Pharmacodynamics and Clinical Implications of the Main Bioactive Peptides: A Review
Abstract
:1. Introduction
2. Animal Sources
3. Plant Sources
4. New Sources
4.1. From Production to Commercialisation
4.2. Stability and Bioavailability
5. Applications
5.1. Anti-Inflammatory Activity
5.2. Anti-Hypertensive Activity
5.3. Lipid-Lowering Activity
5.4. Anti-Cancer Activity
5.5. Immunomodulatory Activity
5.6. Other Biological Activities
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Krystyna, J.; Lewicki, S.; Złotek, U. Different temperature treatments of millet grains affect the biological activity of protein hydrolyzates. Nutrients 2019, 11, 550. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [Green Version]
- Aluko, R. Functional Foods and Nutraceuticals; Springer: New York, NY, USA, 2012; pp. 37–61. [Google Scholar]
- Karaś, M. Influence of physiological and chemical factors on the absorption of bioactive peptides. Int. J. Food Sci. Technol. 2019, 54, 1486–1496. [Google Scholar] [CrossRef]
- Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2017, 8398, 2147–2163. [Google Scholar]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Lemes, A.C.; Sala, L.; Ores Jda, C.; Braga, A.R.; Egea, M.B.; Fernandes, K.F. A Review of the Latest Advances in Encrypted Bioactive Peptides from Protein-Rich Waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [Green Version]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides-New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef]
- Arulrajah, B.; Muhialdin, B.J.; Zarei, M.; Hasan, H.; Saari, N. Lacto-fermented Kenaf (Hibiscus cannabinus L.) seed protein as a source of bioactive peptides and their applications as natural preservatives. Food Control 2020, 110, 106969. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Moller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mazzocchi, C.; Paolella, S.; FitzGerald, R.J. Release of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from milk protein isolate (MPI) during enzymatic hydrolysis. Food Res. Int. 2017, 94, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Zenezini Chiozzi, R.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Samperi, R.; Lagana, A. Purification and identification of endogenous antioxidant and ACE-inhibitory peptides from donkey milk by multidimensional liquid chromatography and nanoHPLC-high resolution mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5657–5666. [Google Scholar] [CrossRef]
- Sipola, M.; Finckenberg, P.; Vapaatalo, H.; Pihlanto-Leppälä, A.; Korhonen, H.; Korpela, R.; Nurminen, M.-L. Alpha-lactorphin and beta-lactorphin improve arterial function in spontaneously hypertensive rats. Life Sci. 2002, 71, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Colletti, A.; Rosticci, M.; Cagnati, M.; Urso, R.; Giovannini, M.; Borghi, C.; D’Addato, S. Effect of Lactotripeptides (Isoleucine-Proline-Proline/Valine-Proline-Proline) on Blood Pressure and Arterial Stiffness Changes in Subjects with Suboptimal Blood Pressure Control and Metabolic Syndrome: A Double-Blind, Randomized, Crossover Clinical Trial. Metab. Syndr. Relat. Disord. 2016, 14, 161–166. [Google Scholar] [CrossRef]
- Aspri, M.; Leni, G.; Galaverna, G.; Papademas, P. Bioactive properties of fermented donkey milk, before and after in vitro simulated gastrointestinal digestion. Food Chem. 2018, 268, 476–484. [Google Scholar] [CrossRef]
- Sun, X.; Chakrabarti, S.; Fang, J.; Yin, Y.; Wu, J. Low-molecularweight fractions of Alcalase hydrolyzed egg ovomucin extract exert antiinflammatory activity in human dermal fibroblasts through the inhibition of tumor necrosis factor-mediated nuclear factor κB pathway. Nutr. Res. 2016, 36, 648–657. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Wang, F.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Characterization of ACE-inhibitory peptide associated with antioxidant and anticoagulation properties. J. Food Sci. 2011, 76, C1149–C1155. [Google Scholar] [CrossRef]
- Remanan, M.K.; Wu, J. Antioxidant activity in cooked and simulated digested eggs. Food Funct. 2014, 5, 1464–1474. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2016, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Toldra, F.; Sentandreu, M.A.; Nishimura, H.; Arihara, K. Antihypertensive activity of peptides identified in the in vitro gastrointestinal digest of pork meat. Meat Sci. 2012, 91, 382–384. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Samperi, R.; Ventura, S.; Chiozzi, R.Z.; Lagana, A. Identification of potential bioactive peptides generated by simulated gastrointestinal digestion of soybean seeds and soy milk proteins. J. Food Compos. Anal. 2015, 44, 205–213. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Hayashi, M.; Murakami, Y.; Araki, Y.; Otsuka, Y.; Kashiwagi, T.; Shimamura, T.; Ukrda, H. Development of LC-MS/MS analysis of cyclic dipeptides and its application to tea extract. Biosci. Biotechnol. Biochem. 2016, 80, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Cleaner Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Esteve, C.; Marina, M.L.; García, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escrig, A.; Alaiz, M.; Vioque, J.; Ruperez, P. Health-promoting activities of ultra-filtered okara protein hydrolysates released by in vitro gastrointestinal digestion: Identification of active peptide from soybean lipoxygenase. Eur. Food Res. Technol. 2010, 230, 655–663. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Vercruysse, L.; Smagghe, G.; Herregods, G.; Van Camp, J. ACE inhibitory activity in enzymatic hydrolysates of insect protein. J. Agric. Food Chem. 2005, 53, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Wang, C.; Liao, D.; Liu, H.; Zhao, Z.; Zhao, Z. Purification, modification and inhibition mechanism of angiotensin I-converting enzyme inhibitory peptide from silkworm pupa (Bombyx mori) protein hydrolysate. Process Biochem. 2017, 54, 172–179. [Google Scholar] [CrossRef]
- Zieli ´nska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Dellafiora, L.; Paolella, S.; Dall’Asta, C.; Dossena, A.; Cozzini, P.; Galaverna, G. Hybrid in Silico/in Vitro Approach for the Identification of Angiotensin I Converting Enzyme Inhibitory Peptides from Parma Dry-Cured Ham. J. Agric. Food Chem. 2015, 63, 6366–6375. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Zarei, M.; Ebrahimpour, A.; Abdul Hamid, A.; Anwar, F.; Saari, N. Production of defatted palm kernel cake protein hydrolysate as a valuable source of natural antioxidants. Int. J. Mol. Sci. 2012, 13, 8097–8111. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part I: Overview. Biotechnol. J. 2007, 2, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Wen, D.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct. 2015, 6, 622–629. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Zenezini Chiozzi, R.; Laganà, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Shi, C.; Zhou, C.; Sun, X.; Ang, Y.; Dong, X.; Huang, M.; Zhou, G. Purification and characterization of novel antioxidant peptides from duck breast protein hydrolysates. LWT Food Sci. Technol. 2020, 125, 109215. [Google Scholar] [CrossRef]
- Fitzgerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef] [Green Version]
- Boelsma, E.; Kloek, J. IPP-rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutr. J. 2010, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Dellafiora, L.; Pugliese, R.; Bollati, C.; Gelain, F.; Galaverna, G.; Arnoldi, A.; Lammi, C. “Bottom-Up” Strategy for the Identification of Novel Soybean Peptides with Angiotensin-Converting Enzyme Inhibitory Activity. J. Agric. Food Chem. 2020, 68, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Darewicz, M.; Dziuba, B.; Minkiewicz, P.; Dziuba, J. The preventive potential of milk and colostrum proteins and protein fragments. Food Rev. Int. 2011, 27, 357–388. [Google Scholar] [CrossRef]
- Li, R.; Laurent, F.; Taverner, A.; Mackay, J.; De Bank, P.A.; Mrsny, R.J. Intestinal Transcytosis of a Protein Cargo and Nanoparticles Mediated by a Non-Toxic Form of Pseudomonas aeruginosa Exotoxin A. Pharmaceutics 2021, 13, 1171. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, E.; Lee, V.H.L. Aminopeptidase Activity in the Jejunal and Heal Peyer’s Patches of the Albino Rabbit. Pharm. Res. 1992, 9, 535–540. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug. Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Bechaux, J.; de La Pomélie, J.; Théron, J.; SantéLhoutellier, V.; Gatellier, P. Iron-catalysed chemistry in the gastrointestinal tract: Mechanisms, kinetics and consequences. A review. Food Chem. 2018, 268, 27–39. [Google Scholar] [CrossRef]
- Philippart, M.; Schmidt, J.; Bittner, B. Oral Delivery of Therapeutic Proteins and Peptides: An Overview of Current Technologies and Recommendations for Bridging from Approved Intravenous or Subcutaneous Administration to Novel Oral Regimens. Drug Res. 2016, 66, 113–120. [Google Scholar] [CrossRef]
- Lee, J.K.; Li-Chan, E.C.Y.; Jeon, J.K.; Byun, H.G. Development of functional materials from seafood by-products by membrane separation technology. In Seafood Processing By-Products; Kim, S.K., Ed.; Springer: New York, NY, USA, 2014; pp. 35–62. [Google Scholar]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Shori, A.B.; Baba, A.S. Comparative antioxidant activity, proteolysis and in vitro α-amylase and α-glucosidase inhibition of Allium sativumyogurts made from cow and camel milk. J. Saud. Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Ledwoń, P.; Errante, F.; Papini, A.M.; Rovero, P.; Latajka, R. Peptides as Active Ingredients: A Challenge for Cosmeceutical Industry. Chem. Biodivers. 2021, 18, e2000833. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides and their health promoting effects: A potential role in atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef]
- Malinowski, J.; Klempt, M.; Clawin-Rädecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NFκB inhibitory peptide from tryptic β-casein hydrolysate. Food Chem. 2014, 165, 129–133. [Google Scholar] [CrossRef]
- Aihara, K.; Ishii, H.; Yoshida, M. Casein-derived tripeptide, Val-Pro-Pro (VPP), modulates monocyte adhesion to vascular endothelium. J. Atheroscler. Thromb. 2009, 16, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Mine, Y.; Wu, J. The potential of food proteinderived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric. 2016, 96, 2303–2311. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 2013, 8, e82829. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toalá, J.E.; Santiago-Lòpez, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; Gonzàlez-Còrdova, A.F.; Hernàndez-Mendoza. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Holmer-Jensen, J.; Karhu, T.; Mortensen, L.S.; Pedersen, S.B.; Herzig, K.H.; Hermansen, K. Differential effects of dietary protein sources on postprandial low-grade inflammation after a single high fat meal in obese non-diabetic subjects. Nutr. J. 2011, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- McInnes, G.T. Lowering blood pressure for cardiovascular risk reduction. J. Hypertens. Suppl. 2005, 23, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Lawes, C.M.; Vanders, H.S.; Rodgers, A. Global burden of bloodpressure related disease. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Borghi, C.; Cicero, A.F. Nutraceuticals with clinically detectable blood pressure lowering effect: A review of available randomized clinical trials and their meta-analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.; Colletti, A. Nutraceuticals and blood pressure control: Results from clinical trials and meta-analyses. High Blood Press. Cardiovasc. Prev. 2015, 22, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Aubin, F.; Azais-Braesco, V.; Borghi, C. Do the lactotripeptides isoleucine–proline–proline and valine–proline–proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2013, 26, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.; Gerocarni, B.; Laghi, L.; Borghi, C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: A meta-analysis of current available clinical trials. J. Hum. Hypertens. 2011, 25, 425–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D. Surampalli RY Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Bioactive properties of milk proteins in humans: A review. Peptides 2015, 73, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.Y.; Szeto, I.M.Y.; Makinen, K.; Gao, Q.; Wang, J.; Li-Qin, Q.; Zhao, Y. Effect of probiotic fermented milk on blood pressure: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1188–1194. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Nirupama, G.; Mohammad, B.; Hossain, D.K.R.; Nigel, P.B. A review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules 2015, 20, 10884–10909. [Google Scholar]
- Ford, E.S.; Li, C.; Pearson, W.S.; Zhao, G.; Mokdad, A.H. Trends in hypercholesterolemia, treatment and control among United States adults. Int. J. Cardiol. 2010, 140, 226–235. [Google Scholar] [CrossRef]
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; et al. Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective metaanalysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar]
- Zdrojewski, T.; Solnica, B.; Cybulska, B.; Bandosz, P.; Rutkowski, M.; Stokwiszewski, J.; Gaciong, Z.; Banach, M.; Wojtyniak, B.; Pencina, M.; et al. Prevalence of lipid abnormalities in Poland. The NATPOL 2011 survey. Kardiol. Pol. 2016, 74, 213–223. [Google Scholar] [CrossRef]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin peptides lower low-density lipoprotein (LDL) cholesterol through an up-regulation of the LDL receptor/sterol regulatory element binding protein 2 (SREBP2) pathway at HepG2 cell line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.; Soares Freitas, R.A.; Corrêa Carlos, A.C.; Siguemoto, É.S.; Fontanari, G.G.; Arêas, J.A. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2014, 168, 288–293. [Google Scholar] [CrossRef]
- Herrera Chalé, F.; Ruiz Ruiz, J.C.; Betancur Ancona, D.; Acevedo Fernández, J.J.; Segura Campos, M.R. The hypolipidemic effect and antithrombotic activity of Mucuna pruriens protein hydrolysates. Food Funct. 2016, 7, 434–444. [Google Scholar] [CrossRef]
- Lule, V.K.; Garg, S.; Pophaly, S.D.; Hitesh; Tomar, S.K. Potential health benefits of lunasin: A multifaceted soy-derived bioactive peptide. J. Food. Sci. 2015, 80, R485–R494. [Google Scholar] [CrossRef]
- Otvos, L., Jr. Peptide-based drug design: Here and now. Methods Mol. Biol. 2008, 494, 1–8. [Google Scholar]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucl. Acids. Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Chemopreventive properties of peptide lunasin: A review. Protein Pept. Lett. 2013, 20, 424–432. [Google Scholar]
- Dia, V.P.; de Mejia, E.G. Lunasin potentiates the effect of oxaliplatin preventing outgrowth of colon cancer metastasis, binds to α5β1 integrin and suppresses FAK/ERK/NF-κB signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Lewis, D.; Tung, C.Y.; Han, L.; Henriquez, S.M.P.; Voiles, L.; Lupov, I.P.; Pelloso, D.; Sinn, A.L.; Pollok, K.E.; et al. Soypeptide lunasin in cytokine immunotherapy for lymphoma. Cancer Immunol. Immunother. 2014, 63, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from ricebran. Peptides 2010, 31, 1629–1634. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, H.J.; de Lumen, B.O. Contents and bioactivities of lunasin, bowman-birk inhibitor, and isoflavones in soybean seed. J. Agric. Food Chem. 2005, 53, 7686–7690. [Google Scholar] [CrossRef]
- Malkowicz, S.B.; McKenna, W.G.; Vaughn, D.J.; Wan, X.S.; Propert, K.J.; Rockwell, K.; Marks, S.H.; Wein, A.J.; Kennedy, A.R. Effects of Bowman-Birk inhibitorconcentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate 2001, 48, 16–28. [Google Scholar] [CrossRef]
- Valadez-Vega, C.; Alvarez-Manilla, G.; Riverón-Negrete, L.; GarcíaCarrancá, A.; Morales-González, J.A.; Zuñiga-Pérez, C.; Zuñiga-Pérez, C.; Madrigal-Santillán, E.; Esquivel-Soto, J.; Esquivel-Chirino, C.; et al. Detection of cytotoxic activity of lectin on human colon adenocarcinoma (Sw480) and epithelial cervical carcinoma (C33-A). Molecules 2011, 16, 2107–2118. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Zhang, W.; Tang, H.; Ye, Q.; Liao, Q.; Zhou, Y.; Wu, M.; Xiong, W.; Zheng, Y.; Guo, X.; et al. Lactotransferrin acts as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT through multiple mechanisms. Oncogene 2013, 32, 4273–4283. [Google Scholar] [CrossRef] [Green Version]
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W., Jr.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer 2004, 111, 398–403. [Google Scholar] [CrossRef]
- Azuma, N.; Suda, H.; Iwasaki, H.; Yamagata, N.; Saeki, T.; Kanamoto, R.; Iwami, K. Antitumorigenic effects of several food proteins in a rat model with colon cancer and their reverse correlation with plasma bile acid concentration. J. Nutr. Sci. Vitaminol. 2000, 46, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Ledesma-Martínez, E.; Aguíñiga-Sánchez, I.; Weiss-Steider, B.; Rivera-Martínez, A.R.; Santiago-Osorio, E. Casein and Peptides Derived from Casein as Antileukaemic Agents. J. Oncol. 2019, 2019, 8150967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, Z.; Pei, X.; Han, H.; Wang, J.; Wang, L.; Long, Z.; Shen, X.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from chum salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigon, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; He, H.L.; Wang, G.F.; Wu, H.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, M.; Zhao, Q.; Jiang, Y. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem. 2007, 101, 1658–1663. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Brownson, C. A possible new role for the anti-ageing peptide carnosine. Cell. Mol. Life Sci. 2000, 7, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nebot, M.J.; Recio, I.; Hernandez-Ledesma, B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food. Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Tamura, Y.; Koizumi, T. Antioxidant activity of fish sauces including puffer (Lagocephalus wheeleri) fish sauce measured by the oxygen radical absorbance capacity method. Mol. Med. Rep. 2010, 3, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2010, 11, 347–356. [Google Scholar] [CrossRef]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [Green Version]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Jo, C.; Kang, K.S.; Lee, M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008, 107, 327–336. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptidebased drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Franck, P.; Moneret Vautrin, D.A.; Dousset, B.; Kanny, G.; Nabet, P.; Guénard-Bilbaut, L.; Parisot, L. The allergenicity of soybean-based products is modified by food technologies. Int. Arch. Allergy Immunol. 2002, 128, 212–219. [Google Scholar] [CrossRef]
- Yoshikawa, M. Bioactive peptides derived from natural proteins with respect to diversity of their receptors and physiological effects. Peptides 2015, 72, 208–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.J.; Xu, S.; Wang, H.M.; Ling, Y.; Dong, J.; Xia, R.D.; Sun, X.H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [Green Version]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Sayd, T.; Dufour, C.; Chambon, C.; Buffière, C.; Remond, D.; Santé-Lhoutellier, V. Combined in vivo and in silico approaches for predicting the release of bioactive peptides from meat digestion. Food Chem. 2018, 249, 111–118. [Google Scholar] [CrossRef]
- Rutherfurd-Markwick, K.J. Food proteins as a source of bioactive peptides with diverse functions. Br. J. Nutr. 2012, 108, S149–S157. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Egashira, Y.; Ono, S.; Mochizuki, S.; Shimmura, Y.; Suzuki, Y.; Nagata, M.; Hashimoto, K.; Kiyono, T.; Park, E.Y.; et al. Identification of a hepatoprotective peptide in wheat gluten hydrolysate against D-galactosamine-induced acute hepatitis in rats. J. Agric. Food Chem. 2013, 61, 6304–6310. [Google Scholar] [CrossRef] [PubMed]

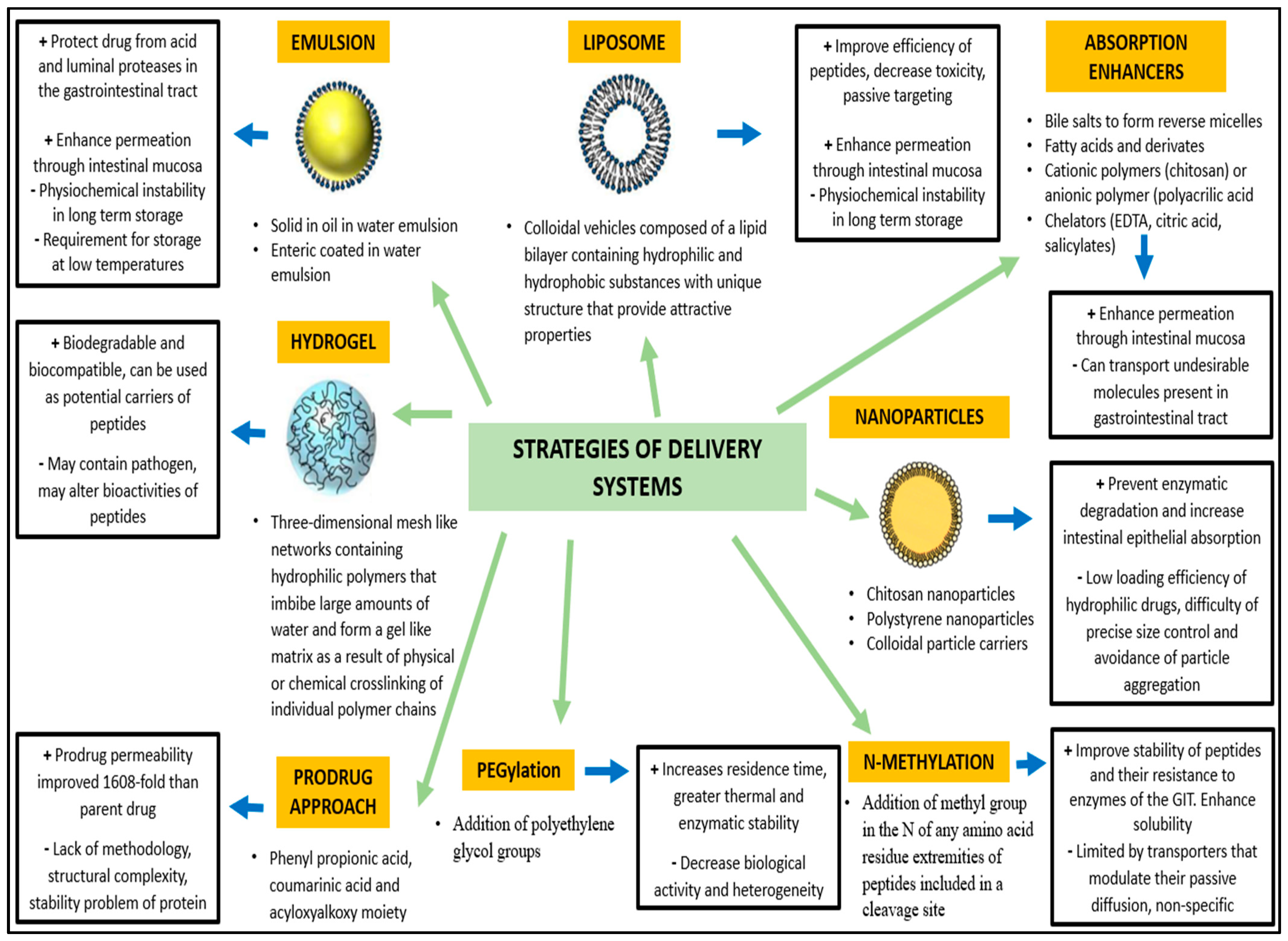
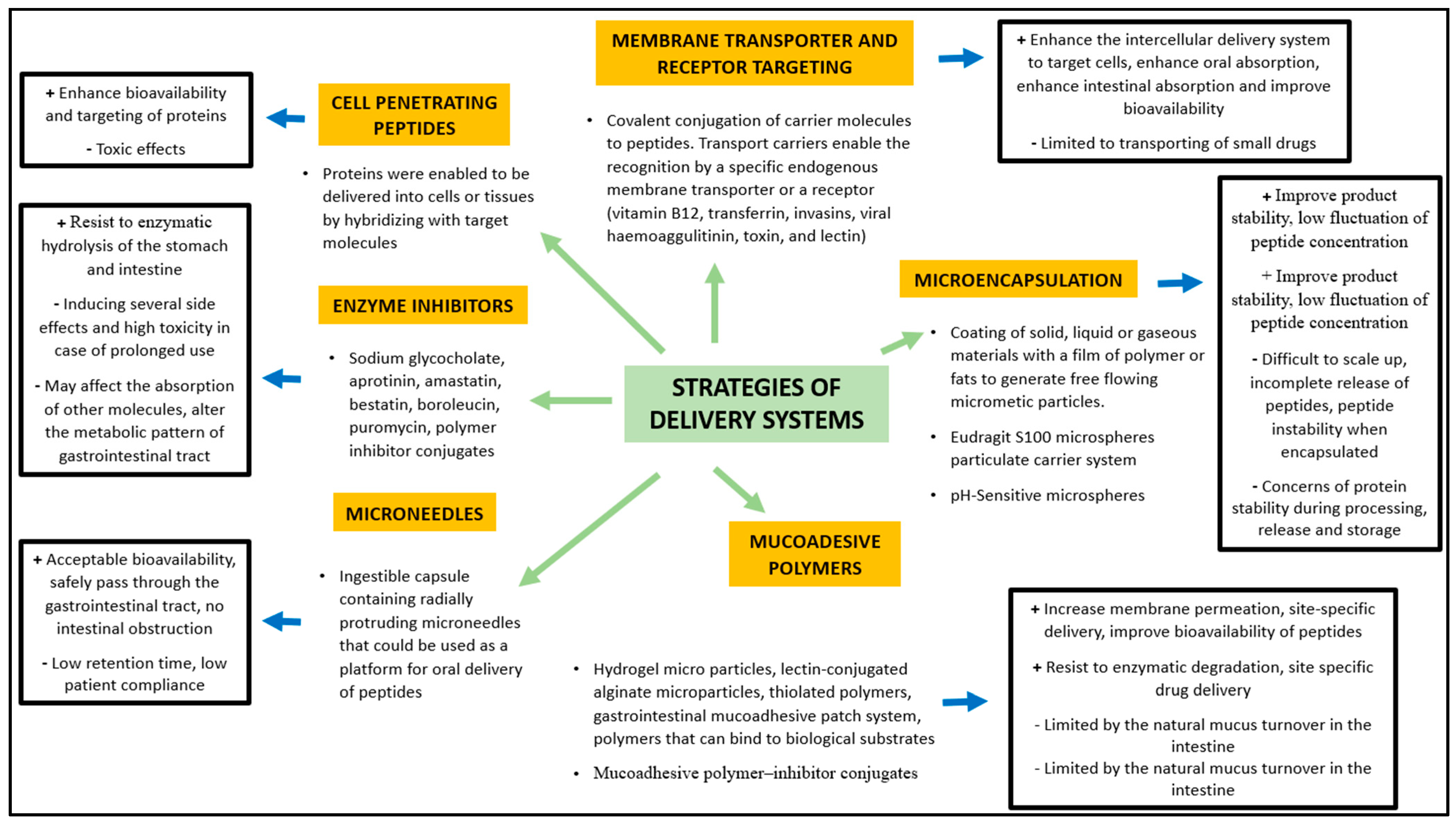
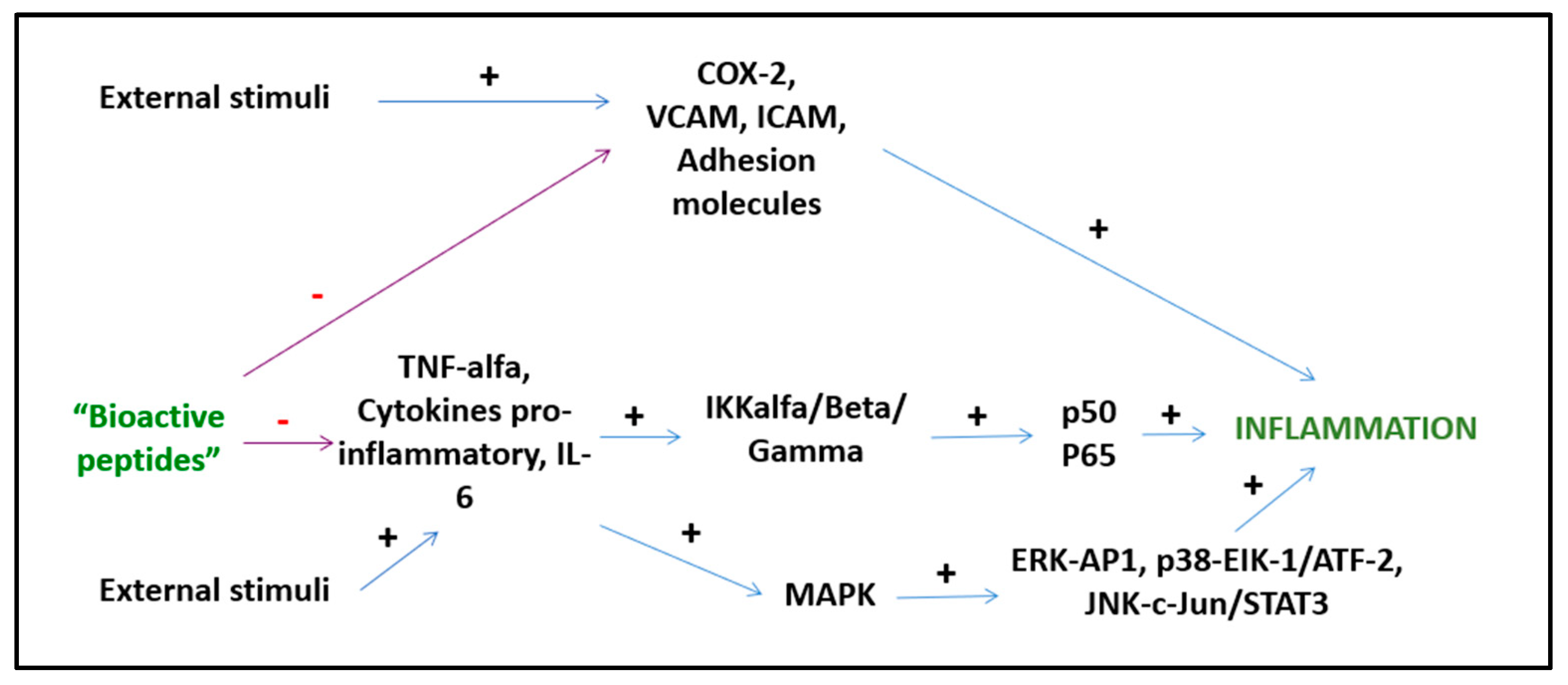
| Level of Evidence | |
|---|---|
| Hypertension | A (Meta-analysis of RCTs) |
| Dyslipidemia | A (Meta-analysis of RCTs) |
| Metabolic Syndrome | C (Single RCT or open label clinical trials) |
| Obesity | C (Single RCT or open label clinical trials) |
| Type 2 diabetes | C (Single RCT or open label clinical trials) |
| Immune dysfunction | D (Preclinical studies, no evidence in humans) |
| Cancer | D (Preclinical studies, no evidence in humans) |
| Pain | D (Preclinical studies, no evidence in humans) |
| Infections | D (Preclinical studies, no evidence in humans) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colletti, A.; Favari, E.; Grandi, E.; Cicero, A.F.G. Pharmacodynamics and Clinical Implications of the Main Bioactive Peptides: A Review. Nutraceuticals 2022, 2, 404-419. https://doi.org/10.3390/nutraceuticals2040030
Colletti A, Favari E, Grandi E, Cicero AFG. Pharmacodynamics and Clinical Implications of the Main Bioactive Peptides: A Review. Nutraceuticals. 2022; 2(4):404-419. https://doi.org/10.3390/nutraceuticals2040030
Chicago/Turabian StyleColletti, Alessandro, Elda Favari, Elisa Grandi, and Arrigo F. G. Cicero. 2022. "Pharmacodynamics and Clinical Implications of the Main Bioactive Peptides: A Review" Nutraceuticals 2, no. 4: 404-419. https://doi.org/10.3390/nutraceuticals2040030
APA StyleColletti, A., Favari, E., Grandi, E., & Cicero, A. F. G. (2022). Pharmacodynamics and Clinical Implications of the Main Bioactive Peptides: A Review. Nutraceuticals, 2(4), 404-419. https://doi.org/10.3390/nutraceuticals2040030








