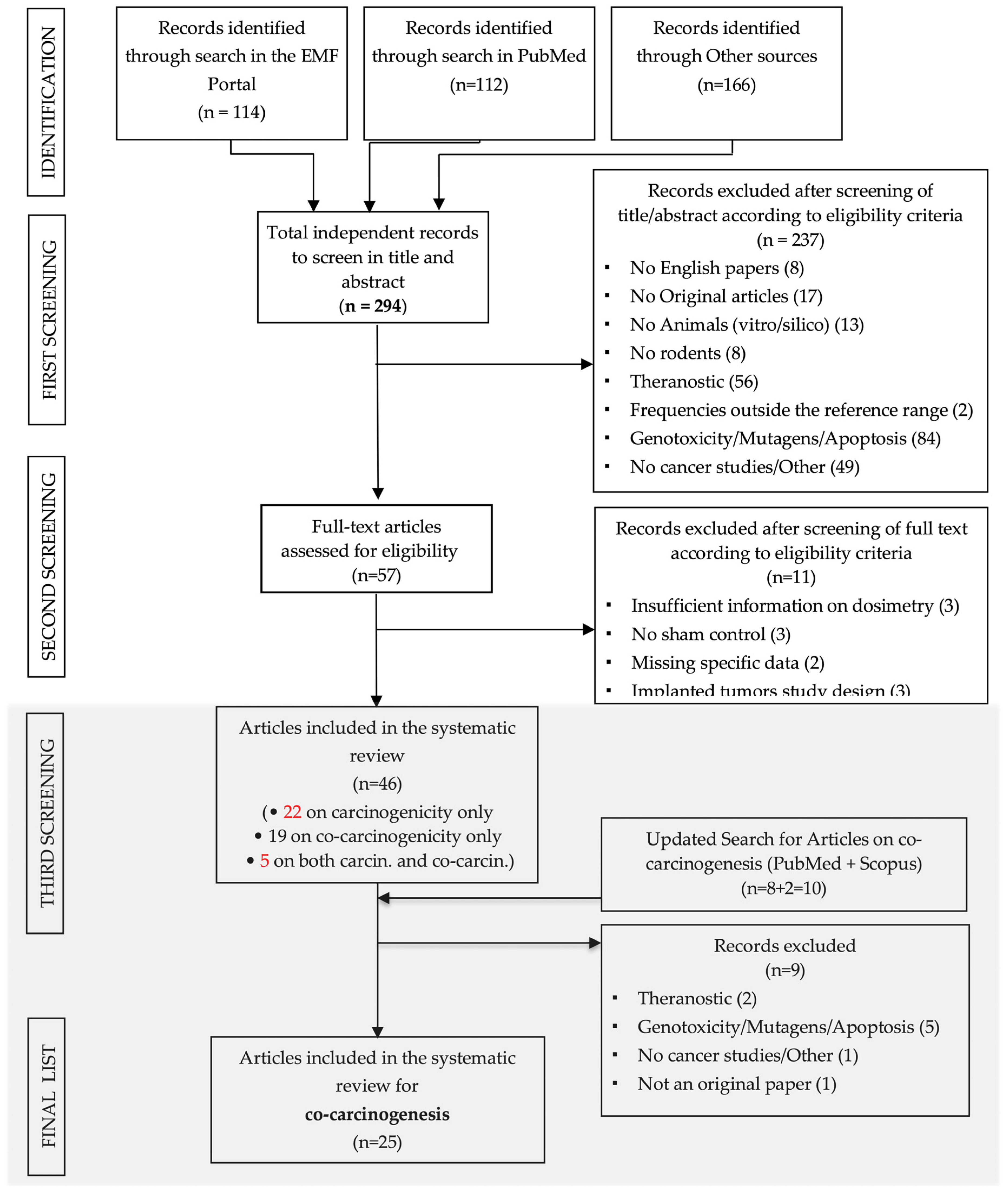
2.2. Search Strategy
2.3. Selection Process
2.4. Data Extraction Form
- General information sheet on the experimental protocol:
- Authors, publication year, title, journal,
- Study design details, including the number of experimental and control groups, the number of animals per group, information on randomization, and blinding,
- Characteristics of the animal model, such as species, strain, sex, genotype of animals (wild-type or transgenic),
- Details of exposure duration (Long Term Exposure, longer than 52 weeks; Medium Term Exposure, longer than 9 weeks; Short Term Exposure),
- Timing of treatment,
- RF–EMF exposure details,
- Type of well-known carcinogen agent, dosage administration,
- Primary outcome(s), encompassing all tumor-related outcome measures. Numerical data were extracted from the text, tables, and figures (by using digital rulers where necessary). Notably, data related to animal survival were mainly derived from Kaplan–Meier curves, which were provided in most of the analyzed papers,
- Methods employed to assess the endpoints,
- Details on data analysis and the statistical evaluation process,
- Information concerning animal deaths during the experimental period or instances where animals were euthanized due to suffering.
- Results sheet where all raw data on tumor incidences, survival and latency were collected.
- Risk of Bias (RoB) Sheet: this sheet also included a report on potential conflicts of interest present in all the included papers.
2.5. Risk of Bias (RoB) Evaluation
- Adequate randomization of administered dose or level of exposure,
- Allocation of animals in treatment groups unknown to operators,
- Evaluation of the experimental protocol,
- Conducting treatment and analysis in a “blind” manner for animal groups (blind or double-blind),
- Assessment of the exposure conditions,
- The use of standardized and validated methods for determining the outcomes, and appropriate statistical methodologies,
- Comprehensive reporting of all anticipated outcomes,
- Calculation and justification of any losses of animals during the experimentation, whether due to death or for reasons other than those possibly foreseen by the experimental protocol,
- The presence of any potential conflicts of interest.
2.6. Meta-Analysis: Strategy
2.7. Quality Assessment (Confidence Ratings and Level of Evidence for Health Effects)
3. Results
3.1. General Description of the Selected Studies
- 7,12-dimethylbenz[a]anthracene (DMBA) was used in 8 papers (25 treated/sham comparisons), with one of these combining DMBA treatment with 12-O-tetradecanoyl phorbol-13-acetate (TPA),
- Ethylnitrosourea (ENU) was employed in 9 papers (17 treated/sham comparisons), where pregnant females were treated with a single ENU administration to assess the effects of RF–EMF on the development of tumors, especially in the central nervous system, induced by the transplacental transmission of the agent,
- Diethylnitrosamine (DEN) was used in 2 papers (2 treated/sham comparisons),
- 3,4-benzopyrene (BaP) was featured in 2 papers (11 treated/sham comparisons),
- 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX) was employed in 1 paper (2 treated/sham comparisons),
- Dimethylhydrazine (DMH) was used in 1 paper (1 treated/sham comparisons),
- X-rays were employed in 1 paper (2 treated/sham comparisons),
- UV radiation was used in 1 paper (4 treated/sham comparisons).
3.2. RoB of the Selected Papers
3.3. Incidence Analysis
3.3.1. Subgroup-Analysis
3.3.2. Regression Analysis
3.3.3. Quality Assessment (Confidence Ratings and Level of Evidence for Health Effects)
3.4. Survival Analyses
Quality Assessment (Confidence Ratings and Level of Evidence for Health Effects) for Survival
3.5. Latency Analysis
3.6. Qualitative Summary of the Excluded Works from the Meta-Analysis
- Imaida et al. 1998a [22] and Imaida et al. 1998b [21]: these papers investigated the promotion role of RF–EMF exposure to 929.2 MHz or 1439 GHz, respectively, in rats treated with DEN. The animals received a single dose of DEN (200 mg/kg) and, after two weeks, they were exposed 90 min/day, 5 days/week, for 6 weeks to RF–EMF. After treatment, all animals were subjected to a partial hepatectomy, and the co-carcinogenic potential of the co-exposure was assessed analyzing the glutathione S-transferase placental form (GST-P) positive foci induction in the livers. The results indicated that the exposure to 929.2 MHz, as well as to 1.439 GHz RF–EMF, has no promoting effect on rat liver carcinogenesis. It was decided not to include the results of these articles in the meta-analysis due to the experimental design which, having foreseen the partial hepatectomy, made the data non-comparable with those reported by the other included papers. Furthermore, the tumor onset was not evaluated in terms of incidence, survival, or latency.
- Mason et al. 2001 [38]: This paper investigated the effects of single or repeated (2 exposures/week for 12 weeks) exposure to 94 GHz RF–EMF combined with DMBA or DMBA + TPA on mice skin. The authors reported the incidence data of skin tumors only through graphs. The results showed that, in any case, RF–EMF exposure did not promote or co-promote papilloma development. Due to the very high incidence of tumors in the positive control (TPA treatment), it was impossible to extrapolate neoplasm incidence numerical data related to sham and co-exposed samples.
- Wu et al. 1994 [23]: This paper investigated the effects of the combined exposure to RF–EMF and dimethylhydrazine (DMH) to assay the onset of colon tumor. Mice were treated with DMH (as tumor initiator) once per week for 14 weeks and with TPA (as a tumor promoter) once per week for 10 weeks beginning 3 weeks after the initial treatment with DMH. The animals were irradiated dorsally with RF–EMF 2.45 GHz for 3 h daily, 6 days per week, over a period of 5 months. The authors report the lack of tumor onset in both sham and treated samples. Because colon cancer was not assessed in any of the other papers included in this review, data were not included in the meta-analysis.
- Imaida et al. 2001 [20]: this paper assessed the effects of the co-exposure to DMBA, TPA and 1.5 GHz RF–EMF on mouse skin. Animals were treated with a topical application of DMBA on pre-shaved dorsal skin and divided in three groups: one group was exposed to 1.5 GHz RF–EMF 90 min a day, 5 days a week for 19 weeks, one group was placed in the exposure system without exposure, and the third group, the positive control, was weekly treated with TPA, a known tumor promoter in DMBA-induced skin carcinogenesis. The presence of tumors was only observed in the positive control sample, while the onset of tumors was not observed either in the DMBA sham control and in the animals treated with DMBA and RF–EMF.
- Paulraj et al. 2011 [28]: This paper investigated the co-carcinogenic effect of the exposure to 112 MHz or 2.45 GHz RF–EMF 2 h/day, 3 days a week for 16 weeks and a single dose of DMBA on mice skin. There was no tumor development in mice exposed to DMBA, as well as to DMBA and RF–EMF.
- Huang et al. 2005 [27]: this paper assessed the effects of the co-exposure to DMBA (a single administration), TPA and 849 or 1763 MHz RF–EMF on mouse skin. RF–EMF exposure was conducted for 2 cycles of 45 min exposure with a 15 min interval each day, 5 days a week for 19 weeks. There was no evidence of tumor onset either in the animals treated with DMBA or in the mice treated with DMBA and RF–EMF.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Working Group on the Evaluation of Carcinogenic Risks to Humans. In Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Pinto, R.; Ardoino, L.; Villani, P.; Marino, C. In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Cancer: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 2071. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Ardoino, L.; Giardullo, P.; Villani, P.; Marino, C. Protocol for a systematic review of the in vivo studies on radiofrequency (100 kHz–300 GHz) electromagnetic field exposure and cancer. Syst. Rev. 2022, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- NTP-OHAT (Ed.) Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Toxicology Program-Office of Health Assessment and Translation, US Department of health and Human Services: Washington, DC, USA, 2019. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Kuster, N.; Schönborn, F. Recommended minimal requirements and development guidelines for exposure setups of bio-experiments addressing the health risk concern of wireless communications. Bioelectromagnetics 2000, 21, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Paffi, A.; Merla, C.; Pinto, R.; Lovisolo, G.A.; Liberti, M.; Marino, C.; Repacholi, M.; Apollonio, F. Microwave exposure systems for in vivo biological experiments: A systematic review. IEEE Trans. Microw. Theory Tech. 2013, 61, 1980–1993. [Google Scholar] [CrossRef]
- WHO (Ed.) WHO Handbook for Guideline Development, 2nd ed.; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- van Rhee, H.; Suurmond, R.; Hak, T. User Manual for Meta-Essentials: Workbooks for Meta-Analysis. Erasmus Res. Inst. Manag. 2015. [Google Scholar] [CrossRef]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hooijmans, C.; Inthout, J.; Ritskes-Hoitinga, M.; Rovers, M. Meta-Analyses of Animal Studies: An Introduction of a Valuable Instrument to Further Improve Healthcare. ILAR J./Natl. Res. Counc. Inst. Lab. Anim. Resour. 2014, 55, 418–426. [Google Scholar] [CrossRef]
- Hooijmans, C.R.R.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Vesterinen, H.; Sena, E.; Egan, K.; Hirst, T.C.; Churolov, L.; Currie, G.; Antonic, A.; Howells, D.W.; Macleod, M.R. Meta-analysis of data from animal studies: A practical guide. J. Neurosci. Methods 2013, 221, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, T.; Ernst, H.; Streckert, J.; Zhou, Y.; Taugner, F.; Hansen, V.; Dasenbrock, C. Indication of cocarcinogenic potential of chronic UMTS-modulated radiofrequency exposure in an ethylnitrosourea mouse model. Int. J. Radiat. Biol. 2010, 86, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Adey, W.R.; Byus, C.V.; Cain, C.D.; Higgins, R.J.; Jones, R.A.; Kean, C.J.; Kuster, N.; MacMurray, A.; Stagg, R.B.; Zimmerman, G. Spontaneous and nitrosourea-induced primary tumors of the central nervous system in Fischer 344 rats exposed to frequency-modulated microwave fields. Cancer Res. 2000, 60, 1857–1863. [Google Scholar] [PubMed]
- Adey, W.R.; Byus, C.V.; Cain, C.D.; Higgins, R.J.; Jones, R.A.; Kean, C.J.; Kuster, N.; MacMurray, A.; Stagg, R.B.; Zimmerman, G.; et al. Spontaneous and nitrosourea-induced primary tumors of the central nervous system in Fischer 344 rats chronically exposed to 836 MHz modulated microwaves. Radiat. Res. 1999, 152, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Imaida, K.; Kuzutani, K.; Wang, J.; Fujiwara, O.; Ogiso, T.; Kato, K.; Shirai, T. Lack of promotion of 7,12-dimethylbenz[a]anthracene-initiated mouse skin carcinogenesis by 1.5 GHz electromagnetic near fields. Carcinogenesis 2001, 22, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Imaida, K.; Taki, M.; Watanabe, S.; Kamimura, Y.; Ito, T.; Yamaguchi, T.; Ito, N.; Shirai, T. The 1.5 GHz electromagnetic near-field used for cellular phones does not promote rat liver carcinogenesis in a medium-term liver bioassay. Jpn. J. Cancer Res. 1998, 89, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Imaida, K.; Taki, M.; Yamaguchi, T.; Ito, T.; Watanabe, S.; Wake, K.; Aimoto, A.; Kamimura, Y.; Ito, N.; Shirai, T. Lack of promoting effects of the electromagnetic near-field used for cellular phones (929.2 MHz) on rat liver carcinogenesis in a medium-term liver bioassay. Carcinogenesis 1998, 19, 311–314. [Google Scholar] [CrossRef]
- Wu, R.Y.; Chiang, H.; Shao, B.J.; Li, N.G.; Fu, Y.D. Effects of 2.45-GHz microwave radiation and phorbol ester 12-O-tetradecanoylphorbol-13-acetate on dimethylhydrazine-induced colon cancer in mice. Bioelectromagnetics 1994, 15, 531–538. [Google Scholar] [CrossRef]
- Zook, B.C.; Simmens, S.J. The effects of pulsed 860 MHz radiofrequency radiation on the promotion of neurogenic tumors in rats. Radiat. Res. 2006, 165, 608–615. [Google Scholar] [CrossRef]
- Heikkinen, P.; Ernst, H.; Huuskonen, H.; Komulainen, H.; Kumlin, T.; Mäki-Paakkanen, J.; Puranen, L.; Juutilainen, J. No effects of radiofrequency radiation on 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone-induced tumorigenesis in female Wistar rats. Radiat. Res. 2006, 166, 397–408. [Google Scholar] [CrossRef]
- Heikkinen, P.; Kosma, V.M.; Hongisto, T.; Huuskonen, H.; Hyysalo, P.; Komulainen, H.; Kumlin, T.; Lahtinen, T.; Lang, S.; Puranen, L.; et al. Effects of mobile phone radiation on X-ray-induced tumorigenesis in mice. Radiat. Res. 2001, 156, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Q.; Lee, J.S.; Kim, T.H.; Pack, J.K.; Jang, J.J.; Seo, J.S. Effect of radiofrequency radiation exposure on mouse skin tumorigenesis initiated by 7,12-dimethybenz[alpha]anthracene. Int. J. Radiat. Biol. 2005, 81, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, R.; Behari, J. Effects of low-level microwave radiation on carcinogenesis in Swiss Albino mice. Mol. Cell. Biochem. 2011, 348, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Ichihara, T.; Wake, K.; Watanabe, S.; Yamanaka, Y.; Kawabe, M.; Taki, M.; Fujiwara, O.; Wang, J.; Takahashi, S.; et al. Lack of promoting effects of chronic exposure to 1.95-GHz W-CDMA signals for IMT-2000 cellular system on development of N-ethylnitrosourea-induced central nervous system tumors in F344 rats. Bioelectromagnetics 2007, 28, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Kawabe, M.; Ichihara, T.; Fujiwara, O.; Taki, M.; Watanabe, S.; Wake, K.; Yamanaka, Y.; Imaida, K.; Asamoto, M.; et al. Chronic exposure to a 1.439 GHz electromagnetic field used for cellular phones does not promote N-ethylnitrosourea induced central nervous system tumors in F344 rats. Bioelectromagnetics 2005, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zook, B.C.; Simmens, S.J. Effects of a cell phone radiofrequency (860 MHz) on the latency of brain tumors in rats. Int. Congr. Ser. 2002, 1236, 137–139. [Google Scholar] [CrossRef]
- Bartsch, H.; Bartsch, C.; Seebald, E.; Deerberg, F.; Dietz, K.; Vollrath, L.; Mecke, D. Chronic exposure to a GSM-like signal (mobile phone) does not stimulate the development of DMBA-induced mammary tumors in rats: Results of three consecutive studies. Radiat. Res. 2002, 157, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hruby, R.; Neubauer, G.; Kuster, N.; Frauscher, M. Study on potential effects of “902-MHz GSM-type Wireless Communication Signals” on DMBA-induced mammary tumours in Sprague-Dawley rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 649, 34–44. [Google Scholar] [CrossRef]
- Lerchl, A.; Klose, M.; Grote, K.; Wilhelm, A.F.; Spathmann, O.; Fiedler, T.; Streckert, J.; Hansen, V.; Clemens, M. Tumor promotion by exposure to radiofrequency electromagnetic fields below exposure limits for humans. Biochem. Biophys. Res. Commun. 2015, 459, 585–590. [Google Scholar] [CrossRef]
- Yu, D.; Shen, Y.; Kuster, N.; Fu, Y.; Chiang, H. Effects of 900 MHz GSM wireless communication signals on DMBA-induced mammary tumors in rats. Radiat. Res. 2006, 165, 174–180. [Google Scholar] [CrossRef]
- Heikkinen, P.; Kosma, V.M.; Alhonen, L.; Huuskonen, H.; Komulainen, H.; Kumlin, T.; Laitinen, J.T.; Lang, S.; Puranen, L.; Juutilainen, J. Effects of mobile phone radiation on UV-induced skin tumourigenesis in ornithine decarboxylase transgenic and non-transgenic mice. Int. J. Radiat. Biol. 2003, 79, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Zook, B.C.; Simmens, S.J. The effects of 860 MHz radiofrequency radiation on the induction or promotion of brain tumors and other neoplasms in rats. Radiat. Res. 2001, 155, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.A.; Walters, T.J.; DiGiovanni, J.; Beason, C.W.; Jauchem, J.R.; Dick, E.J., Jr.; Mahajan, K.; Dusch, S.J.; Shields, B.A.; Merritt, J.H.; et al. Lack of effect of 94 GHz radio frequency radiation exposure in an animal model of skin carcinogenesis. Carcinogenesis 2001, 22, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Szudziński, A.; Pietraszek, A.; Janiak, M.; Wrembel, J.; Kałczak, M.; Szmigielski, S. Acceleration of the development of benzopyrene-induced skin cancer in mice by microwave radiation. Arch. Dermatol. Res. 1982, 274, 303–312. [Google Scholar] [CrossRef]
- Anane, R.; Dulou, P.E.; Taxile, M.; Geffard, M.; Crespeau, F.L.; Veyret, B. Effects of GSM-900 microwaves on DMBA-induced mammary gland tumors in female Sprague-Dawley rats. Radiat. Res. 2003, 160, 492–497. [Google Scholar] [CrossRef]
- Szmigielski, S.; Szudzinski, A.; Pietraszek, A.; Bielec, M.; Janiak, M.; Wrembel, J.K. Accelerated development of spontaneous and benzopyrene-induced skin cancer in mice exposed to 2450-MHz microwave radiation. Bioelectromagnetics 1982, 3, 179–191. [Google Scholar] [CrossRef]



| ID Paper [Ref] | ID Study Number | Study (Treatment-Sham Comparison) | Species Animal Type Prone/Wild Type | No of Animals/Groups | Sex | Carcinogenic Agent (CA) | Dose of CA | Frequency (MHz) Modulation | SAR (W/Kg) | WbSAR/ Local SAR | Duration (w) | Timing (h/d, d/w) | Organ | Type of Tumor (Mal/Ben) | Outcome Measure | Note |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [19] | 1 | Adey 1999 | Rats F344 Ut WT | 116 | M + F | ENU | 4 mg/kg at gestation day 18 | 836 TDMA | 1–1.60 | local | 94 | 2 h/d 4 d/w | CNS/Brain | Malignant tumors | Incidence Survival | SAR values related to growth |
| 2 [18] | 2 | Adey 2000 | Rats F344 Ut WT | 90 | M + F | ENU | 4 mg/kg at gestation day 18 | 836 TDMA | 0.74–1.60 | local | 96 | 2 h/d 4 d/w | CNS/Brain | Malignant tumors | Incidence Survival | SAR values related to growth |
| 3 [40] | 3 | Anane 2003 (1) | Rats Spraque-Dawley WT | 16 | F | DMBA | 10 mg sigle dose | 900 TDMA | 1.4 | wb | 9 | 2 h/d 5 d/w | Breast | Malignant tumors | Incidence/latency Survival | |
| 4 | Anane 2003 (2) | 16 * | 2.2 | |||||||||||||
| 5 | Anane 2003 (3) | 16 * | 3.5 | |||||||||||||
| 6 | Anane 2003 (4) | 16 | 0.1 | |||||||||||||
| 7 | Anane 2003 (5) | 16 * | 0.7 | |||||||||||||
| 8 | Anane 2003 (6) | 16 * | 1.4 | |||||||||||||
| 4 [32] | 9 | Bartsch 2002 (1) | Rats Spraque-Dawley WT | 20 | F | DMBA | 8.75 mg single dose | 900 TDMA | 0.0175–0.07 | wb | Until all animals developed tumors | 24 h/d 7 d/w | Breast | Malignant, Benign tumors | Incidence/Latency | |
| 10 | Bartsch 2002 (2) | 20 | ||||||||||||||
| 11 | Bartsch 2002 (3) | 20 | ||||||||||||||
| 5 [26] | 12 | Heikkinen 2001 (1) | Mice CBA/S WT | 50 | F | RX | 4 Gy total (three equal fractions of 1.33 Gy at 1-week intervals | 902.5 CW | 1.5 | wb | 78 | 1.5 h/d 5 d/w | All | Malignant, Benign tumors | Incidence Survival | |
| 13 | Heikkinen 2001 (2) | 50 | 902.5 TDMA | 0.35 | ||||||||||||
| 6 [36] | 14 | Heikkinen 2003 (1) | Mice K2 ODC-transgenic | 20 | F | UV | 1.2 Human MED 3 times a week | 894 DAMPS | 0.5 | wb | 52 | 1.5 h/d 5 d/w | Skin | Malignant, Benign tumors | Incidence Survival | |
| 15 | Heikkinen 2003 (2) | 26 | 894 DAMPS | |||||||||||||
| 16 | Heikkinen 2003 (3) | 21 | 902 TDMA | |||||||||||||
| 17 | Heikkinen 2003 (4) | 26 | 902 TDMA | |||||||||||||
| 7 [25] | 18 | Heikkinen 2006 (1) | Rats Winstar WT | 72 | F | MX | 1.7 mg/kg daily | 900 TDMA | 0.3 0.9 | wb | 104 | 2 h/d 5 d/w | All | Malignant, Benign tumors | Incidence Survival | |
| 19 | Heikkinen 2006 (2) | 72 * | ||||||||||||||
| 8 [33] | 20 | Hruby 2008 (1) | Rats Spraque-Dawley WT | 100 | F | DMBA | 17 mg/kg single dose | 902 TDMA | 0.4 1.3 4 | wb | 27 | 4 h/d 5 d/w | Breast | Malignant, Benign tumors | Incidence Survival | |
| 21 | Hruby 2008 (2) | 100 * | ||||||||||||||
| 22 | Hruby 2008 (3) | 100 * | ||||||||||||||
| 9 [27] | 23 | Huang 2005 (1) | Mice ICR WT | 20 | M | DMBA | 100 mg single dose | 894 CDMA | 0.4 | wb | 19 | 1.5 h/d 5 d/w | Skin | Malignant, Benign tumors | Incidence | The incidence is 0 in all groups. The paper is not included in the meta-analysis |
| 24 | Huang 2005 (2) | 20 * | 1763 CDMA | |||||||||||||
| 10 [20] | 25 | Imaida 2001 | Mice ICR WT | 48 | F | DMBA | 100 mg single dose | 1500 TDMA | 0.084 | wb | 19 | 1.5 h/d 5 d/w | Skin | Malignant, Benign tumors | Incidence | SAR 2 W/kg skin near field exposure |
| 11 [22] | 26 | Imaida 1998a | Rats F344 WT | 92 | M | DEN | 200 mg/kg single dose | 1500 TDMA | 0.680–0.453 | wb | 6 | 1.5 h/d 5 d/w | Liver | GST-P Positive Liver Foci | Nor incidence or latency | SAR 1.91–0.937 W/kg in the liver near field exposure |
| 12 [21] | 27 | Imaida 1998b | Rats F344 WT | 96 | M | DEN | 200 mg/kg single dose | 929.2 TDMA | 0.80–0.58 | wb | 6 | 1.5 h/d 5 d/w | Liver | GST-P Positive Liver Foci | Nor incidence or latency | SAR 2–1.7 W/kg in the liver near field exposure |
| 13 [34] | 28 | Lerchl 2015 (1) | Mice B6C3F1 hybrids (Ut) WT | 96 | F | ENU | 40 mg/kg at gestation day 14 | 1966 CDMA | 0.04 0.4 2 | wb | 72 | 24 h/d 7 d/w | All | Malignant, Benign tumors | Incidence Survival | |
| 29 | Lerchl 2015 (2) | 96 * | ||||||||||||||
| 30 | Lerchl 2015 (3) | 96 * | ||||||||||||||
| 14 [38] | 31 | Mason 2001 (1) | Mice SENCAR WT | 55 | F | DMBA | 10 nmol DMBA single dose | 94 GHz CW | 1 W/cm2 | local | 0 | 10 sec | Skin | Skin Papilloma | Incidence | Data from graph not readable |
| 32 | Mason 2001 (2) | 35 | DMBA | 10 nmol DMBA single dose | 333 mW/cm2 | 12 | Skin Papilloma | Incidence | Data from graph not readable | |||||||
| 33 | Mason 2001 (3) | 35 | DMBA + TPA | 10 nmol DMBA single dose 0.85 nmol TPA twice a week | 333 mW/cm2 | 12 | Skin Papilloma | Incidence | Data from graph not readable | |||||||
| 34 | Mason 2001 (4) | 35 | DMBA | 10 nmol DMBA single dose | 333 mW/cm2 | 12 | Not specified | Epidermal tickness | Nor incidence or latency | |||||||
| 35 | Mason 2001 (5) | 35 | DMBA + TPA | 10 nmol DMBA single dose 0.85 nmol TPA twice a week | 333 mW/cm2 | 12 | Not specified | Epidermal tickness | Nor incidence or latency | |||||||
| 15 [28] | 36 | Paulraj 2010 | Mice Swiss Albino WT | 18 | M | DMBA | 100 mg single dose | 112 AM 16 Hz | 0.75 | wb | 16 | 2 h/d 3 d/w | Skin | Malignant tumors | Incidence | The incidence is 0 in all groups. The paper is not included in the meta-analysis |
| 37 | Paulraj 2010 | 18 | 2450 CW | 0.1 | 17 | |||||||||||
| 16 [30] | 38 | Shirai 2005 (1) | Rats F344 Ut WT | 100 | M + F | ENU | 4 mg/kg at gestation day 18 | 1439 TDMA | 0.67 | local (head) | 104 | 1.5 h/d 5 d/w | CNS/Brain | Malignant, Benign tumors | Incidence/ Survival | wb SAR provided as lower than 0.4 W/kg always |
| 39 | Shirai 2005 (2) | 100 * | 2 | |||||||||||||
| 17 [29] | 40 | Shirai 2007 (1) | Rats F344 Ut WT | 100 | M + F | ENU | 4 mg/kg at gestation day 18 | 1950 CDMA | 0.67 2 | local (head) | 104 | 1.5 h/d 5 d/w | CNS/Brain | Malignant, Benign tumors | Incidence/ Survival | wb SAR provided as lower than 0.4 W/kg always |
| 41 | Shirai 2007 (2) | 100 * | ||||||||||||||
| 18 [41] | 42 | Szmigielski 1982 (1) | Mice Balb/c WT | 40 | M | BaP | 0.01 mL of 5% 3.4 benzopyrene every 2nd day of the week, over 5 months, starting 1 month after MW exposure | 2450 CW | 2–3 | wb | 13 | 2 h/d 6 d/w | Skin | Malignant, Benign tumors | Latency | The study aim is the latency; incidendence data are only provided for groups 5 and 6 (included in the meta-analysis) |
| 43 | Szmigielski 1982 (2) | 40 | 2–3 | 13 | Latency | |||||||||||
| 44 | Szmigielski 1982 (3) | 40 * | 6–8 | 13 | Latency | |||||||||||
| 45 | Szmigielski 1982 (4) | 40 * | 6–8 | 13 | Latency | |||||||||||
| 46 | Szmigielski 1982 (5) | 40 | 2–3 | 22 | Incidence/latency | |||||||||||
| 47 | Szmigielski 1982 (6) | 40 * | 6–8 | 22 | Incidence/latency | |||||||||||
| 19 [39] | 48 | Szudzinski 1982 (1) | Mice Balb/c WT | 100 | M | BaP | 0.01 mL of 5% 3.4 benzopyrene every 2nd day of the week, over 6 months simultaneously MW exposure | 2450 CW | 2 | wb | 27 | 2 h/d 6 d/w | Skin | Malignant, Benign tumors | Incidence/latency | The study aim is the latency; It has been possible to extrapolate the incidence, at the end of the observatoion period, only for groups 1 and 2. |
| 49 | Szudzinski 1982 (2) | 100 * | 6 | 27 | Incidence/latency | |||||||||||
| 50 | Szudzinski 1982 (3) | 100 | 4 | 4 | Latency | |||||||||||
| 51 | Szudzinski 1982 (4) | 100 | 4 | 9 | Latency | |||||||||||
| 52 | Szudzinski 1982 (5) | 100 * | 4 | 13 | Latency | |||||||||||
| 20 [17] | 53 | Tillman 2010 | Mice B6C3F1 hybrids (Ut) WT | 60 | F | ENU | 40 mg/kg at 14th day of pregnancy | 1966 CDMA | 2.1–5.5 | wb | 75 | 20 h/d 7 d/w | All tumors | Malignant, Benign tumors | Incidence/latency | |
| 21 [23] | 54 | Wu 1994 | Mice Balb WT | 54 | M + F | DMH | 15 mg/kg once a week for 14 weeks 20 mg/kg once a week for next 8 weeks | 2450 CW | 10–12 | wb | 22 | 3 h/d 6 d/w | Colon | Malignant tumors | Incidence | The paper is the only one focused on the Colon and it doesn’t provide any other data |
| 22 [35] | 55 | Yu 2006 (1) | Rats Spraque-Dawley WT | 100 | F | DMBA | 35 mg/kg single dose | 900 TDMA | 0.44 | wb | 26 | 4 h/d 5 d/w | Breast | Malignant, Benign tumors | Incidence/latency Survival | This paper is not included in the meta-analysis of benign tumors. Benign tumors number is underestimated because rats with carcinoma and benign tumors are counted only in carcinomas group |
| 56 | Yu 2006 (2) | 100 | 1.33 | |||||||||||||
| 57 | Yu 2006 (3) | 100 * | 4 | |||||||||||||
| 23 [37] | 58 | Zook 2001 (1) | Rats Spraque-Dawley (Ut) WT | 60 | M + F | ENU | 10 mg/kg at 15th day of pregnancy | 860 PRF | 1 | local (head) | 56 # | 6 h/d 5 d/w | CNS/Brain | Malignant tumors | Incidence/ Survival | # Animals are sacrificed at 394 days because 75% and over were found death |
| 59 | Zook 2001 (2) | 60 | 2.5 mg/kg at 15th day of pregnancy | 95 | Data from the paper are used only for brain tumor because other data requires a pre-processing process that is too complex and with many factors of uncertainty | |||||||||||
| 60 | Zook 2001 (3) | 60 | 95 | |||||||||||||
| 61 | Zook 2001 (4) | 120 | 860 CW | 95 | ||||||||||||
| 24 [31] | 62 | Zook 2002 (1) | Rats Spraque-Dawley (Ut) WT | 180 | M + F | ENU | 6.3 mg/kg at 15th day of pregnancy | 860 PRF | 1 | local (head) | 39 | 6 h/d 5 d/w | CNS/Brain | Malignant tumors | Incidence/latency | |
| 63 | Zook 2002 (2) | 10 mg/kg at 15th day of pregnancy | 39 | |||||||||||||
| 25 [24] | 65 | Zook 2006 | Rats Spraque-Dawley (Ut) WT | 360 | M + F | ENU | 6.3 and 10 mg/kg at 15th day of pregnancy | 860 PRF | 1 | local (head) | 39 | 6 h/d 5 d/w | CNS/Brain | Malignant tumors | Incidence/latency | ENU doses pooled; data on survival were not used because they were inconsistent with other data |
| Paper | Item Score (- -, -, +, ++) | Quality Category (1–3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Adey 1999 [18] | + | + | + | + | - | ++ | ++ | ++ | - | 2 |
| Adey 2000 [19] | + | + | + | + | - | ++ | ++ | ++ | - | 2 |
| Anane 2003 [40] | + | + | + | ++ | ++ | ++ | - - | + | + | 1 |
| Bartsch 2002 [32] | + | + | + | ++ | ++ | + | ++ | ++ | - - | 2 |
| Heikkinen 2006 [25] | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | - | 1 |
| Heikkinen 2003[36] | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | - | 1 |
| Heikkinen 2001 [26] | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | - | 1 |
| Hruby 2008 [33] | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | 1 |
| Huang 2005 [27] | + | + | + | - | + | + | ++ | ++ | ++ | 2 |
| Imaida 2001 [20] | + | + | + | - | - | - | ++ | - | - - | 3 |
| Imaida 1998 a [22] | + | + | + | - | - | - | ++ | - | - - | 3 |
| Imaida 1998 b [21] | + | + | + | - | - | - | ++ | - | - - | 3 |
| Lerchl 2015 [34] | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | 1 |
| Mason 2001 [38] | ++ | + | ++ | ++ | ++ | ++ | + | + | ++ | 2 |
| Paulraj 2010 [28] | + | - | + | - | + | + | - | ++ | + | 2 |
| Shirai 2005 [30] | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | 1 |
| Shirai 2007 [29] | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | 1 |
| Szimigielski 1982 [41] | - - | - - | ++ | - - | + | ++ | - | - | ++ | 3 |
| Szudzinski 1982 [39] | - - | - - | ++ | - - | + | ++ | - | ++ | ++ | 3 |
| Tillmann 2010 [17] | ++ | ++ | - | ++ | ++ | ++ | ++ | ++ | ++ | 2 |
| Wu 1994 [23] | ++ | - - | + | - - | + | ++ | ++ | ++ | ++ | 2 |
| Yu 2006 [35] | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - - | 1 |
| Zook 2006 [24] | ++ | - - | + | ++ | ++ | ++ | - - | ++ | - - | 2 |
| Zook 2002 [31] | ++ | - - | + | ++ | ++ | ++ | ++ | ++ | - - | 1 |
| Zook 2001 [37] | ++ | - - | + | ++ | ++ | ++ | - - | ++ | - - | 2 |
| 1. Randomized exposure level; | ||||||||||
| 2. Allocation concealment of study groups; | ||||||||||
| 3. Evaluation in the study design or analysis of possible important confounding and modifying variables; | ||||||||||
| 4. Blinding of research personnel; | ||||||||||
| 5. Confidence in the exposure characterization (dosimetry); | ||||||||||
| 6. Confidence in the outcome assessment; | ||||||||||
| 7. All measured outcomes reported; | ||||||||||
| 8. Attrition/exclusion rate; | ||||||||||
| 9. Possible conflicts of interest: “- -” was assigned to papers stemming from projects directly financed by telecommunication companies, while a rating of “-” was given to studies funded by consortia including both public institutions and companies. | ||||||||||
| Papers | Inclusion in MA | Presence of Effects |
|---|---|---|
| Adey 2000 [18] | Yes | No effects |
| Adey 1999 [19] | Yes | No effects |
| Anane 2003 [40] | Yes | No effects |
| Bartsch 2002 [32] | Yes | No effects |
| Heikkinen 2006 [25] | Yes | No effects |
| Heikkinen 2003 [36] | Yes | No effects |
| Heikkinen 2001 [26] | Yes | No effects |
| Hruby 2008 [33] | Yes |
|
| Huang 2005 [27] | No | No effects |
| Imaida 2001 [20] | No | No effects |
| Imaida 1998a [22] | No | No effects |
| Imaida 1998b [21] | No | No effects |
| Lerchl 2015 [34] | Yes |
|
| Mason 2001 [38] | No | No effects |
| Paularj 2010 [28] | No | No effects |
| Shirai 2007 [29] | Yes | No effects |
| Shirai 2005 [30] | Yes | No effects |
| Szmigielski 1982 [41] | Yes | Acceleration of cancer development |
| Szudzinski 1982 [39] | Yes | Acceleration of cancer development |
| Tillmann 2010 [17] | Yes |
|
| Wu 1994 [23] | No | No effects |
| Yu 2006 [35] | Yes | No effects |
| Zook 2006 [24] | Yes | No effects |
| Zook 2002 [31] | Yes | No effects |
| Zook 2001 [37] | Yes | No effects |
| Organ/Tumor | Number of Papers | Number of Comparisons Exposed/Sham | Meta-Analysis | Risk Ratio | LL | UL | Two Tailed p-Value | Tau2 | I2 (%) | z-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Malignant Tumors | ||||||||||
| Adrenals | 1 | 2 | NO | |||||||
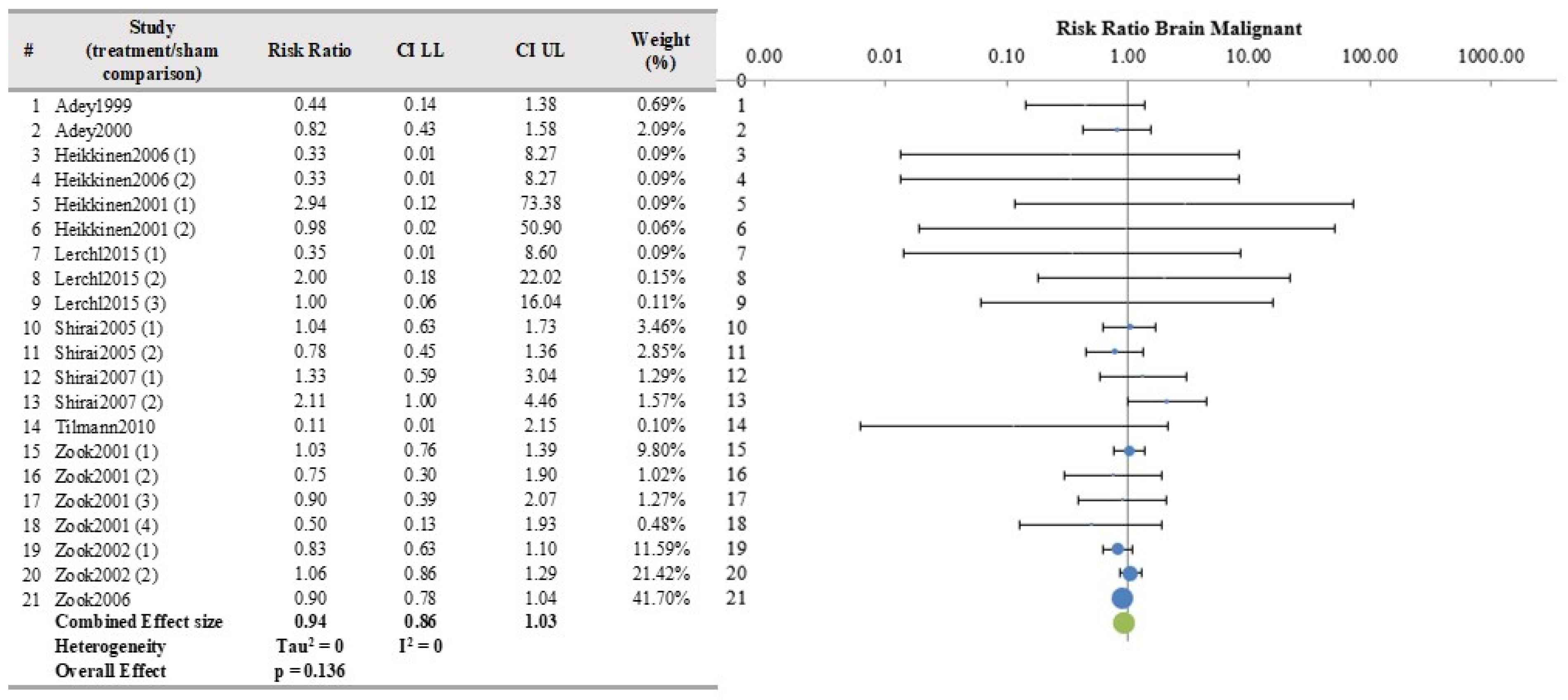
| Brain | 11 | 21 | YES | 0.939 | 0.860 | 1.025 | 0.1356 | 0 | 0 | −1.49 |
| Female Genital system | 2 | 4 | NO | |||||||
| Heart | 1 | 2 | NO | |||||||
| Histiocytic Sarcoma | 4 | 8 | YES | 0.749 | 0.401 | 1.398 | 0.2730 | 0 | 0 | −1.10 |
| Hypophysis | 1 | 2 | NO | |||||||
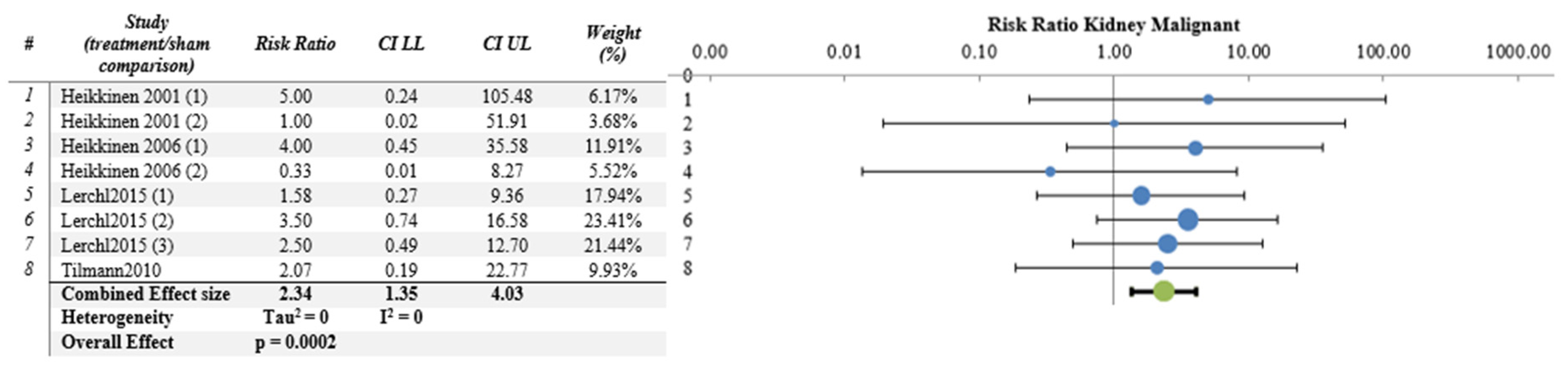
| Kidney | 4 | 8 | YES | 2.335 | 1.352 | 4.033 | 0.0002 | 0 | 0 | 3.67 |
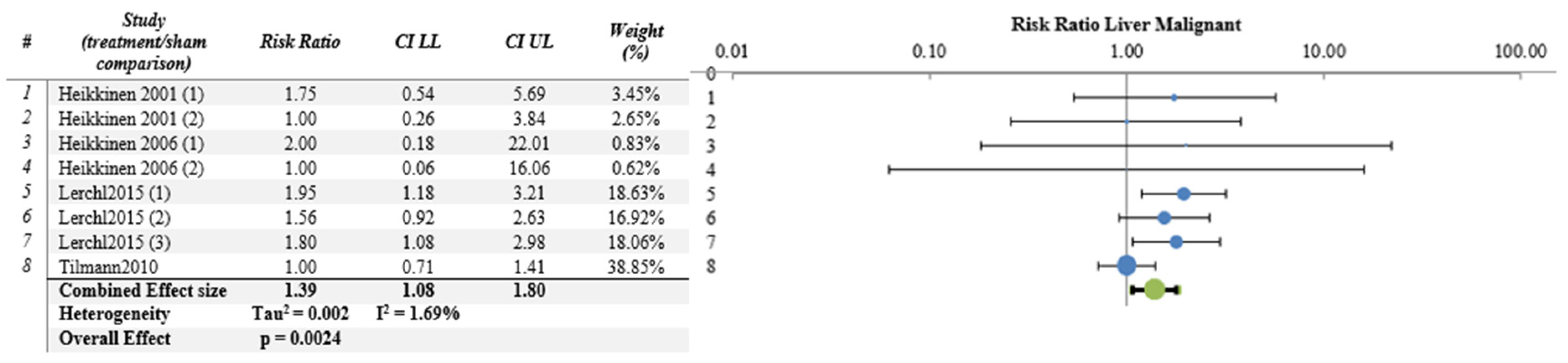
| Liver | 4 | 8 | YES | 1.392 | 1.075 | 1.802 | 0.0024 | 0.0020 | 1.70 | 3.03 |
| Lung | 4 | 8 | YES | 1.057 | 0.912 | 1.224 | 0.3749 | 0.0110 | 48.80 | 0.89 |
| Lymphoma | 4 | 8 | YES | 1.302 | 0.873 | 1.941 | 0.1189 | 0.0130 | 5.40 | 1.56 |
| Breast | 5 | 17 | YES | 1.062 | 0.931 | 1.210 | 0.3377 | 0.0150 | 26.40 | 0.96 |
| Mesenteric lymph node | 1 | 2 | NO | |||||||
| Pancreas | 1 | 2 | NO | |||||||
| Sensor organs (Harderian gl.) | 1 | 2 | NO | |||||||
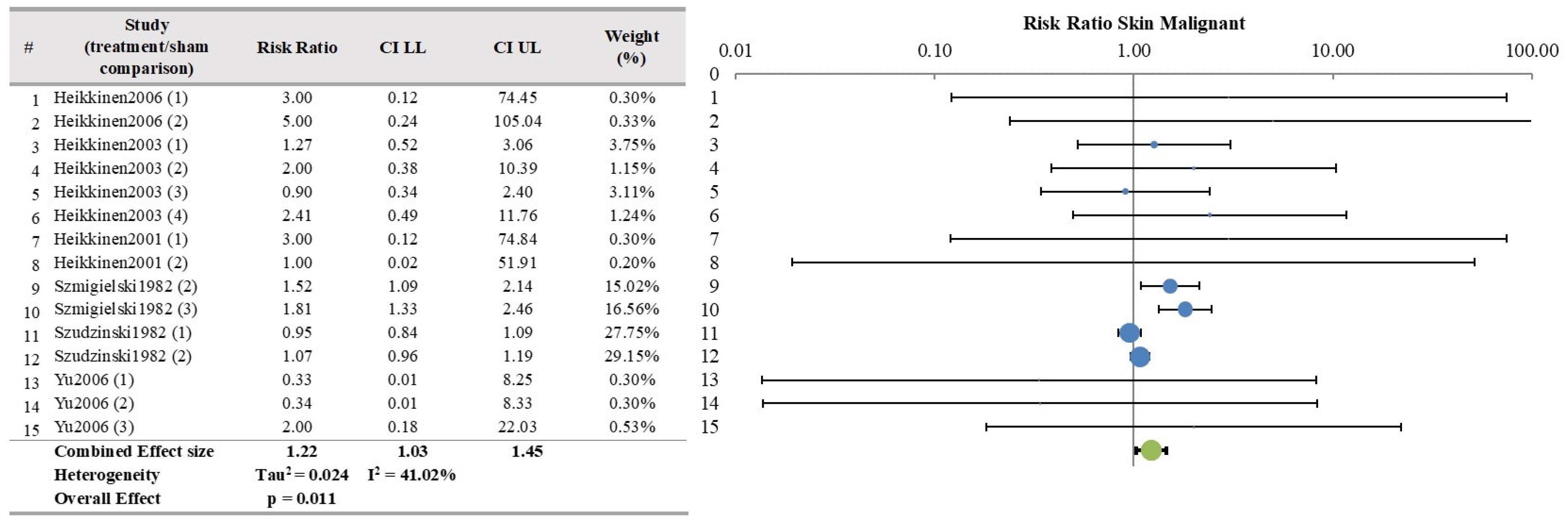
| Skin | 6 | 15 | YES | 1.224 | 1.031 | 1.452 | 0.0116 | 0.0240 | 41.00 | 2.53 |
| Spleen | 3 | 6 | YES | 0.589 | 0.123 | 2.810 | 0.3849 | 0.0018 | 0.08 | −0.87 |
| Thymus | 1 | 2 | NO | |||||||
| Benign Tumors | ||||||||||
| Adrenals | 1 | 2 | NO | |||||||
| Brain | 5 | 10 | YES | 0.537 | 0.242 | 1.192 | 0.0776 | 0 | 0 | −1.76 |
| Female Genital system | 2 | 4 | NO | |||||||
| Heart | 1 | 2 | NO | |||||||
| Hypophysis | 1 | 2 | NO | |||||||
| Kidney | 4 | 8 | YES | 0.845 | 0.472 | 1.512 | 0.4934 | 0 | 0 | −0.68 |
| Liver | 4 | 8 | YES | 1.045 | 0.787 | 1.388 | 0.7137 | 0.058 | 50.2 | 0.37 |
| Lung | 4 | 8 | YES | 1.651 | 1.351 | 2.017 | 4 × 10−9 | 0 | 0 | 5.91 |
| Breast | 3 | 8 | YES | 0.887 | 0.678 | 1.160 | 0.2905 | 0.036 | 52.3 | −1.06 |
| Mesenteric lymph node | 1 | 2 | NO | |||||||
| Pancreas | 1 | 2 | NO | |||||||
| Sensor organs (Harderian gl.) | 1 | 2 | NO | |||||||
| Skin | 3 | 8 | YES | 0.644 | 0.395 | 1.050 | 0.0333 | 0 | 0 | −2.13 |
| Spleen | 3 | 6 | YES | 1.030 | 0.419 | 2.528 | 0.9338 | 0 | 0 | 0.08 |
| Thymus | 1 | 2 | NO | |||||||
| Thyroid | 1 | 2 | NO | |||||||
| Studies (Groups/Papers) | Design | RoB | Inconsistency | Imprecision | Publication Bias | Total Exposed Animals | Total Sham Animals | Relative Effect RR (CI 95%) | Quality of Evidence | Health Evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Malignant Tumors | |||||||||||
| Brain | 21/11 | No concern | Some concern (−1) | No (I2 = 0) | No serious | No | 2096 | 1585 | 0.94 (0.86–1.03) | Moderate | Inadequate |
| Histiocytic sarcoma | 8/4 | Some concern (−1) | No concern | No (I2 = 0) | No serious | No | 587 | 278 | 0.75 (0.40–1.40) | Moderate | Inadequate |
| Kidney | 8/4 | Some concern (−1) | No concern | No (I2 = 0) | No serious | No | 585 | 278 | 2.34 (1.34–4.03) | Moderate | Moderate |
| Liver | 8/4 | Some concern (−1) | No concern | No (I2 = 1.7) | No serious | No | 586 | 278 | 1.39 (1.08–1.80) | Moderate | Moderate |
| Lung | 8/4 | Some concern (−1) | No concern | No (I2 = 48.8) | No serious | No | 587 | 278 | 1.06 (0.91–1.22) | Moderate | Inadequate |
| Lymphoma | 8/4 | Some concern (−1) | No concern | No (I2 = 5.4) | No serious | No | 587 | 278 | 1.30 (0.87–1.94) | Moderate | Inadequate |
| Breast | 8/4 | Some concern (−1) | No concern | No (I2 = 26.4) | No serious | No | 899 | 364 | 1.06 (0.93–1.21) | Moderate | Inadequate |
| Skin | 15/6 | Some concern (−1) | Some concern (−1) | No (I2 = 41) | No serious | No | 917 | 452 | 1.22 (1.03–1.45) | Low | Inadequate |
| Spleen | 6/3 | Some concern (−1) | No concern | No (I2 = 0.08) | Seriuos (-1) | No | 487 | 228 | 0.59 (0.12–2.81) | Low | Inadequate |
| Benign Tumors | |||||||||||
| Brain | 10/5 | Some concern (−1) | Some concern (−1) | No (I2 = 0) | No serious | No | 886 | 428 | 0.54 (0.24–1.19) | Low | Inadequate |
| Kidney | 8/4 | Some concern (−1) | No concern | No (I2 = 0) | No serious | No | 585 | 278 | 0.84 (0.47–1.51) | Moderate | Inadequate |
| Liver | 8/4 | Some concern (−1) | No concern | Yes (−1) (I2 = 50.2) | No serious | No | 586 | 278 | 1.05 (0.79–1.39) | Low | Inadequate |
| Lung | 8/4 | Some concern (−1) | No concern | No (I2 = 0) | No serious | No | 587 | 278 | 1.65 (1.35–2.02) | Moderate | Moderate |
| Breast | 8/4 | Some concern (−1) | No concern | Yes (−1) (I2 = 52.3) | No serious | No | 504 | 232 | 0.89 (0.68–1.16) | Low | Inadequate |
| Skin | 8/3 | No concern | No concern | No (I2 = 0) | No serious | No | 338 | 212 | 0.64 (0.39–1.05) | High | Evidence of no health effect |
| Spleen | 6/3 | Some concern (−1) | No concern | No (I2 = 0) | No serious | No | 487 | 228 | 1.03 (0.42–2.53) | Moderate | Inadequate |
| Studies (Groups/Papers) | Design | RoB | Inconsistency | Imprecision | Publication Bias | Total Exposed Animals | Total Sham Animals | Relative Effect RR (CI 95%) | Quality of Evidence | Health Evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|
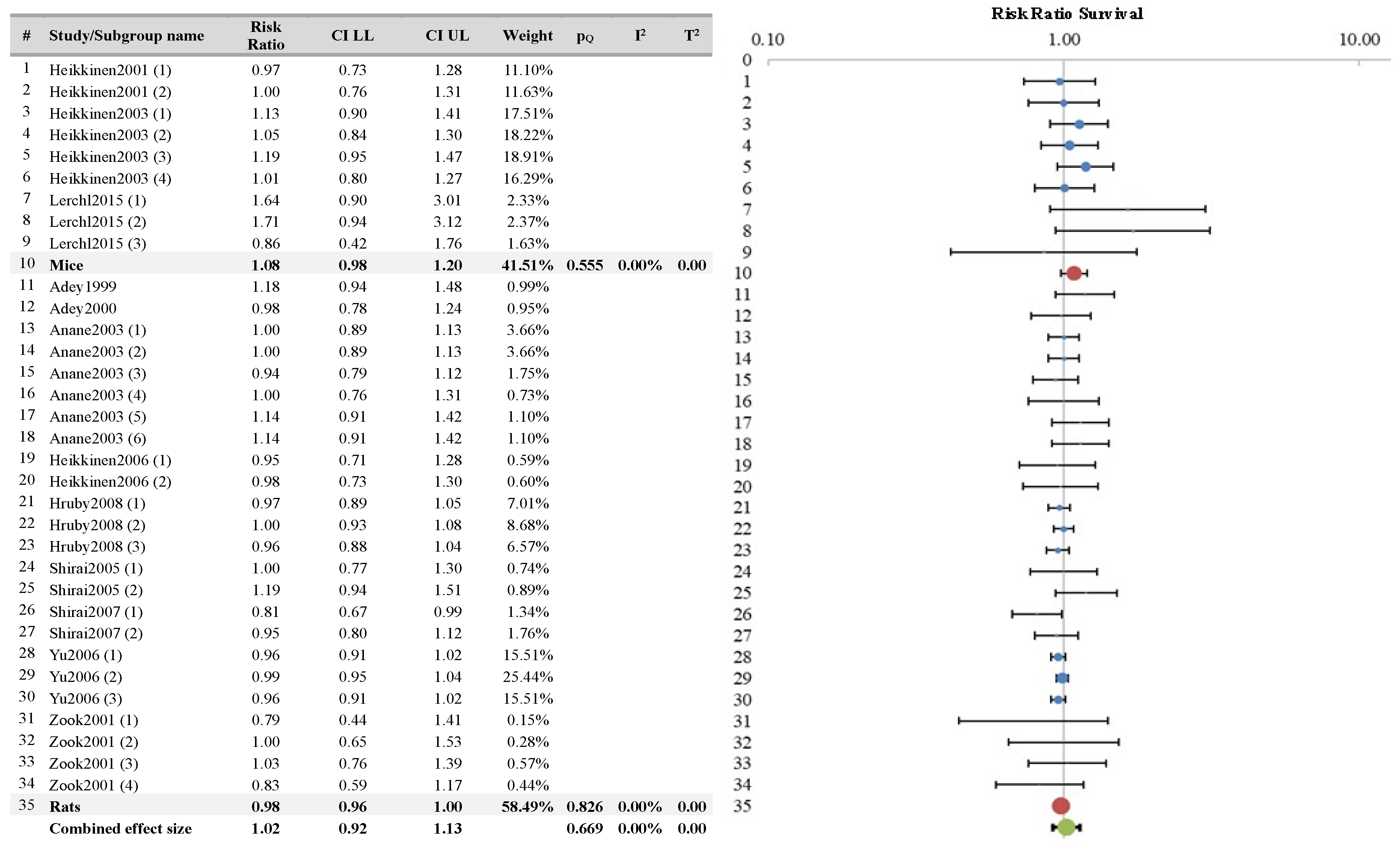
| Survival | 33/12 | No concern | No concern | No (I2 = 0) | No serious | No | 2109 | 1085 | 0.98 (0.96–1.01) | High | Evidence no health effect |
| Survival with DMBA | 12/3 | No concern | No concern | No (I2 = 0) | No serious | No | 696 | 332 | 0.98 (0.96–1.00) | High | Evidence no health effect |
| Survival with ENU | 13/6 | No concern | No concern | No (I2 = 28) | No serious | No | 1074 | 684 | 1.00 (0.90–1.12) | High | Evidence no health effect |
| Survival with MX | 2/1 | No concern | No concern | No (I2 = 0) | No serious | No | 144 | 72 | 0.96 (0.82–1.13) | High | Evidence no health effect |
| Survival with RX | 2/1 | No concern | No concern | No (I2 = 0) | No serious | No | 100 | 50 | 0.99 (0.82–1.19) | High | Evidence no health effect |
| Survival with UV | 4/1 | No concern | No concern | No (I2 = 0) | No serious | No | 95 | 45 | 1.09 (0.97–1.23) | High | Evidence no health effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, R.; Ardoino, L.; Giardullo, P.; Villani, P.; Marino, C. A Systematic Review on the In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Co-Carcinogenesis. Int. J. Environ. Res. Public Health 2024, 21, 1020. https://doi.org/10.3390/ijerph21081020
Pinto R, Ardoino L, Giardullo P, Villani P, Marino C. A Systematic Review on the In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Co-Carcinogenesis. International Journal of Environmental Research and Public Health. 2024; 21(8):1020. https://doi.org/10.3390/ijerph21081020
Chicago/Turabian StylePinto, Rosanna, Lucia Ardoino, Paola Giardullo, Paola Villani, and Carmela Marino. 2024. "A Systematic Review on the In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Co-Carcinogenesis" International Journal of Environmental Research and Public Health 21, no. 8: 1020. https://doi.org/10.3390/ijerph21081020
APA StylePinto, R., Ardoino, L., Giardullo, P., Villani, P., & Marino, C. (2024). A Systematic Review on the In Vivo Studies on Radiofrequency (100 kHz–300 GHz) Electromagnetic Field Exposure and Co-Carcinogenesis. International Journal of Environmental Research and Public Health, 21(8), 1020. https://doi.org/10.3390/ijerph21081020





