Effects of 12 Months of Structured Physical Activity Program and 18-Month Follow-Up Period on Body Composition, Physical Capacities, and Physical Activity Levels in Adults with Obesity
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
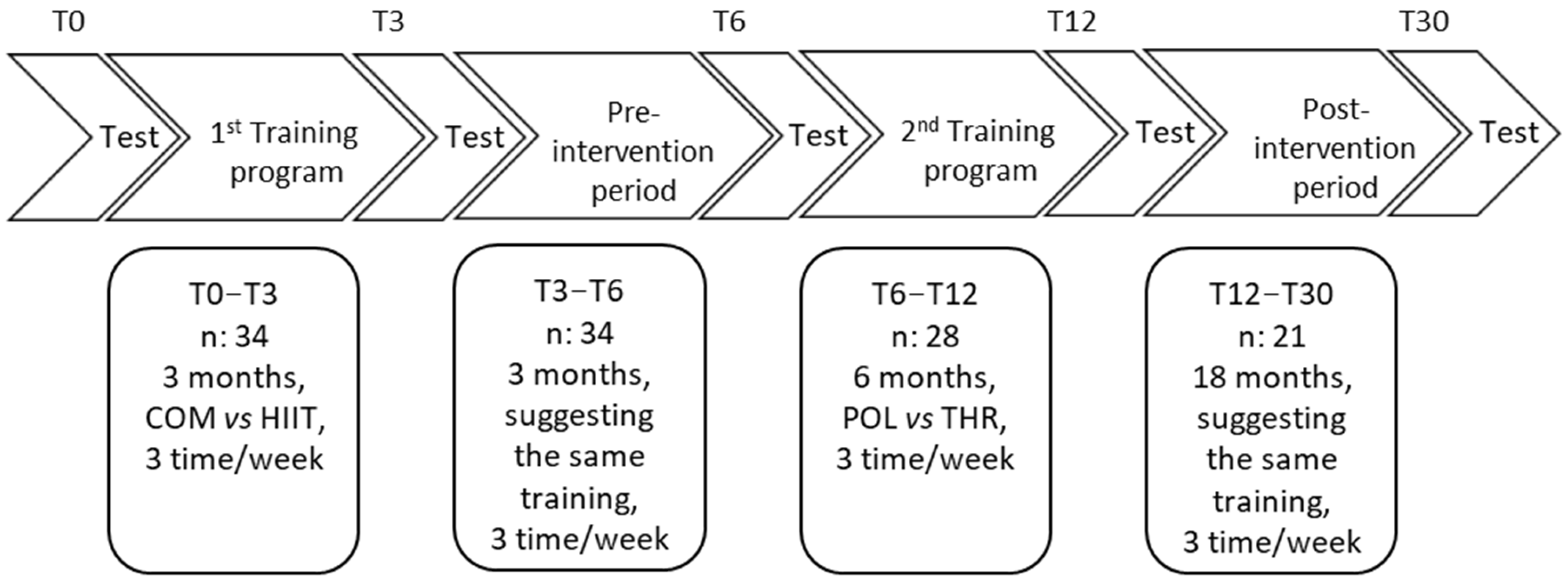
2.2. Experimental Design
2.3. Training Protocols
2.4. Measurements
2.4.1. Anthropometric Characteristics and Body Composition
2.4.2. Graded Exercise Test (GRAD)
2.4.3. Dietary and Physical Activity Habits
2.5. Statistical Analyses
3. Results
3.1. Characteristics of All Participants Assessed in This Study
3.2. Characteristics of the 15 Participants That Were Included in All Time Points of This Study
3.3. Univariate and Multivariate Analysis of 15 Participants That Were Included in All Time Points of This Study
4. Discussion
4.1. Limitations
4.2. Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Body mass |
| BMI | Body mass index |
| V̇O2max | Max oxygen uptake |
| IPAQ_TOT | International Physical Activity Questionnaire |
| FM | Fat mass |
| FFM | Fat-free mass |
| POL | Polarized training |
| GET | Gas exchange threshold |
| RCP | Respiratory compensation point |
| HR | Heart rate |
| HIIT | High intensity interval training |
| COMB | Combined training |
| THR | Threshold training |
| GRAD | Graded exercise test |
| CRF | Cardiorespiratory fitness |
References
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A.; Fitchett, D.H.; Gilbert, R.E.; Gupta, M.; Mancini, G.J.; McFarlane, P.A.; Ross, R.; Teoh, H.; Verma, S.; Anand, S.; et al. Cardiometabolic risk in Canada: A detailed analysis and position paper by the Cardiometabolic risk working group. Can. J. Cardiol. 2011, 27, e1–e33. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, M.; Cai, J.; Gong, W.; Liu, Y.; Liu, Z. Effect of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Fat Loss and Cardiorespiratory Fitness in the Young and Middle-Aged a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 4741. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef]
- Keating, S.E.; Johnson, N.A.; Mielke, G.I.; Coombes, J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes. Rev. 2017, 18, 943–964. [Google Scholar] [CrossRef]
- Wewege, M.; Van Den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Andreato, L.V.; Esteves, J.V.; Coimbra, D.R.; Moraes, A.J.P.; de Carvalho, T. The influence of high-intensity interval training on anthropometric variables of adults with overweight or obesity: A systematic review and network meta-analysis. Obes. Rev. 2019, 20, 142–155. [Google Scholar] [CrossRef]
- Vaccari, F.; Passaro, A.; D’amuri, A.; Sanz, J.M.; Di Vece, F.; Capatti, E.; Magnesa, B.; Comelli, M.; Mavelli, I.; Grassi, B.; et al. Effects of 3-month high-intensity interval training vs. moderate endurance training and 4-month follow-up on fat metabolism, cardiorespiratory function and mitochondrial respiration in obese adults. Eur. J. Appl. Physiol. 2020, 120, 1787–1803. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S. What is Best Practice for Training Intensity and Duration Distribution in Endurance Athletes? Int. J. Sports Physiol. Perform. 2010, 5, 276–291. [Google Scholar] [CrossRef] [PubMed]
- D’alleva, M.; Giovanelli, N.; Graniero, F.; Billat, V.L.; Fiori, F.; Marinoni, M.; Parpinel, M.; Lazzer, S. Effects of 24-week polarized training vs. threshold training in obese male adults. Int. J. Sports Med. 2023, 45, 282–291. [Google Scholar] [CrossRef] [PubMed]
- D’Alleva, M.; Vaccari, F.; Graniero, F.; Giovanelli, N.; Floreani, M.; Fiori, F.; Marinoni, M.; Parpinel, M.; Lazzer, S. Effects of 12-week combined training versus high intensity interval training on cardiorespiratory fitness, body composition and fat metabolism in obese male adults. J. Exerc. Sci. Fit. 2023, 21, 193–201. [Google Scholar] [CrossRef]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.F.; Little, J.P.; Renner, K.; Bishop, D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016, 30, 3413–3423. [Google Scholar] [CrossRef]
- Bishop, D.J.; Botella, J.; Granata, C. CrossTalk opposing view: Exercise training volume is more important than training intensity to promote increases in mitochondrial content. J. Physiol. 2019, 597, 4115–4118. [Google Scholar] [CrossRef]
- Georgiev, A.; Granata, C.; Roden, M. The role of mitochondria in the pathophysiology and treatment of common metabolic diseases in humans. Am. J. Physiol. Physiol. 2022, 322, C1248–C1259. [Google Scholar] [CrossRef]
- Tsai, A.G.; Wadden, T.A. Systematic Review: An Evaluation of Major Commercial Weight Loss Programs in the United States. Ann. Intern. Med. 2005, 142, 56–66. [Google Scholar] [CrossRef]
- Quist, J.; Winther, J.; Friis, A.; Gram, A.; Blond, M.; Rosenkilde, M.; Jespersen, A.; Stallknecht, B. Maintenance of cardiorespiratory fitness, body composition, and a physically active lifestyle after structured exercise interventions in individuals with overweight and obesity: A mixed-method follow-up study. Public Health Pract. 2022, 4, 100293. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.; Nordby, P.; Stallknecht, B. Maintenance of improvements in fitness and fatness 1 year after a 3-month lifestyle intervention in overweight men. Eur. J. Clin. Nutr. 2016, 70, 1212–1214. [Google Scholar] [CrossRef]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9 (Suppl. 4), 228S–233S. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, T.; Bruun, J.M.; Madsen, E.L.; Richelsen, B. Weight loss maintenance in severely obese adults after an intensive lifestyle intervention: 2- to 4-year follow-up. Obesity 2007, 15, 413–420. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Close, G.L.; MacLaren, D.P.M.; Gregson, W.; Drust, B.; Morton, J.P. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. J. Sports Sci. 2011, 29, 547–553. [Google Scholar] [CrossRef]
- Martland, R.; Mondelli, V.; Gaughran, F.; Stubbs, B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J. Sports Sci. 2020, 38, 430–469. [Google Scholar] [CrossRef]
- Lukaski, H.C. Methods for the assessment of human body composition: Traditional and new. Am. J. Clin. Nutr. 1987, 46, 537–556. [Google Scholar] [CrossRef]
- Gray, D.S.; Bray, G.A.; Gemayel, N.; Kaplan, K. Effect of obesity on bioelectrical impedance. Am J Clin Nutr. 1989, 50, 255–260. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Howley, E.T.; David, R.B.; Hugh, G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Parpinel, M.; Salvini, S.; Franceschi, S.; Palli, D.; Boyle, P. The update of the Italian Food Composition Database. J. Food Compos. Anal. 2004, 17, 509–522. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, C.E.; Searle, S.R. Generalized, Linear, and Mixed Models; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Atkinson, G.; Nevill, A.M. Statistical Methods For Assessing Measurement Error (Reliability) in Variables Relevant to Sports Medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar] [CrossRef]
- Collins, K.A.; Huffman, K.M.; Wolever, R.Q.; Smith, P.J.; Siegler, I.C.; Ross, L.M.; Hauser, E.R.; Jiang, R.; Jakicic, J.M.; Costa, P.T.; et al. Determinants of Dropout from and Variation in Adherence to an Exercise Intervention: The STRRIDE Randomized Trials. Transl. J. ACSM 2022, 7, e000190. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, T.K.; Qiu, W.; Zhang, X.; Zhou, S.; Liu, Y.; He, Y. Comparable Effects of High-Intensity Interval Training and Prolonged Continuous Exercise Training on Abdominal Visceral Fat Reduction in Obese Young Women. J. Diabetes Res. 2017, 2017, 5071740. [Google Scholar] [CrossRef]
- Wills, A.C.; Arreola, E.V.; Olaiya, M.T.; Curtis, J.M.; Hellgren, M.I.; Hanson, R.L.; Knowler, W.C. Cardiorespiratory Fitness, BMI, Mortality, and Cardiovascular Disease in Adults with Overweight/Obesity and Type 2 Diabetes. Med. Sci. Sports Exerc. 2022, 54, 994–1001. [Google Scholar] [CrossRef]
- Mølmen, K.S.; Almquist, N.W.; Skattebo, Ø. Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle: A Systematic Review and Meta-Regression. Sports Med. 2024, 55, 115–144. [Google Scholar] [CrossRef]
- Williamson, D.A.; Bray, G.A.; Ryan, D.H. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015, 23, 2319–2320. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.S.; Rosenkilde, M.; Petersen, M.B.; Gram, A.S.; Sjödin, A.; Stallknecht, B. Effects of active commuting and leisure-time exercise on fat loss in women and men with overweight and obesity: A randomized controlled trial. Int. J. Obes. 2018, 42, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsson, E.; Uddén, J.; Neovius, M.; Ekelund, U.; Rössner, S. Increased physical activity in abdominally obese women through support for changed commuting habits: A randomized clinical trial. Int. J. Obes. 2009, 33, 645–652. [Google Scholar] [CrossRef]
- Schleh, M.W.; Ahn, C.; Ryan, B.J.; Chugh, O.K.; Luker, A.T.; Luker, K.E.; Gillen, J.B.; Ludzki, A.C.; Van Pelt, D.W.; Pitchford, L.M.; et al. Both moderate- and high-intensity exercise training increase intramyocellular lipid droplet abundance and modify myocellular distribution in adults with obesity. Am. J. Physiol. Metab. 2023, 325, E466–E479. [Google Scholar] [CrossRef]
- Poon, E.T.-C.; Siu, P.M.-F.; Wongpipit, W.; Gibala, M.; Wong, S.H.-S. Alternating high-intensity interval training and continuous training is efficacious in improving cardiometabolic health in obese middle-aged men. J. Exerc. Sci. Fit. 2022, 20, 40–47. [Google Scholar] [CrossRef]
- Rosenkilde, M.; Petersen, M.B.; Gram, A.S.; Quist, J.S.; Winther, J.; Kamronn, S.D.; Milling, D.H.; Larsen, J.E.; Jespersen, A.P.; Stallknecht, B. The GO-ACTIWE randomized controlled trial—An interdisciplinary study designed to investigate the health effects of active commuting and leisure time physical activity. Contemp. Clin. Trials 2017, 53, 122–129. [Google Scholar] [CrossRef]
- Barbieri, A.; Fuk, A.; Gallo, G.; Gotti, D.; Meloni, A.; La Torre, A.; Filipas, L.; Codella, R. Cardiorespiratory and metabolic consequences of detraining in endurance athletes. Front. Physiol. 2023, 14, 1334766. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Calbet, J.A.L. Progress Update and Challenges on V.O2max Testing and Interpretation. Front. Physiol. 2020, 11, 1070. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef]
- Campos, Y.; Casado, A.; Vieira, J.G.; Guimarães, M.; Sant’ana, L.; Leitão, L.; da Silva, S.F.; de Azevedo, P.H.S.M.; Vianna, J.; Domínguez, R. Training-intensity Distribution on Middle- and Long-distance Runners: A Systematic Review. Int. J. Sports Med. 2022, 43, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; González-Mohíno, F.; González-Ravé, J.M.; Foster, C. Training Periodization, Methods, Intensity Distribution, and Volume in Highly Trained and Elite Distance Runners: A Systematic Review. Int. J. Sports Physiol. Perform. 2022, 17, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.J.; Granata, C.; Eynon, N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Bækkerud, F.H.; Solberg, F.; Leinan, I.M.; Wisløff, U.; Karlsen, T.; Rognmo, Ø. Comparison of Three Popular Exercise Modalities on VO2max in Overweight and Obese. Med. Sci. Sports Exerc. 2016, 48, 491–498. [Google Scholar] [CrossRef]
- Tan, R.; Nederveen, J.P.; Gillen, J.B.; Joanisse, S.; Parise, G.; Tarnopolsky, M.A.; Gibala, M.J. Skeletal muscle fiber-type-specific changes in markers of capillary and mitochondrial content after low-volume interval training in overweight women. Physiol. Rep. 2018, 6, e13597. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Apovian, C.M.; Barr-Anderson, D.J.; Courcoulas, A.P.; Donnelly, J.E.; Ekkekakis, P.; Hopkins, M.; Lambert, E.V.; Napolitano, M.A.; Volpe, S.L. Physical Activity and Excess Body Weight and Adiposity for Adults. American College of Sports Medicine Consensus Statement. Transl. J. Am. Coll. Sports Med. 2024, 9, e000266. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Latner, J.D.; Rozzell-Voss, K.N.; Huang, P.-C.; Tsai, Y.-C.; Pakpour, A.H.; Weng, H.-C. Association of quality of life with internet use and weight stigma across individuals with obesity and those without obesity: Role of self-perception. Acta Psychol. 2024, 244, 104203. [Google Scholar] [CrossRef]
- Pojednic, R.; D’Arpino, E.; Halliday, I.; Bantham, A. The Benefits of Physical Activity for People with Obesity, Independent of Weight Loss: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4981. [Google Scholar] [CrossRef]
| T0 (n: 34) | T3 (n: 34) | T6 (n: 28) | T12 (n: 21) | T30 (n: 15) | p Value | |
|---|---|---|---|---|---|---|
| Age (y) | 39.5 ± 7.0 | 39.8 ± 7.0 | 41.3 ± 6.3 | 41.7 ± 6.3 | 43.1 ± 6.9 | 0.386 |
| Body mass (kg) | 105.3 ± 13.2 | 102.2 ± 13.4 * | 98.7 ± 10.0 *† | 96.1 ± 10.8 *†£ | 99.0 ± 11.4 | 0.001 |
| BMI (kg m−2) | 33.9 ± 4.2 | 32.9 ± 4.2 * | 31.6 ± 2.6 *† | 30.7 ± 2.6 *†£ | 31.3 ± 3.7 | 0.001 |
| Fat-free mass (kg) | 64.2 ± 7.1 | 65.0 ± 6.9 | 63.1 ± 5.3† | 63.9 ± 5.9 | 65.7 ± 4.6 | 0.009 |
| Fat mass (kg) | 41.1 ± 8.5 | 37.2 ± 8.6 * | 35.5 ± 6.2 * | 32.2 ± 6.9 *†£ | 33.3 ± 9.8 | 0.001 |
| Fat mass (%) | 38.8 ± 4.1 | 36.3 ± 4.2 * | 35.8 ± 3.5 * | 33.0 ± 4.6 *†£ | 33.1 ± 6.6 | 0.001 |
| V̇O2max (L·min−1) | 3.02 ± 0.44 | 3.49 ± 0.46 * | 3.79 ± 0.44 *† | 4.01 ± 0.45 *† | 3.59 ± 0.64 *& | 0.001 |
| V̇O2max (mL·kg−1 FFM ·min−1) | 47.2 ± 5.8 | 53.9 ± 6.2 * | 60.1 ± 5.8 *† | 63.1 ± 6.7 *† | 54.5 ± 7.0 *& | 0.001 |
| T0 (n: 34) | T3 (n: 34) | T6 (n: 28) | T12 (n: 21) | T30 (n: 15) | p Value | |
|---|---|---|---|---|---|---|
| IPAQ_TOT (MET-min·week−1) | 1413 (6812) | 1815 (7029) | 1662 (4704) | 1906 (7380) | 1857 (8748) | 0.066 |
| IPAQ_VIG (MET-min·week−1) | 1020 (2160) | 1080 (3552) | 1280 (2592) | 1134 (2722) †£ | 1440 (4560) | 0.002 |
| IPAQ_MOD (MET-min·week−1) | 440 (920) | 380 (2880) | 400 (2780) † | 420 (2919) †£ | 720 (1800) | 0.066 |
| IPAQ_WALK (MET-min·week−1) | 553 (5544) | 594 (3366) | 495 (3881) † | 624 (3534) †£ | 396 (5412) | 0.326 |
| SF12_PI (pt) | 49.8 ± 7.5 | 51.3 ± 6.2 | 52.6 ± 4.0 | 54.0 ± 6.5 †£ | 50.1 ± 7.8 | 0.038 |
| SF12_MI (pt) | 43.7 ± 10.0 | 45.0 ± 10.7 | 46.7 ± 9.7 | 47.2 ± 11.2 | 48.6 ± 11.0 | 0.191 |
| Energy intake (kJ·day−1) | 8700 ± 2280 | 7546 ± 2100 * | 7830 ± 2071 *† | 7434 ± 1692 * | 8805 ± 1809 † | 0.001 |
| T0 (n: 15) | T3 (n: 15) | T6 (n: 15) | T12 (n: 15) | T30 (n: 15) | p Value | |
|---|---|---|---|---|---|---|
| Age (y) | 39.8 ± 8.1 | 40.1 ± 8.1 * | 40.8 ± 8.1 * | 41.2 ± 8.1 *£ | 42.8 ± 8.1 *†£& | 0.001 |
| Body mass (kg) | 102.4 ± 8.8 | 99.5 ± 8.4 | 97.4 ± 9.7 | 94.1 ± 10.8 *†£ | 99.0 ± 11.4 | 0.042 |
| BMI (kg m−2) | 33.0 ± 1.6 | 32.1 ± 1.8 | 31.3 ± 2.3 | 30.2 ± 2.5 *†£ | 31.5 ± 3.7 | 0.023 |
| Fat-free mass (kg) | 63.6 ± 4.6 | 65.2 ± 4.0 | 62.9 ± 5.3 † | 63.4 ± 5.8 | 65.7 ± 4.6 | 0.054 |
| Fat Mass (kg) | 38.7 ± 5.4 | 34.2 ± 5.1 * | 34.3 ± 6.3 * | 30.7 ± 7.1 *†£ | 33.3 ± 9.8 | 0.044 |
| Fat Mass (%) | 37.7 ± 3.0 | 34.7 ± 3.3 * | 35.1 ± 4.1 * | 32.4 ± 4.7 *£ | 33.1 ± 6.6 | 0.044 |
| V̇O2max (L·min−1) | 3.07 ± 0.46 | 3.69 ± 0.35 * | 3.93 ± 0.48 * | 3.98 ± 0.55 * | 3.67 ± 0.60 * | 0.001 |
| V̇O2max (mL·kg−1 FFM·min−1) | 48.3 ± 6.6 | 56.6 ± 4.7 * | 62.4 ± 5.5 *† | 62.8 ± 6.4 *† | 54.5 ± 7.0 *£& | 0.001 |
| T0 (n: 15) | T3 (n: 15) | T6 (n: 15) | T12 (n: 15) | T30 (n: 15) | p Value | |
|---|---|---|---|---|---|---|
| IPAQ_TOT (MET-min week−1) | 396 (2888) | 1194 (5100) | 998 (5448) | 1254 (5355) *†£ | 1356 (9144) * | 0.006 |
| IPAQ_VIG (MET-min week−1) | 584 (2400) | 720 (3840) | 645 (2760) | 378 (2016) | 960 (4800) *£ | 0.037 |
| IPAQ_MOD (MET-min week−1) | 60 (720) | 240 (2400) | 198 (2195) | 252 (2520) * | 200 (2160) & | 0.001 |
| IPAQ_WALK (MET-min week−1) | 198 (1040) | 396 (1980) | 325 (1912) | 416 (2079) | 132 (5544) | 0.101 |
| SF12_PI (pt) | 48.8 ± 7.0 | 51.9 ± 3.1 | 49.9 ± 2.8 | 54.5 ± 3.4 *†£ | 50.1 ± 7.8 | 0.037 |
| SF12_MI (pt) | 41.4 ± 8.9 | 46.3 ± 7.4 | 42.1 ± 5.3 | 48.7 ± 7.6 †£ | 48.6 ± 11.0 * | 0.013 |
| Energy intake (kJ·day−1) | 9012 ± 1619 | 8014 ± 1521 | 8845 ± 1356 | 8046 ± 1431 | 8805 ± 1809 | 0.047 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| BMI (kg m−2) | Beta Coefficient | 95% CI | p-Value | Beta Coefficient | 95% CI | p-Value |
| Age (y) | −0.21 | −0.32, −0.10 | <0.001 | −0.19 | −0.32, −0.05 | 0.005 |
| IPAQ_TOT (MET-min·week−1) | −0.42 | −0.83, −0.02 | 0.041 | −0.23 | −0.55, 0.09 | 0.157 |
| Energy intake (kJ·day−1) | 0.02 | −0.47, 0.51 | 0.936 | |||
| SF 12 MI (pt) | −0.09 | −0.16, −0.01 | 0.022 | 0.01 | −0.07, 0.08 | 0.929 |
| SF 12 PI (pt) | −0.16 | −0.26, −0.06 | 0.002 | −0.12 | −0.23, −0.01 | 0.032 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| FM (%) | Beta Coefficient | 95% CI | p-Value | Beta Coefficient | 95% CI | p-Value |
| Age (y) | −0.29 | −0.66, 0.09 | 0.136 | |||
| IPAQ_TOT (MET-min·week−1) | −1.34 | −2.33, −0.34 | 0.008 | −1.36 | −2.16, −0.56 | 0.001 |
| Energy intake (kJ·day−1) | −1.01 | −2.20, 0.17 | 0.094 | −2.09 | −3.05, −1.13 | <0.001 |
| SF 12 MI (pt) | −0.26 | −0.43, −0.08 | 0.004 | −0.24 | −0.43, −0.06 | 0.010 |
| SF 12 PI (pt) | −0.20 | −0.46, 0.06 | 0.137 | |||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| V̇O2max (L min−1) | Beta Coefficient | 95% CI | p-Value | Beta Coefficient | 95% CI | p-Value |
| Age (y) | −0.04 | −0.08, 0.01 | 0.017 | −0.08 | −0.11, −0.05 | <0.001 |
| IPAQ_TOT (MET-min·week−1) | 0.16 | 0.05, 0.26 | 0.003 | 0.15 | 0.08, 0.22 | <0.001 |
| Energy intake (kJ·day−1) | −0.08 | −0.20, 0.05 | 0.228 | |||
| SF 12 MI (pt) | 0.04 | 0.02, 0.07 | <0.001 | 0.03 | 0.01, 0.05 | 0.001 |
| SF 12 PI (pt) | 0.04 | 0.01, 0.07 | 0.004 | 0.02 | −0.01, 0.05 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mari, L.; D’Alleva, M.; Graniero, F.; Azzini, V.; Fiori, F.; Marinoni, M.; De Martino, M.; Rejc, E.; Zaccaron, S.; Stafuzza, J.; et al. Effects of 12 Months of Structured Physical Activity Program and 18-Month Follow-Up Period on Body Composition, Physical Capacities, and Physical Activity Levels in Adults with Obesity. Int. J. Environ. Res. Public Health 2025, 22, 665. https://doi.org/10.3390/ijerph22050665
Mari L, D’Alleva M, Graniero F, Azzini V, Fiori F, Marinoni M, De Martino M, Rejc E, Zaccaron S, Stafuzza J, et al. Effects of 12 Months of Structured Physical Activity Program and 18-Month Follow-Up Period on Body Composition, Physical Capacities, and Physical Activity Levels in Adults with Obesity. International Journal of Environmental Research and Public Health. 2025; 22(5):665. https://doi.org/10.3390/ijerph22050665
Chicago/Turabian StyleMari, Lara, Mattia D’Alleva, Francesco Graniero, Valeria Azzini, Federica Fiori, Michela Marinoni, Maria De Martino, Enrico Rejc, Simone Zaccaron, Jacopo Stafuzza, and et al. 2025. "Effects of 12 Months of Structured Physical Activity Program and 18-Month Follow-Up Period on Body Composition, Physical Capacities, and Physical Activity Levels in Adults with Obesity" International Journal of Environmental Research and Public Health 22, no. 5: 665. https://doi.org/10.3390/ijerph22050665
APA StyleMari, L., D’Alleva, M., Graniero, F., Azzini, V., Fiori, F., Marinoni, M., De Martino, M., Rejc, E., Zaccaron, S., Stafuzza, J., Isola, M., Parpinel, M., & Lazzer, S. (2025). Effects of 12 Months of Structured Physical Activity Program and 18-Month Follow-Up Period on Body Composition, Physical Capacities, and Physical Activity Levels in Adults with Obesity. International Journal of Environmental Research and Public Health, 22(5), 665. https://doi.org/10.3390/ijerph22050665







