Effects of Ursolic Acid on Colorectal Cancer: A Review of Recent Evidence
Abstract
1. Introduction
2. Effects of Ursolic Acid against Colorectal/Colon Cancer
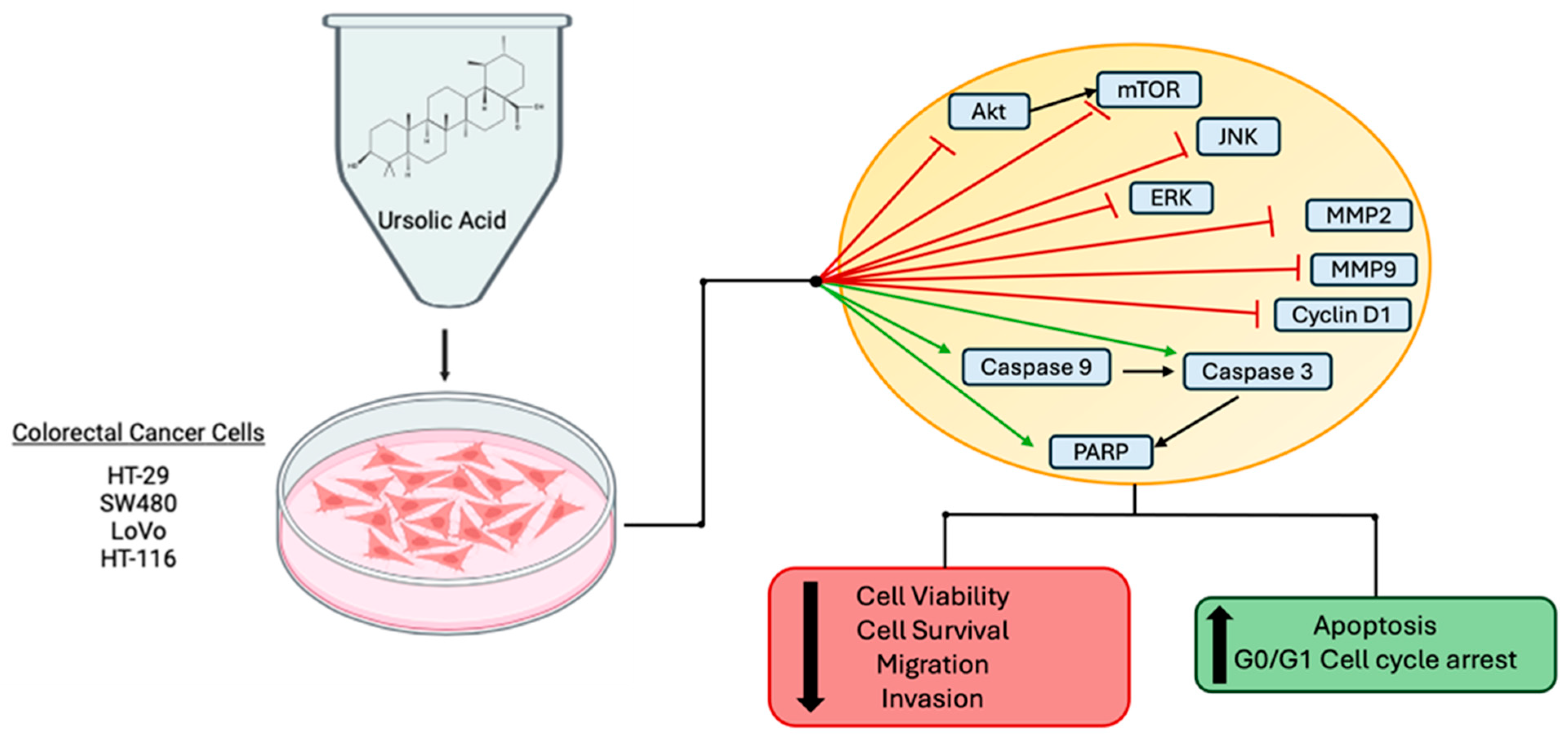
2.1. Effects of Ursolic Acid against Colorectal/Colon Cancer: In Vitro Evidence
2.2. Effects of UA in Combination with Chemotherapy Agents and Radiation
2.3. Effects of Ursolic Acid on Animal Models of Colorectal Cancer
2.4. Bioavailability and Potential Toxicity of UA
3. Discussion/Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.A.; Morreau, H.; de Miranda, N.F.C.C.; van Wezel, T. The Missing Heritability of Familial Colorectal Cancer. Mutagenesis 2020, 35, 221–231. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and Biological Hallmarks of Colorectal Cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef] [PubMed]
- Dariya, B.; Aliya, S.; Merchant, N.; Alam, A.; Nagaraju, G.P. Colorectal Cancer Biology, Diagnosis, and Therapeutic Approaches. Crit. Rev. Oncog. 2020, 25, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M.; Markowitz, S.D. The Molecular Pathogenesis of Colorectal Cancer and Its Potential Application to Colorectal Cancer Screening. Dig. Dis. Sci. 2015, 60, 762–772. [Google Scholar] [CrossRef]
- Kasi, A.; Handa, S.; Bhatti, S.; Umar, S.; Bansal, A.; Sun, W. Molecular Pathogenesis and Classification of Colorectal Carcinoma. Curr. Color. Cancer Rep. 2020, 16, 97–106. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Liu, Y.; Lau, H.C.-H.; Cheng, W.Y.; Yu, J. Gut Microbiome in Colorectal Cancer: Clinical Diagnosis and Treatment. Genom. Proteom. Bioinform. 2023, 21, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Saoudi González, N.; Salvà, F.; Ros, J.; Baraibar, I.; Rodríguez-Castells, M.; García, A.; Alcaráz, A.; Vega, S.; Bueno, S.; Tabernero, J.; et al. Unravelling the Complexity of Colorectal Cancer: Heterogeneity, Clonal Evolution, and Clinical Implications. Cancers 2023, 15, 4020. [Google Scholar] [CrossRef]
- Ashique, S.; Bhowmick, M.; Pal, R.; Khatoon, H.; Kumar, P.; Sharma, H.; Garg, A.; Kumar, S.; Das, U. Multi Drug Resistance in Colorectal Cancer- Approaches to Overcome, Advancements and Future Success. Adv. Cancer Biol.-Metastasis 2024, 10, 100114. [Google Scholar] [CrossRef]
- Sellers, R.S.; Morton, D. The Colon: From Banal to Brilliant. Toxicol. Pathol. 2014, 42, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.D.L.; Otegbeye, E.E.; Zong, X.; Demb, J.; Nickel, K.B.; Olsen, M.A.; Mutch, M.; Davidson, N.O.; Gupta, S.; Cao, Y. Red-Flag Signs and Symptoms for Earlier Diagnosis of Early-Onset Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2023, 115, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Briggs, N.L.; Ton, M.; Malen, R.C.; Reedy, A.M.; Cohen, S.A.; Phipps, A.I.; Burnett-Hartman, A.N.; Newcomb, P.A. Colorectal Cancer Pre-Diagnostic Symptoms Are Associated with Anatomic Cancer Site. BMC Gastroenterol. 2024, 24, 65. [Google Scholar] [CrossRef]
- Bissery, M.C.; Nohynek, G.; Sanderink, G.J.; Lavelle, F. Docetaxel (Taxotere): A Review of Preclinical and Clinical Experience. Part I: Preclinical Experience. Anticancer Drugs 1995, 6, 339–355. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Panda, S.S.; Thangaraju, M.; Lokeshwar, B.L. Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules 2022, 27, 8981. [Google Scholar] [CrossRef] [PubMed]
- Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Bobinas, C.; Janulis, V. Variation of Triterpenes in Apples Stored in a Controlled Atmosphere. Molecules 2021, 26, 3639. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.R.; Neto, C.C. Ursolic Acid and Its Esters: Occurrence in Cranberries and Other Vaccinium Fruit and Effects on Matrix Metalloproteinase Activity in DU145 Prostate Tumor Cells: Anti-Tumor Activity and Content of Ursolic Acid from Vaccinium Fruit. J. Sci. Food Agric. 2011, 91, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Triterpenic Content and Chemometric Analysis of Virgin Olive Oils from Forty Olive Cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef]
- Guinda, Á.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Petkova, N.; Tumbarski, Y.; Vrancheva, R.; Stoyanova, M. Lavender Waste-Promising Source of Triterpenoids and Polyphenols with Antioxidant and Antimicrobial Activity. Ind. Technol. 2018, 5, 26–32. [Google Scholar]
- Silva, M.G.V.; Vieira, I.G.P.; Mendes, F.N.P.; Albuquerque, I.L.; dos Santos, R.N.; Silva, F.O.; Morais, S.M. Variation of Ursolic Acid Content in Eight Ocimum Species from Northeastern Brazil. Molecules 2008, 13, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, R.H. Triterpenoids Isolated from Apple Peels Have Potent Antiproliferative Activity and May Be Partially Responsible for Apple’s Anticancer Activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical Composition and Antioxidant and Anti-Inflammatory Potential of Peels and Flesh from 10 Different Pear Varieties (Pyrus Spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants–Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; İPekci, B.; Koca, İ.; Odabaş, H.İ. Assessing Ursolic Acid Contents of Some Commonly Consumed Herbs Grown in Turkey. Gümüşhane Üniversitesi Fen Bilim. Enstitüsü Derg. 2022, 12, 301–308. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Kocjan, R.; Nowak, R. Effect of Different Extraction Techniques on Quantification of Oleanolic and Ursolic Acid in Lamii Albi Flos. Ind. Crops Prod. 2013, 44, 373–377. [Google Scholar] [CrossRef]
- Kowalski, R. Studies of Selected Plant Raw Materials as Alternative Sources of Triterpenes of Oleanolic and Ursolic Acid Types. J. Agric. Food Chem. 2007, 55, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, H.; Sui, Z.; Jing, F.; Quan, X.; Zhao, W.; Liu, G. Ursolic Acid Exhibits Anti-Inflammatory Effects through Blocking TLR4-MyD88 Pathway Mediated by Autophagy. Cytokine 2019, 123, 154726. [Google Scholar] [CrossRef] [PubMed]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Yadav, D.K. Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int. J. Mol. Sci. 2021, 22, 12162. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, L.; Tan, Y.; Xu, W.; Liu, S.; Zhou, J.; Hu, Y.; Lin, J.; Yao, X.; Mi, P.; et al. Recent Progress in the Development of Ursolic Acid Derivatives as Anti-Diabetes and Anti-Cardiovascular Agents. Chem. Biol. Drug Des. 2023, 102, 1643–1657. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Soon, C.Y.; Tan, J.B.L.; Wong, S.K.; Hui, Y.W. Ursolic Acid: An Overview on Its Cytotoxic Activities against Breast and Colorectal Cancer Cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef]
- Kornel, A.; Nadile, M.; Tsiani, E. Evidence of the Beneficial Effects of Ursolic Acid against Lung Cancer. Molecules 2022, 27, 7466. [Google Scholar] [CrossRef] [PubMed]
- Kornel, A.; Nadile, M.; Retsidou, M.I.; Sakellakis, M.; Gioti, K.; Beloukas, A.; Sze, N.S.K.; Klentrou, P.; Tsiani, E. Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 7414. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic Acid, a Potential Anticancer Compound for Breast Cancer Therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, W.-J.; Yang, Q.-Y. Effects of Ursolic Acid and Oleanolic Acid on Human Colon Carcinoma Cell Line HCT15. World J. Gastroenterol. 2002, 8, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Xuan, Y.; Zheng, S.; Dong, Q.; Zhang, S. Ursolic Acid Inhibits Proliferation and Induces Apoptosis of HT-29 Colon Cancer Cells by Inhibiting the EGFR/MAPK Pathway. J. Zhejiang Univ. Sci. B 2009, 10, 668–674. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Preto, A.; Seruca, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Luteolin, Quercetin and Ursolic Acid Are Potent Inhibitors of Proliferation and Inducers of Apoptosis in Both KRAS and BRAF Mutated Human Colorectal Cancer Cells. Cancer Lett. 2009, 281, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Limami, Y.; Pinon, A.; Leger, D.Y.; Mousseau, Y.; Cook-Moreau, J.; Beneytout, J.-L.; Delage, C.; Liagre, B.; Simon, A. HT-29 Colorectal Cancer Cells Undergoing Apoptosis Overexpress COX-2 to Delay Ursolic Acid-Induced Cell Death. Biochimie 2011, 93, 749–757. [Google Scholar] [CrossRef]
- Limami, Y.; Pinon, A.; Leger, D.Y.; Pinault, E.; Delage, C.; Beneytout, J.-L.; Simon, A.; Liagre, B. The P2Y2/Src/P38/COX-2 Pathway Is Involved in the Resistance to Ursolic Acid-Induced Apoptosis in Colorectal and Prostate Cancer Cells. Biochimie 2012, 94, 1754–1763. [Google Scholar] [CrossRef]
- Rawat, L.; Nayak, V. Ursolic Acid Disturbs ROS Homeostasis and Regulates Survival-Associated Gene Expression to Induce Apoptosis in Intestinal Cancer Cells. Toxicol. Res. 2021, 10, 369–375. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Wei, L.; Hong, Z.; Sferra, T.J.; Peng, J. Ursolic Acid Inhibits Colorectal Cancer Angiogenesis through Suppression of Multiple Signaling Pathways. Int. J. Oncol. 2013, 43, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Wei, L.; Shen, A.; Sferra, T.J.; Hong, Z.; Peng, J. Ursolic Acid Promotes Colorectal Cancer Cell Apoptosis and Inhibits Cell Proliferation via Modulation of Multiple Signaling Pathways. Int. J. Oncol. 2013, 43, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, C.; Jou, D.; Lü, J.; Zhang, C.; Lin, L.; Lin, J. Ursolic Acid Inhibits the Growth of Colon Cancer-Initiating Cells by Targeting STAT3. Anticancer Res. 2013, 33, 4279–4284. [Google Scholar]
- Wang, J.; Liu, L.; Qiu, H.; Zhang, X.; Guo, W.; Chen, W.; Tian, Y.; Fu, L.; Shi, D.; Cheng, J.; et al. Ursolic Acid Simultaneously Targets Multiple Signaling Pathways to Suppress Proliferation and Induce Apoptosis in Colon Cancer Cells. PLoS ONE 2013, 8, e63872. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, E.A.; Jung, J.H.; Park, J.E.; Kim, D.S.; Shim, B.S.; Kim, S.-H. Ursolic Acid Induces Apoptosis in Colorectal Cancer Cells Partially via Upregulation of MicroRNA-4500 and Inhibition of JAK2/STAT3 Phosphorylation. Int. J. Mol. Sci. 2018, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, Q.-Y.; Liu, J.; Peng, J.; Chen, Y.-Q.; Sferra, T.J.; Lin, J.-M. Ursolic Acid Suppresses the Invasive Potential of Colorectal Cancer Cells by Regulating the TGF-Β1/ZEB1/miR-200c Signaling Pathway. Oncol. Lett. 2019, 18, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.; Yi, F.; Duan, C.; Wang, Q.; He, N.; Zhu, L.; Li, Q.; Deng, W. Ursolic Acid Inhibits Tumor Growth via Epithelial-to-Mesenchymal Transition in Colorectal Cancer Cells. Biol. Pharm. Bull. 2019, 42, 685–691. [Google Scholar] [CrossRef]
- Yang, M.; Hu, C.; Cao, Y.; Liang, W.; Yang, X.; Xiao, T. Ursolic Acid Regulates Cell Cycle and Proliferation in Colon Adenocarcinoma by Suppressing Cyclin B1. Front. Pharmacol. 2020, 11, 622212. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Wu, M.; Liu, L.; Chen, Y.; Chen, X.; Zhuang, M.; Xie, Q.; Cheng, Y.; Li, J.; Shen, Z.; et al. Targeting NUFIP1 Suppresses Growth and Induces Senescence of Colorectal Cancer Cells. Front. Oncol. 2021, 11, 681425. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wang, S.-S.; Shen, K.-P.; Chen, L.; Peng, X.; Chen, J.-F.; An, H.-M.; Hu, B. Ursolic Acid Induces Apoptosis and Anoikis in Colorectal Carcinoma RKO Cells. BMC Complement. Med. Ther. 2021, 21, 52. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Yang, H.; Ren, S.; Sun, Q.; Zhao, H.; Ming, T.; Tang, S.; Tao, Q.; Zeng, S.; et al. Ursolic Acid Inhibits the Activation of Smoothened-Independent Non-Canonical Hedgehog Pathway in Colorectal Cancer by Suppressing AKT Signaling Cascade. Phytother. Res. 2022, 36, 3555–3570. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, S.; Tao, Q.; Ming, T.; Lei, J.; Liang, Y.; Peng, Y.; Wang, M.; Liu, M.; Yang, H.; et al. Ursolic Acid Suppresses Colorectal Cancer by Down-Regulation of Wnt/β-Catenin Signaling Pathway Activity. J. Agric. Food Chem. 2023, 71, 3981–3993. [Google Scholar] [CrossRef]
- Zhang, M.; Xiang, F.; Sun, Y.; Liu, R.; Li, Q.; Gu, Q.; Kang, X.; Wu, R. Ursolic Acid Inhibits the Metastasis of Colon Cancer by Downregulating ARL4C Expression. Oncol. Rep. 2024, 51, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Leng, P.; Xu, W.; Sun, J.-L.; Ni, B.-B.; Liu, G.-W. Investigating the Multitarget Pharmacological Mechanism of Ursolic Acid Acting on Colon Cancer: A Network Pharmacology Approach. Evid. Based Complement. Altern. Med. 2021, 2021, 9980949. [Google Scholar] [CrossRef]
- Wang, J.; Guo, W.; Chen, W.; Yu, W.; Tian, Y.; Fu, L.; Shi, D.; Tong, B.; Xiao, X.; Huang, W.; et al. Melatonin Potentiates the Antiproliferative and Pro-Apoptotic Effects of Ursolic Acid in Colon Cancer Cells by Modulating Multiple Signaling Pathways. J. Pineal. Res. 2013, 54, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.R.; Lima, C.F.; Pedro, D.F.N.; Wilson, J.M.; Kristiansen, K.; Pereira-Wilson, C. Ursolic Acid Induces Cell Death and Modulates Autophagy through JNK Pathway in Apoptosis-Resistant Colorectal Cancer Cells. J. Nutr. Biochem. 2013, 24, 706–712. [Google Scholar] [CrossRef]
- Shan, J.; Xuan, Y.; Zhang, Q.; Zhu, C.; Liu, Z.; Zhang, S. Ursolic Acid Synergistically Enhances the Therapeutic Effects of Oxaliplatin in Colorectal Cancer. Protein. Cell 2016, 7, 571–585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shan, J.-Z.; Xuan, Y.-Y.; Zhang, Q.; Huang, J.-J. Ursolic Acid Sensitized Colon Cancer Cells to Chemotherapy under Hypoxia by Inhibiting MDR1 through HIF-1α. J. Zhejiang Univ. Sci. B 2016, 17, 672–682. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Shi, H.; Chen, H.; Tao, J.; Shen, R.; Wang, T. Ursolic Acid Enhances the Therapeutic Effects of Oxaliplatin in Colorectal Cancer by Inhibition of Drug Resistance. Cancer Sci. 2018, 109, 94–102. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wang, S.-S.; Shen, K.-P.; Huang, X.-W.; Li, M.; Chen, L.; Peng, X.; An, H.-M.; Hu, B. Ursolic Acid Potentiated Oxaliplatin to Induce Apoptosis in Colorectal Cancer RKO Cells. Pharmazie 2020, 75, 246–249. [Google Scholar]
- Piet, M.; Paduch, R. Ursolic and Oleanolic Acids in Combination Therapy Inhibit Migration of Colon Cancer Cells through Down-Regulation of the uPA/uPAR-Dependent MMPs Pathway. Chem. Biol. Interact. 2022, 368, 110202. [Google Scholar] [CrossRef]
- Hu, D.; Meng, R.Y.; Nguyen, T.V.; Chai, O.H.; Park, B.H.; Lee, J.-S.; Kim, S.M. Inhibition of Colorectal Cancer Tumorigenesis by Ursolic Acid and Doxorubicin Is Mediated by Targeting the Akt Signaling Pathway and Activating the Hippo Signaling Pathway. Mol. Med. Rep. 2023, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Y.; Liu, Y.; Luo, Z.; Law, B.Y.K.; Zheng, Y.; Huang, X.; Li, W. Ursolic Acid Enhances the Antitumor Effects of Sorafenib Associated with Mcl-1-Related Apoptosis and SLC7A11-Dependent Ferroptosis in Human Cancer. Pharmacol. Res. 2022, 182, 106306. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Tak, J.K.; Kim, S.T.; Nam, W.S.; Kim, S.Y.; Park, K.M.; Park, J.-W. Sensitization of Ionizing Radiation-Induced Apoptosis by Ursolic Acid. Free Radic. Res. 2012, 46, 339–345. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, V.R.; Sung, B.; Reuter, S.; Kannappan, R.; Deorukhkar, A.; Diagaradjane, P.; Wei, C.; Baladandayuthapani, V.; Krishnan, S.; et al. Ursolic Acid Inhibits Growth and Metastasis of Human Colorectal Cancer in an Orthotopic Nude Mouse Model by Targeting Multiple Cell Signaling Pathways: Chemosensitization with Capecitabine. Clin. Cancer Res. 2012, 18, 4942–4953. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.; Zu, Y.; Zhao, C.; Sun, X.; Zhang, Z.; Zhang, L. Physicochemical Properties and Oral Bioavailability of Ursolic Acid Nanoparticles Using Supercritical Anti-Solvent (SAS) Process. Food Chem. 2012, 132, 319–325. [Google Scholar] [CrossRef]
- Yin, M.-C.; Lin, M.-C.; Mong, M.-C.; Lin, C.-Y. Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, G.; Si, D.; Liu, C. Quantitation of Ursolic Acid in Human Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Pharmacokinetic Study. J. Chromatogr. B 2011, 879, 219–224. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhou, S.-Y.; Qian, Z.-Z.; Zhang, H.-L.; Qiu, L.-H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.-S.; Wang, H.-Q. Evaluation of Toxicity and Single-Dose Pharmacokinetics of Intravenous Ursolic Acid Liposomes in Healthy Adult Volunteers and Patients with Advanced Solid Tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef]
- Mishra, V.; Soren, A.D.; Yadav, A.K. Toxicological Evaluations of Betulinic Acid and Ursolic Acid; Common Constituents of Houttuynia Cordata Used as an Anthelmintic by the Naga Tribes in North-East India. Future J. Pharm. Sci. 2021, 7, 39. [Google Scholar] [CrossRef]
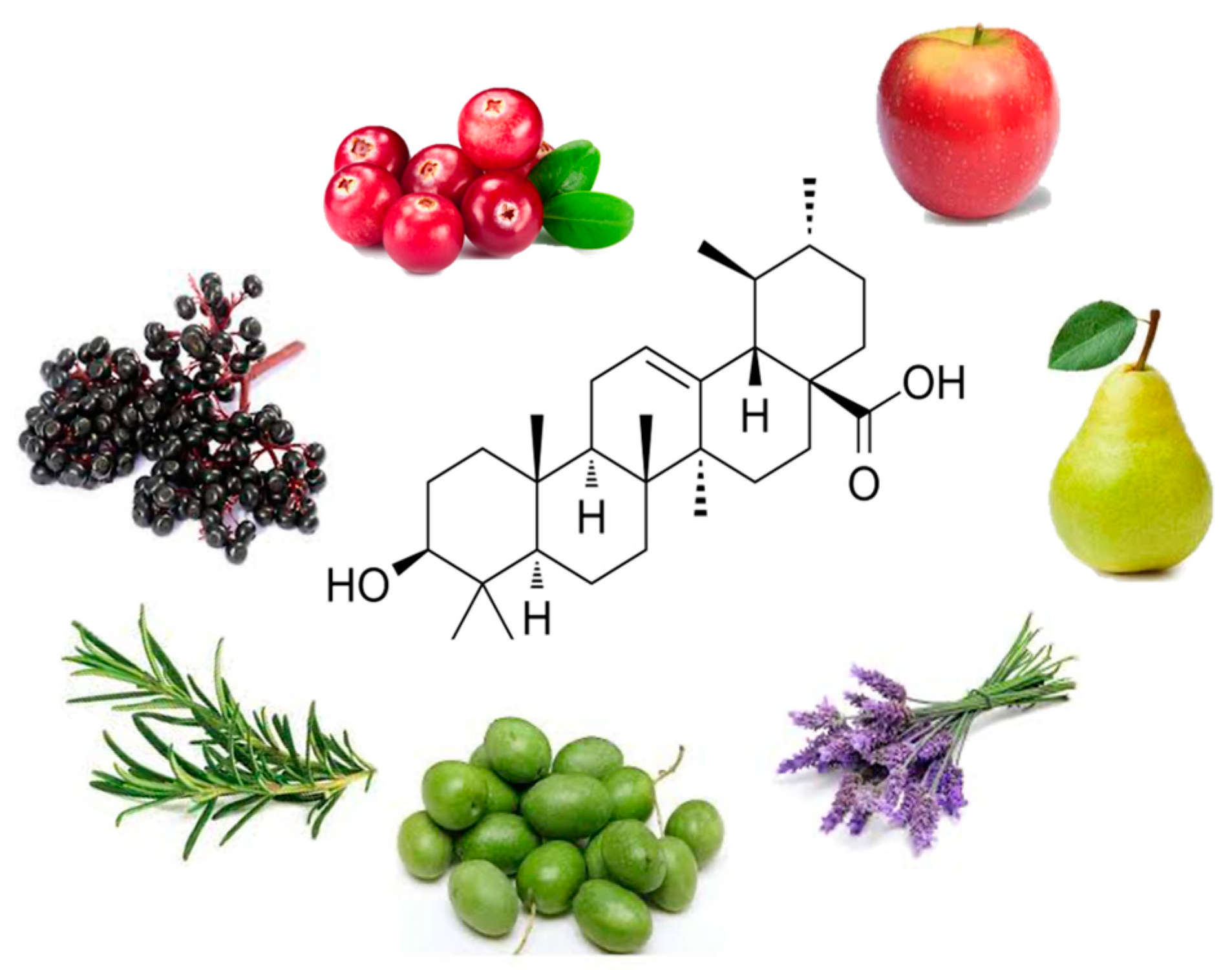


| Source Common and Botanical Name | Concentration of UA (FW = Fresh Weight) (DW = Dry Weight) | Reference | |
|---|---|---|---|
| Fruits | Apple (peel) Malus | 1.52 mg/g DW | [28] |
| Apple (whole fruit) Malus | 0.77 ± 0.1 mg/g to 1.85 ± 0.17 mg/g | [22] | |
| Cranberry Vaccinium macrocarpon | 0.46–1.09 mg/g FW | [23] | |
| Pear pyrus | 0.3481 mg/g (mature fruit) 0.1293 mg/g FW (young fruit) | [29,30] | |
| Herbs | Basil Ocimum tenuiflorum | 20.2 mg/g DW | [27] |
| Rosemary Rosmarinus officinalis | 15.8–29.5 mg/g | [31] | |
| Thyme Thymus vulgaris | 9.4 mg/g DW | [31,32] | |
| Oregano Origanum vulgare | 2.8 mg/g DW | [31] | |
| Sage Salvia officinalus | 18 mg/g DW | [31] | |
| Flowering plants | Lavender Lavandula | 106.7–153.1 mg/g F.W. 3.463–6.484 mg/g D.W. 10.5 mg/g (flowers) | [26,31] |
| White deadnettle Lamii albi flos | 39.1–110.4 mg/g D.W. | [33] | |
| Oleander leaves Nerium oleander | 12.7 mg/g DW | [31] | |
| Rosinweed Silphium sp. Flowers | 17.95–22.05 mg/g D.W. | [34] | |
| Olive leaves Olea europeae | 1.8 mg/g DW | [31] | |
| Other | Arabica coffee leaves Coffea arabic | 18 mg/g DW | [31] |
| Cell Type | Dose/Duration | Effects | Mechanism | Reference |
|---|---|---|---|---|
| HCT15 | UA 30 µM | ↓ Cell viability Cell cycle arrest (G0/G1 phase) | Not examined | [45] |
| HT-29 | UA 10, 20 and 40 µM | ↓ Cell proliferation ↑ Apoptosis | ↓ p-EGFR ↓ p-ERK ½ ↓ p-p38 ↓ p-JNK ↓ Bcl-2 ↓ Bcl-xL ↑ Cleaved caspase 3 ↑ Cleaved caspase 9 | [46] |
| HCT15 CO115 | UA 2.5 and 4 µM (HCT15) and UA 10 and 15 µM (CO115) | ↓ Cell proliferation ↑ Apoptosis | ↓ p-AKT ↓ KRAS | [47] |
| HT-29 | UA 20 and 30 µM | ↓ Cell proliferation ↑ Apoptosis | ↑ Caspase 3 activity ↑ DNA fragmentation ↑ Cleaved Parp ↑ PGE2 concentration ↓ p-ERK ↑ p-p38 ↑COX-2 | [48] |
| HT-29 | UA 25 µM | ↑ ATP in cytosol ↑ P2Y2 mRNA ↑ COX-2 protein ↑ DNA fragmentation ↑p-p38↑ p-Src protein | [49] | |
| HCT-116 | UA 5, 34.7 and 50 µM | ↓ Cell viability ↑ Apoptosis ↑ ROS ↓ Cell migration | ↓ BCL-2 protein ↓ Survivin protein ↓ NFkB ↓ SP1 protein ↑ BAX mRNA ↑ P21 mRNA ↑ P53 mRNA ↓ FN1 mRNA ↓CDH2 ↓↓CTNNB1 ↓ Twist | [50] |
| HT-29 | UA 20, 40 and 80 µM | ↓ SHH protein and mRNA ↓ Gli-1 protein and mRNA ↓ VEGF-A protein and mRNA ↓ bFGF protein and mRNA | [51] | |
| HUVEC | UA 20, 40 and 80 µM | ↓ Cell viability ↓ Cell migration | Not examined | |
| HT-29 | UA 20, 40 and 80 µM | ↓ Cell viability ↓ Cell survival ↓ Cell cycle progression ↑ Apoptosis | ↓ Cells in s-phase ↓ Cyclin D1 protein and mRNA ↓ CDK4 protein and mRNA ↑ p21 protein and mRNA ↑ DNA fragmentation ↓ Bcl-2 protein and mRNA ↑ Bax protein and mRNA ↓ p-Erk1/2 protein ↓ p-JNK protein ↓ p-p38 protein | [52] |
| HCT116 HT29 SW480 | UA 25 µM | ↓ Cell viability ↓ Tumor sphere formation | ↓ p-STAT3 protein ↑ Cleaved caspase 3 | [53] |
| SW480 LoVo | UA 20, 40 and 60 µM | ↓ Cell viability ↓ Colony formation ↓ Cell migration ↑ Apoptosis | ↓ MMP9 mRNA ↑ CDH1 mRNA ↓ p-Akt ↓ p-mTOR ↑ p-PTEN ↓ p-JNK ↓ p-ERK ↓ COX-2 protein and mRNA ↓ PGE2 ↑ NF-kB translocation ↑ p300 translocation ↑ Cleaved PARP ↑ Cleaved caspase -3, -8 and -9 | [54] |
| HCT116 HT29 | UA 20, 40, 60 and 80 µM | ↑ Apoptosis ↓ Cell viability | ↑ TUNEL positive cells ↑ Cleaved PARP ↑ Cleaved caspase-3 ↓ p-JAK2 ↓ p-STAT3 ↓ STAT3 nuclear translocation ↓ miR-4500 mRNA expression | [55] |
| HCT116 HCT-8 | UA 40 µM | ↓ Cell viability ↓ Cell migration ↓ Cell invasion | ↓ TGF-β1 protein ↓ p-Smad2/3 ↓ p-FAK ↓ ZEB1 ↓ N-cadherin ↑ miR-200a mRNA ↑ miR-200c mRNA | [56] |
| SW620 HCT116 | UA 10, 30 and 60 µM | ↓ Cell viability ↓ Clone formation ↓ Cell migration ↓ EMT | ↑ Caspase 3 activity ↓ Mesenchymal phenotype ↑ E-cad protein ↓ Integrin protein ↓ Vimentin protein ↓ Twist protein ↓ Zeb1 protein | [57] |
| SW-480 HCT116 | UA 10 µM | ↓ Cell viability ↑ Cell injury Cell cycle arrest (S phase) | ↓ CCNB1 mRNA and protein ↓ CDK1 mRNA and protein ↓ CDK2 mRNA ↓ CCND1 mRNA and protein ↓ CCNA2 mRNA and protein ↓ CDC20 mRNA and protein ↓ CKS2 mRNA ↓ CCNB2 mRNA | [58] |
| HT-29 HCT116 | UA 2.5–40 µM | ↓ Cell viability ↓ Cell number ↓ Colony formation ↑ Apoptosis | ↓ NUFIP1 mRNA | [59] |
| RKO | UA 14, 17 and 20 µM Conventional conditions | ↓ Cell viability ↑ Apoptosis ↑ ROS Cell cycle arrest (G0/G1 phase) | ↑ Casp-3, -8 and -9 activity ↑ Bax protein ↓ Bcl-2 protein | [60] |
| UA 25, 28, 31 µM 24 h Poly-HEMA coated plates | ↓ Cell viability ↑ Apoptosis ↑ Anoikis | ↓ p-FAK ↓ p-PI3K ↓ p-Akt ↓ N-cadherin ↑ E-cadherin | ||
| HCT-116hSMO− | UA 20 µM | ↓ Cell proliferation ↓ Migration ↑ Apoptosis | ↓ Bcl-2 protein and mRNA ↑ Bax protein and mRNA ↑ Caspase -3 and -9 mRNA ↓ c-Myc protein and mRNA ↓ GLI1 protein and mRNA ↓ SHH protein and mRNA ↓ SUFU protein and mRNA ↓ p-Akt protein | [61] |
| SW620 | UA 7.5, 15 and 30 µM | ↓ Cell proliferation ↓ Migration ↑ Apoptosis Cell cycle arrest–G0/G1 phase | ↓ c-Myc protein ↓ Cyclin D1 protein ↓ Wnt4 mRNA and protein ↓ TCF4 mRNA and protein ↓ LEF1 mRNA and protein ↑ GSK3β mRNA and protein ↓ p-GSK3-β protein ↓ β-catenin mRNA and protein ↑ p-β-catenin | [62] |
| HCT-116 and SW480 | UA 15 µM | ↓ Migration ↓ Invasion | ↓ p-Akt ↓ p-mTOR ↓ ARL4C ↓ MMP2 | [63] |
| N/A | UA–computational model | ↓ Cell proliferation ↑ Apoptosis ↓ Angiogenesis | [64] |
| Cell Type | Dose/Duration | Effects | Mechanism | Reference |
|---|---|---|---|---|
| SW480 LoVo | UA 20, 40 and 60 µM Melatonin 1 mM | ↓ Cell viability ↓ Cell migration ↑ Apoptosis | ↓ MMP9 mRNA expression ↑ Cleaved PARP ↑ Cleaved caspase -3 and -9 ↓ COX-2 protein and mRNA ↑ p300 cytoplasmic translocation ↑ NF-kB cytoplasmic translocation | [65] |
| HCT15 | UA 4 µM 5-FU 100 µM | ↑ Apoptosis | ↑ p-JNK p46 protein ↓ p-mTOR protein ↑ LC3-I protein ↑ LC3-II protein ↑ p62 protein ↑ p53 levels | [66] |
| SW480 SW620 LoVo RKO | UA 10 µmol/L Oxaliplatin 0.4 µmol/L | ↓ Cell viability ↑ Apoptosis | ↓ Mitochondrial membrane potential ↑ Cleaved caspase -3, -8 and -9 ↓ p-B-Raf ↓ p-MEK1/2 ↓ p-ERK1/2 ↓ p-Akt ↓ p-p38 ↓ p-JNK ↓ p-IKKα ↓ p-IkBα ↓ p-p65 ↓ p-NF-kB (plasma and nucleus) | [67] |
| RKO LoVo SW480 | UA 20 and 40 µM/L 5-FU 4 and 8 µM/L Oxaliplatin 0.5, 1 and 1.5 µM/L | ↓ Cell viability ↑ Chemosensitivity (hypoxia) ↑ Apoptosis | ↓ MDR1 protein and mRNA expression ↓ HIF-1α protein and mRNA ↓ VEGF | [68] |
| HCT8 SW480 | UA 20 µmo/L Oxa 0.4 µmol/L | ↓ Cell viability ↑ Apoptosis | ↑ Cleaved caspase -3 ↑ ROS ↑ NAPDH protein ↓ P-gp mRNA and protein ↓ MRP mRNA and protein ↓ BCRP mRNA and protein | [69] |
| RKO | UA 15 µM Oxa 2.5 µM 48 h | ↓ Cell survival ↑ Apoptosis | ↑ Caspase -3, -8 and -9 activity ↑ Cleaved PARP ↓ Survivin protein ↓ XIAP protein | [70] |
| HT-29 SW 620 | UA 5 µg/mL OA 100 µg/mL CPT-11 0.075 µg/ml | ↓ Cell viability ↓ Migration | N/A | [71] |
| HCT116 HT-29 | UA 15 µM DOX 1.5 µM | ↓ Cell proliferation ↑ Apoptosis ↓ Colony formation ↓ Cell migration G1 cell cycle arrest | ↑ Cleaved caspase -9 ↑ Cleaved PARP ↑ E-cadherin ↓ MMP-9 ↓ uPA ↓ CDK4 and CDK6 ↓ cyclin D1 ↓ p-Akt ↓ p-GSK-3β ↓ c-Myc ↑ Rassf1A ↑ Mst1 and Mst2 ↑ Sav1 ↑ p-Mob1 ↑ p-Yap ↓ CTGF | [72] |
| LoVo HCT116 | UA 10 mM Sorafenib 10 mM | ↓ Cell viability ↓ Colony formation ↑ Apoptosis ↑ ROS | ↑ Cleaved PARP ↑ Cleaved caspases 9 and 8 ↑ LC3 I and II ↓ Mcl-1 ↑ Bim ↑ MDA ↓ GSH | [73] |
| CT26 Mouse colon cancer cells | UA 15 µM 15 Gy radiation | ↓ Cell survival ↑ Apoptosis ↑ Ros ↓ GSH | ↓ Casp 3 ↓ Bcl2 ↑ Cleaved PARP | [74] |
| Model | Dose/Duration | Effects | Mechanism | Reference |
|---|---|---|---|---|
| Male BALB/c athymic mice xenografted with HT-29 cells (1.5 × 106) | UA 12.5 mg/kg Daily intraperitoneal injection | ↓ Tumor volume ↓ Tumor weight | ↓ PCNA ↓ cyclin D1 protein and mRNA ↓ CDK4 protein and mRNA ↑ p21 protein and mRNA ↑ TUNEL ↓ Bcl-2 protein and mRNA ↑ Bax protein and mRNA ↓ p-STAT3 ↓ p-Erk1/2 ↓ p-JNK ↓ p-p38 | [52] |
| Male BALB/c athymic mice xenografted with HT-29 cells (1.5 × 106) | UA 12.5 mg/kg Daily intraperitoneal injection | ↓ Tumor volume | ↓ CD31 positive cells ↓ p-STAT3 ↓ p-Akt ↓ p-p70S6K ↓ SHH positive cells and mRNA ↓ Gli-1 positive cells and mRNA ↓ VEGF-A positive and mRNA ↓ bFGF positive cells and mRNA | [51] |
| Chick chorioallantoic membrane | UA 0.25 mg 72 h | ↓ Number of blood vessels | ||
| Female athymic nude mice xenografted with HCT116 cells (1 × 107) | UA 10 mg/mg Daily intraperitoneal injection | ↓ Tumor volume | N/A | [53] |
| Female nude mice xenografted with HCT15 cells (106 cells) | UA 75 mg/kg daily Orally in Nutella | ↓ Tumor size | ↑ p62 (ns) ↑ p-JNK (ns) | [66] |
| Male BALB/c nude mice HCT-116hSMO− cells (1 × 107) | UA 10, 20 or 40 mg/kg Intraperitoneal injection 12 consecutive days | ↓ Tumor weight ↓ Tumor volume | ↓ BCL-2 protein and mRNA ↑ BAX protein and mRNA ↑ Caspase -3 and -9 mRNA ↓ c-Myc protein and mRNA ↓ GLI1 ↓ SHH ↓ SUFU ↓ p-Akt | [61] |
| Nude mice xenografted with SW620 cells (1 × 107) | UA 15, 30 or 60 mg/kg Intragastrical | ↑ Body weight ↓ Tumor weight ↓ Tumor volume ↑ Apoptosis | ↑ GSK3β mRNA and protein ↓ β-catenin mRNA and protein ↓ WNT4 mRNA and protein ↓ TCF4 mRNA and protein ↓ LEF1 mRNA and protein ↑ p-β-catenin ↓ p-GSK3β ↓ Nuclear β-catenin | [62] |
| Male BALB/c-nude mice HCT-116 cells (5 × 106) injected in tail vein | UA 20 mg/kg Intraperitoneal Daily/42 days | ↓ Lung metastasis | ↓ ARL4C | [63] |
| Female nude mice xenografted with SW620 cells | UA 20 mg/kg Oxaliplatin 10 mg/kg | ↓ Tumor weight ↓ Tumor volume | ↓ p-ERK1/2 ↓ p-Akt ↓ p-IKKα ↓ Ki-67 pos cells ↑ TUNEL pos cells | [67] |
| Female nude mice xenografted with HCT8 or SW480 cells (1 × 105) | UA 10 mg/kg Oxaliplatin 10 mg/kg | ↑ Animal survival time ↓ Tumor volume | N/A | [69] |
| Athymic nude mice xenografted with HCT116 cells | UA 10 mg/kg/day DOX 2 mg/kg/twice weekly | ↓ Tumor weight ↓ Tumor volume | ↓ Ki67 ↓ p-Akt ↑ Rassf1A ↑ Mst1 ↑ Mst2 ↑ Sav1 ↑ p-Mob1 ↑ p-Yap ↓ CTGF | [72] |
| Male athymic nu/nu mice Luciferase-transfected HCT116 cells | UA 250 mg/kg Orally, daily Capecitabine 60 mg/kg Orally, 2/week combination | ↓ Tumor growth ↓ Tumor volume ↓ Tumor weight ↓ Metastasis | ↓ Ki67 ↓ CD31 ↓ Nuclear p65 ↓ β-catenin ↓ p-STAT3 ↓ Cyclin D1 protein ↓ cMyc ↓ p-EGFR protein ↓ Bcl-2 protein ↓ Bcl-xl protein ↓ Survivin protein ↓ ICAM-1 ↓ VEGF ↓ MMP9 ↓ p53 ↓ p21 | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornel, A.; Tsiani, E. Effects of Ursolic Acid on Colorectal Cancer: A Review of Recent Evidence. Nutraceuticals 2024, 4, 373-394. https://doi.org/10.3390/nutraceuticals4030022
Kornel A, Tsiani E. Effects of Ursolic Acid on Colorectal Cancer: A Review of Recent Evidence. Nutraceuticals. 2024; 4(3):373-394. https://doi.org/10.3390/nutraceuticals4030022
Chicago/Turabian StyleKornel, Amanda, and Evangelia Tsiani. 2024. "Effects of Ursolic Acid on Colorectal Cancer: A Review of Recent Evidence" Nutraceuticals 4, no. 3: 373-394. https://doi.org/10.3390/nutraceuticals4030022
APA StyleKornel, A., & Tsiani, E. (2024). Effects of Ursolic Acid on Colorectal Cancer: A Review of Recent Evidence. Nutraceuticals, 4(3), 373-394. https://doi.org/10.3390/nutraceuticals4030022








