Obesity Prevention Effects of Avocado (Persea americana) Seed Powder in High-Fat Diet-Induced Obesity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feed Preparation
2.1.1. Nutritional Analysis of Food Pellets
2.1.2. Elemental Analysis of Food Pellets
2.2. In Vivo Assays
2.2.1. Animal Model
2.2.2. Histopathology
2.3. Phytochemical Analysis
2.4. Data Analysis
3. Results
3.1. Nutritional and Elemental Analysis
3.2. In Vivo Study
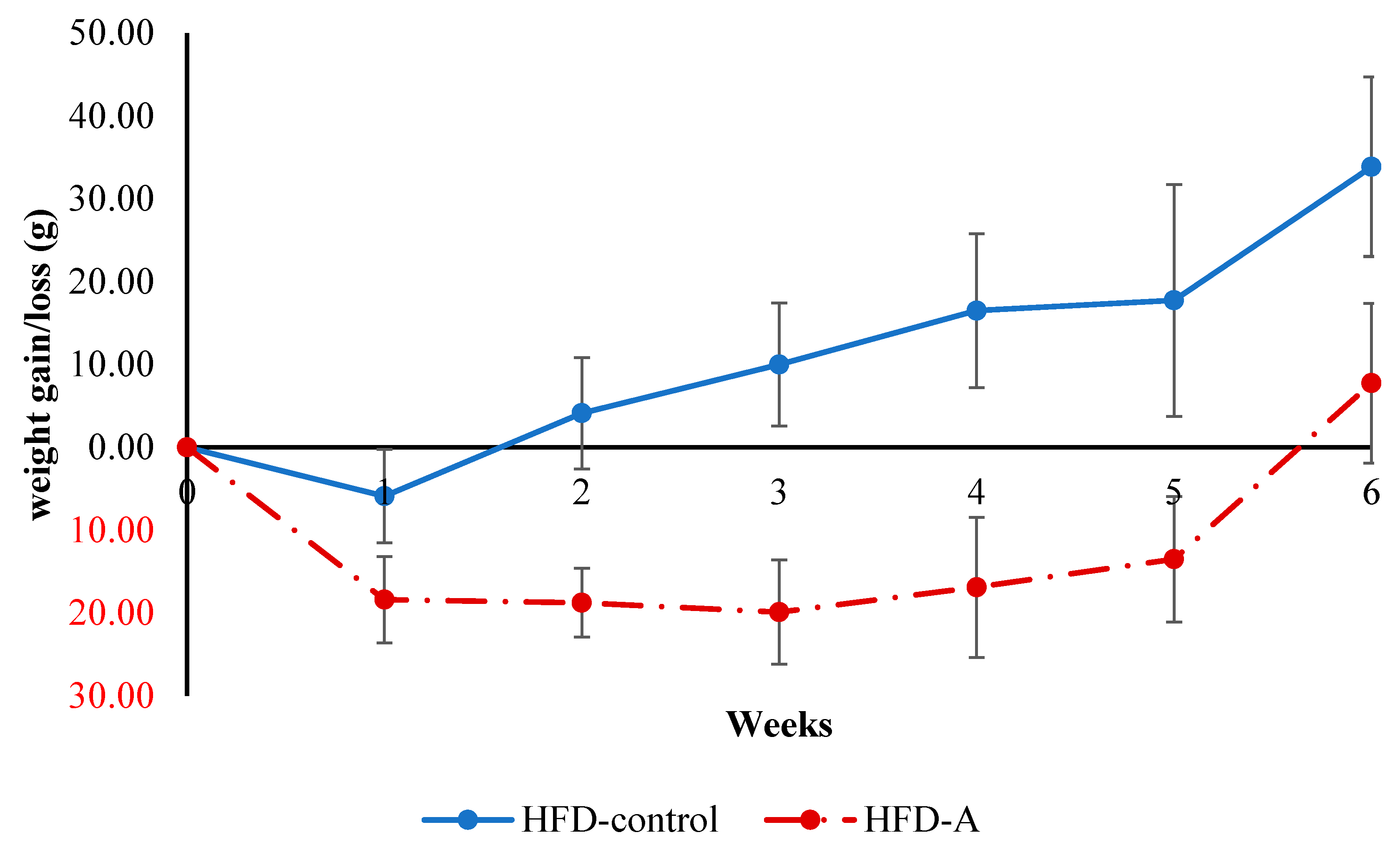
3.2.1. Animal Food Intake and Effects of Avocado Seed Powder on Animal Weights
3.2.2. Effects of Avocado Seed Powder on Animal Organ Weight and Biochemical Parameters
3.2.3. Post-Mortem Results
3.3. Phytochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Tzenios, N. Obesity as a Risk Factor for Different Types of Cancer. EPRA Int. J. Res. Dev. 2023, 8, 97–100. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2021, 23, e13366. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Obesity Day 2022—Accelerating Action to Stop Obesity; WHO: Geneva, Switzerland, 2022.
- Iwu, M.M. Introduction: Therapeutic Agents from Ethnomedicine. In Advances in Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–22. [Google Scholar]
- Ruchi, S. Role of nutraceuticals in health care: A review. Int. J. Green Pharm. 2017, 11. [Google Scholar] [CrossRef]
- Nijhawan, P.; Behl, T. Nutraceuticals in the management of obesity. Obes. Med. 2019, 17, 100168. [Google Scholar] [CrossRef]
- Devalaraja, S.; Jain, S.; Yadav, H. Exotic fruits as therapeutic complements for diabetes, obesity and metabolic syndrome. Food Res. Int. 2011, 44, 1856–1865. [Google Scholar] [CrossRef]
- García-Berumen, C.I.; Vargas-Vargas, M.A.; Ortiz-Avila, O.; Piña–Zentella, R.M.; Ramos-Gómez, M.; Figueroa–García, M.d.C.; Mejía-Zepeda, R.; Rodríguez–Orozco, A.R.; Saavedra–Molina, A.; Cortés-Rojo, C. Avocado oil alleviates non-alcoholic fatty liver disease by improving mitochondrial function, oxidative stress and inflammation in rats fed a high fat–High fructose diet. Front. Pharmacol. 2022, 13, 1089130. [Google Scholar] [CrossRef]
- Ochoa-Zarzosa, A.; Báez-Magaña, M.; Guzmán-Rodríguez, J.J.; Flores-Alvarez, L.J.; Lara-Márquez, M.; Zavala-Guerrero, B.; Salgado-Garciglia, R.; López-Gómez, R.; López-Meza, J.E. Bioactive molecules from native Mexican avocado fruit (Persea americana var. drymifolia): A review. Plant Foods Hum. Nutr. 2021, 76, 133–142. [Google Scholar] [CrossRef]
- Alhassan, A.J.; Sule, M.S.; Atiku, M.K.; Wudil, A.M.; Abubakar, H.; Mohammed, S.A. Effects of aqueous avocado pear (Persea americana) seed extract on alloxan induced diabetes rats. Greener J. Med. Sci. 2012, 2, 005–011. [Google Scholar] [CrossRef]
- Pahua-Ramos, M.E.; Ortiz-Moreno, A.; Chamorro-Cevallos, G.; Hernández-Navarro, M.D.; Garduño-Siciliano, L.; Necoechea-Mondragón, H.; Hernández-Ortega, M. Hypolipidemic effect of avocado (Persea americana Mill) seed in a hypercholesterolemic mouse model. Plant Foods Hum. Nutr. 2012, 67, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Ezejiofor, A.N.; Okorie, A.; Orisakwe, O.E. Hypoglycaemic and tissue-protective effects of the aqueous extract of Persea americana seeds on alloxan-induced albino rats. Malays. J. Med. Sci. 2013, 20, 31–39. [Google Scholar] [PubMed]
- Henry, L.N.; Mtaita, U.Y.; Kimaro, C.C. Nutritional efficacy of avocado seeds. Glob. J. Food Sci. Technol. 2015, 3, 192–196. [Google Scholar]
- Leite, J.J.G.; Brito, H.S.; Cordeiro, R.A.; Brilhante, R.S.N.; Sidrim, J.J.C.; Bertini, L.M.; de Morais, S.M.; Rocha, M.F.G. Chemical composition, toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts. Rev. Soc. Bras. Med. Trop. 2009, 42, 110–113. [Google Scholar] [CrossRef]
- Sebastián, D.F.-S.; Alaniz-Monreal, S.; Rabadán-Chávez, G.; Vázquez-Manjarrez, N.; Hernández-Ortega, M.; Gutiérrez-Salmeán, G. Anthocyanins: Potential Therapeutic Approaches towards Obesity and Diabetes Mellitus Type 2. Molecules 2023, 28, 1237. [Google Scholar] [CrossRef]
- Aloo, S.-O.; Ofosu, F.K.; Kim, N.-H.; Kilonzi, S.M.; Oh, D.-H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Talabi, J.Y.; Osukoya, O.A.; Ajayi, O.O.; Adegoke, G.O. Nutritional and antinutritional compositions of processed Avocado (Persea americana Mill) seeds. Asian J. Plant Sci. Res. 2016, 6, 6–12. [Google Scholar]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Domínguez-Riscart, J.; Millán-Martínez, M.; Lechuga-Sancho, A.M.; González-Domínguez, R. Exploring the association between circulating trace elements, metabolic risk factors, and the adherence to a Mediterranean diet among children and adolescents with obesity. Front. Public Health 2023, 10, 1016819. [Google Scholar] [CrossRef]
- Imafidon, K.; Amaechina, F. Effects of aqueous seed extract of Persea americana Mill. (avocado) on blood pressure and lipid profile in hypertensive rats. Adv. Biol. Res. 2010, 4, 116–121. [Google Scholar]
- Agoreyo, B.; Obansa, E.; Obanor, E. Comparative nutritional and phytochemical analyses of two varieties of Solanum melongena. Sci. World J. 2012, 7, 5–8. [Google Scholar]
- Dolan, S.P.; Capar, S.G. Multi-element analysis of food by microwave digestion and inductively coupled plasma-atomic emission spectrometry. J. Food Compos. Anal. 2002, 15, 593–615. [Google Scholar] [CrossRef]
- Mester, Z.; Sturgeon, R.E. Sample Preparation for Trace Element Analysis; Elsevier: Amsterdam, The Netherlands, 2003; Volume 41. [Google Scholar]
- SAMRC. Guidelines on Ethics for Medical Research: Use of Animals in Research and Training 2004; South African Medical Research Council: Cape Town, South Africa, 2004; pp. 11–16. [Google Scholar]
- South African National Standard. Care and Management of Laboratory Animals—Rodents (Mice, Rats and Hamsters) in the Care and Use of Animals for Scientific Purposes; SABS: Pretoria, South Africa, 2008; pp. 180–187. [Google Scholar]
- Olaokun, O.O.; McGaw, L.J.; van Rensburg, I.J.; Eloff, J.N.; Naidoo, V. Antidiabetic activity of the ethyl acetate fraction of Ficus lutea (Moraceae) leaf extract: Comparison of an in vitro assay with an in vivo obese mouse model. BMC Complement. Altern. Med. 2016, 16, 110. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Mokhele, M.; Tswaledi, D.; Aboyade, O.; Shai, J.; Katerere, D. Investigation of Aloe ferox leaf powder on anti-diabesity activity. S. Afr. J. Bot. 2020, 128, 174–181. [Google Scholar] [CrossRef]
- Rahman, H.A.; Sahib, N.G.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Hamid, A.A. Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complement. Altern. Med. 2017, 17, 122. [Google Scholar] [CrossRef]
- Nilsson, C.; Raun, K.; Yan, F.-F.; O Larsen, M.; Tang-Christensen, M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol. Sin. 2012, 33, 173–181. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef]
- Moon, M.-H.; Jeong, J.-K.; Lee, J.-H.; Park, Y.-G.; Lee, Y.-J.; Seol, J.-W.; Park, S.-Y. Antiobesity activity of a sphingosine 1-phosphate analogue FTY720 observed in adipocytes and obese mouse model. Exp. Mol. Med. 2012, 44, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Hamouda, A.F. Study on the effect of avocado on apoptosis, oxidative stress and injuries induced by diethyl nitrosamine in rat liver. J. Pharm. Pharmacol. 2015, 3, 243–252. [Google Scholar] [CrossRef][Green Version]
- Cha, M.C.; Chou, C.J.; Boozer, C.N. High-fat diet feeding reduces the diurnal variation of plasma leptin concentration in rats. Metabolism 2000, 49, 503–507. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A systematic review of anti-obesity medicinal plants—An update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef]
- Li, C.-P.; Song, Y.-X.; Lin, Z.-J.; Ma, M.-L.; He, L.-P. Essential trace elements in patients with dyslipidemia: A meta-analysis. Curr. Med. Chem. 2024, 31, 3604–3623. [Google Scholar] [CrossRef]
- Nimrouzi, M.; Abolghasemi, J.; Sharifi, M.H.; Nasiri, K.; Akbari, A. Thyme oxymel by improving of inflammation, oxidative stress, dyslipidemia and homeostasis of some trace elements ameliorates obesity induced by high-fructose/fat diet in male rat. Biomed. Pharmacother. 2020, 126, 110079. [Google Scholar] [CrossRef]
- Bouglé, D.; Bouhallab, S.; Bureau, F.; Zunquin, G. Effects of trace elements and calcium on diabetes and obesity, and their complications: Protective effect of dairy products–A mini-review. Dairy Sci. Technol. 2009, 89, 213–218. [Google Scholar] [CrossRef]
- Alagbaoso, C.A.; Tokunbo, I.I.; Osakwe, O.S. Comparative study of antioxidant activity and mineral composition of methanol extract of seeds of ripe and unripe avocado pear (Persea americana, Mill.). NISEB J. 2015, 15, 123–127. [Google Scholar]
- Hwang, H.S.; Kim, H.A.; Lee, S.H.; Yun, J.W. Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar. Biotechnol. 2009, 11, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152. [Google Scholar] [CrossRef] [PubMed]
- El-Awdan, S.A.; Jaleel, G.A.A.; Saleh, D.O. Grape seed extract attenuates hyperglycaemia-induced in rats by streptozotocin. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 203–209. [Google Scholar] [CrossRef][Green Version]
- Angéloco, N.; Deminice, R.; Leme ID, A.; Lataro, R.C.; Jordão, A.A. Bioelectrical impedance analysis and anthropometry for the determination of body composition in rats: Effects of high-fat and high-sucrose diets. Rev. Nutr. 2012, 25, 331–339. [Google Scholar] [CrossRef]
- Chadli, F.K.; Nazih, H.; Krempf, M.; Nguyen, P.; Ouguerram, K. Omega 3 fatty acids promote macrophage reverse cholesterol transport in hamster fed high fat diet. PLoS ONE 2013, 8, e61109. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; Lopez Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and in-flammatory diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Vijayalakshmi, M.A.; Prakash, K.; Bansal, V.S.; Meenakshi, J.; Amit, A. Review article: Herbal approach for obesity management. Am. J. Plant Sci. 2012, 3, 1003–1014. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar] [CrossRef]
- Kasabri, V.; Al-Hallaq, E.K.; Bustanji, Y.K.; Abdul-Razzak, K.K.; Abaza, I.F.; Afifi, F.U. Antiobesity and antihyperglycaemic effects of Adiantum capillus-veneris extracts: In vitro and in vivo evaluations. Pharm. Biol. 2016, 55, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G.; Kang, S.; Im, S.; Pak, Y.K. Cinnamic Acid Attenuates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice. Pharmaceutics 2022, 14, 1675. [Google Scholar] [CrossRef] [PubMed]
- de Melo, T.; Lima, P.; Carvalho, K.; Fontenele, T.; Solon, F.; Tomé, A.; de Lemos, T.; Fonseca, S.d.C.; Santos, F.; Rao, V.; et al. Ferulic acid lowers body weight and visceral fat accumulation via modulation of enzymatic, hormonal and inflammatory changes in a mouse model of high-fat diet-induced obesity. Braz. J. Med. Biol. Res. 2017, 50, e5630. [Google Scholar] [CrossRef] [PubMed]
- Singdam, P.; Naowaboot, J.; Senggunprai, L.; Boonloh, K.; Hipkaeo, W.; Pannangpetch, P. The mechanisms of neochlorogenic acid (3-caffeoylquinic acid) in improving glucose and lipid metabolism in rats with insulin resistance induced by a high fat-high fructose diet. Trends Sci. 2023, 20, 6455. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef] [PubMed]
- Kosińska, A.; Karamac, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, C. Targeting adipocyte apoptosis: A novel strategy for obesity therapy. Biochem. Biophys. Res. Commun. 2012, 417, 1–4. [Google Scholar] [CrossRef]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Hardisty, J.F.; Brix, A.E. Comparative hepatic toxicity: Prechronic/chronic liver toxicity in rodents. Toxicol. Pathol. 2005, 33, 35–40. [Google Scholar] [CrossRef]
- Wintola, O.; Sunmonu, T.; Afolayan, A. Toxicological evaluation of aqueous extract of Aloe ferox Mill. in loperamide-induced constipated rats. Hum. Exp. Toxicol. 2010, 30, 425–431. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; Kootstra-Ros, J.E.; Gansevoort, R.T.; Gregson, J.; Dullaart, R.P.F. Serum alkaline phosphatase and eisk of incident cardiovascular disease: Interrelationship with high sensitivity C-reactive protein. PLoS ONE 2015, 10, e0132822. [Google Scholar] [CrossRef]
- Zakariya, U.A.; Umar, U.A.; Dambazau, S.M.; Sulaiman, A. Comparative Hepatotoxic Effects of Aqueous and Phenolic Extracts of Avocado (Persea americana) Seed in Wistar Albino Rats. Int. J. Biochem. Res. Rev. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Ozolua, R.; Anaka, O.; Okpo, S.; Idogun, S. Acute and sub-acute toxicological assessment of the aqueous seed extract of Persea americana Mill (Lauraceae) in rats. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Peshti, V.; Gil, R.; Naiman, S.; Nahum, L.; Levin, E.; Kronfeld-Schor, N.; Cohen, H.Y. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010, 9, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Saxena, P.N.; Saxena, N. Biochemical and histological changes in rat liver caused by cypermethrin and be-ta-cyfluthrin. Arch. Ind. Hyg. Toxicol. 2013, 64, 57–67. [Google Scholar]


| Analysis | HFD | HFD-A |
|---|---|---|
| Moisture (%) | 9.30 | 8.10 |
| Protein (%) | 16.09 | 13.70 |
| Ash (%) | 5.46 | 4.82 |
| Fat (%) | 19.50 | 24.50 |
| Fiber (%) | 4.60 | 4.70 |
| Trace Elements (mg/L) | B | Cu | Se | Mo | As | Cr | Na | Mg | K | Ca | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFD | 0.22 | 0.60 | 0.23 | <−0.17 | 0.01 | <−0.08 | 34.01 | 17.08 | 69.03 | 42.75 | 0.52 | 1.89 |
| HFD-A | 0.25 | 0.34 | 0.16 | <−0.18 | 0.01 | <−0.07 | 30.26 | 22.52 | >105.0 | 57.08 | 0.76 | 2.52 |
| Week | HFD (Control) Group Weight (g) | HFD-A (Treatment) Group Weight (g) |
|---|---|---|
| 0 | 345.88 ± 9.16 | 306.75 ± 4.06 |
| 1 | 340.00 ± 11.48 | 288.38 ± 6.65 |
| 2 | 350.00 ± 11.77 | 288 ± 6.59 |
| 3 | 355.88 ± 10.37 | 286.88 ± 7.28 |
| 4 | 362.38 ± 12.93 | 289.88 ± 9.51 |
| 5 | 363.63 ± 15.84 | 293.25 ± 9.22 |
| 6 | 379.75 ± 12.67 | 314.5 ± 9.72 |
| Analysis | HFD | HFD-A |
|---|---|---|
| Total cholesterol (mmol/L) | 2.38 ± 0.13 | 2.23 ± 0.32 |
| Triglycerides (mmol/L) | 0.49 ± 0.10 | 0.34 ± 0.05 * |
| HDL (mmol/L) | 0.98 ± 0.05 | 1.00 ± 0.08 |
| LDL (mmol/L) | 0.59 ± 0.08 | 0.69 ± 0.11 |
| ALT (U/L) | 65.75 ± 14.67 | 70.75 ± 12.93 |
| ALP (U/L) | 130.00 ± 22.81 | 175.88 ± 28.40 * |
| AST (U/L) | 83.63 ± 18.95 | 74.29 ± 10.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhele, S.; Aboyade, O.; Katerere, D.R. Obesity Prevention Effects of Avocado (Persea americana) Seed Powder in High-Fat Diet-Induced Obesity in Rats. Nutraceuticals 2024, 4, 417-429. https://doi.org/10.3390/nutraceuticals4030025
Mokhele S, Aboyade O, Katerere DR. Obesity Prevention Effects of Avocado (Persea americana) Seed Powder in High-Fat Diet-Induced Obesity in Rats. Nutraceuticals. 2024; 4(3):417-429. https://doi.org/10.3390/nutraceuticals4030025
Chicago/Turabian StyleMokhele, Shoeshoe, Oluwaseyi Aboyade, and David R. Katerere. 2024. "Obesity Prevention Effects of Avocado (Persea americana) Seed Powder in High-Fat Diet-Induced Obesity in Rats" Nutraceuticals 4, no. 3: 417-429. https://doi.org/10.3390/nutraceuticals4030025
APA StyleMokhele, S., Aboyade, O., & Katerere, D. R. (2024). Obesity Prevention Effects of Avocado (Persea americana) Seed Powder in High-Fat Diet-Induced Obesity in Rats. Nutraceuticals, 4(3), 417-429. https://doi.org/10.3390/nutraceuticals4030025







