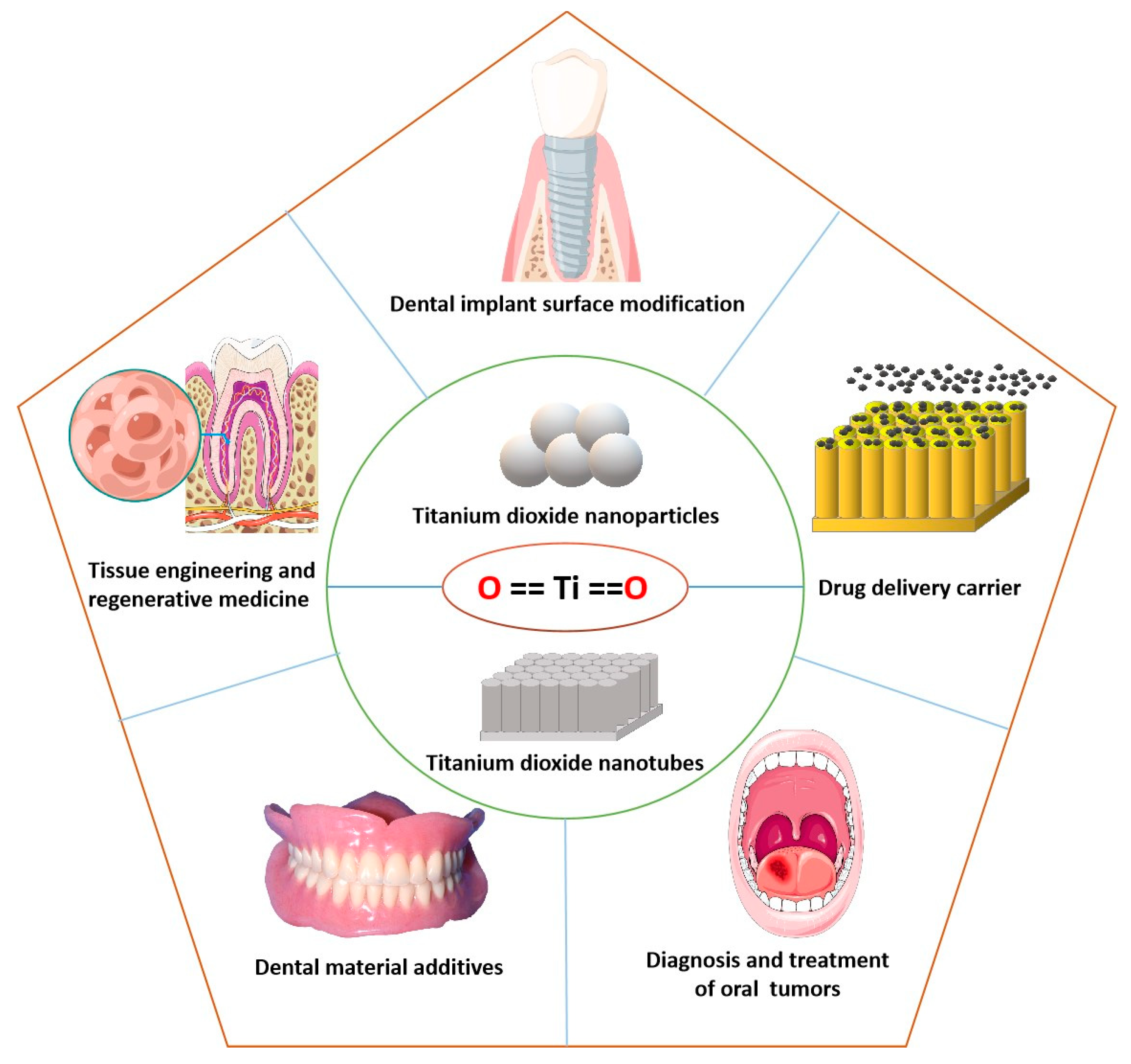
Applications of Titanium Dioxide Nanostructure in Stomatology
Abstract
:1. Introduction
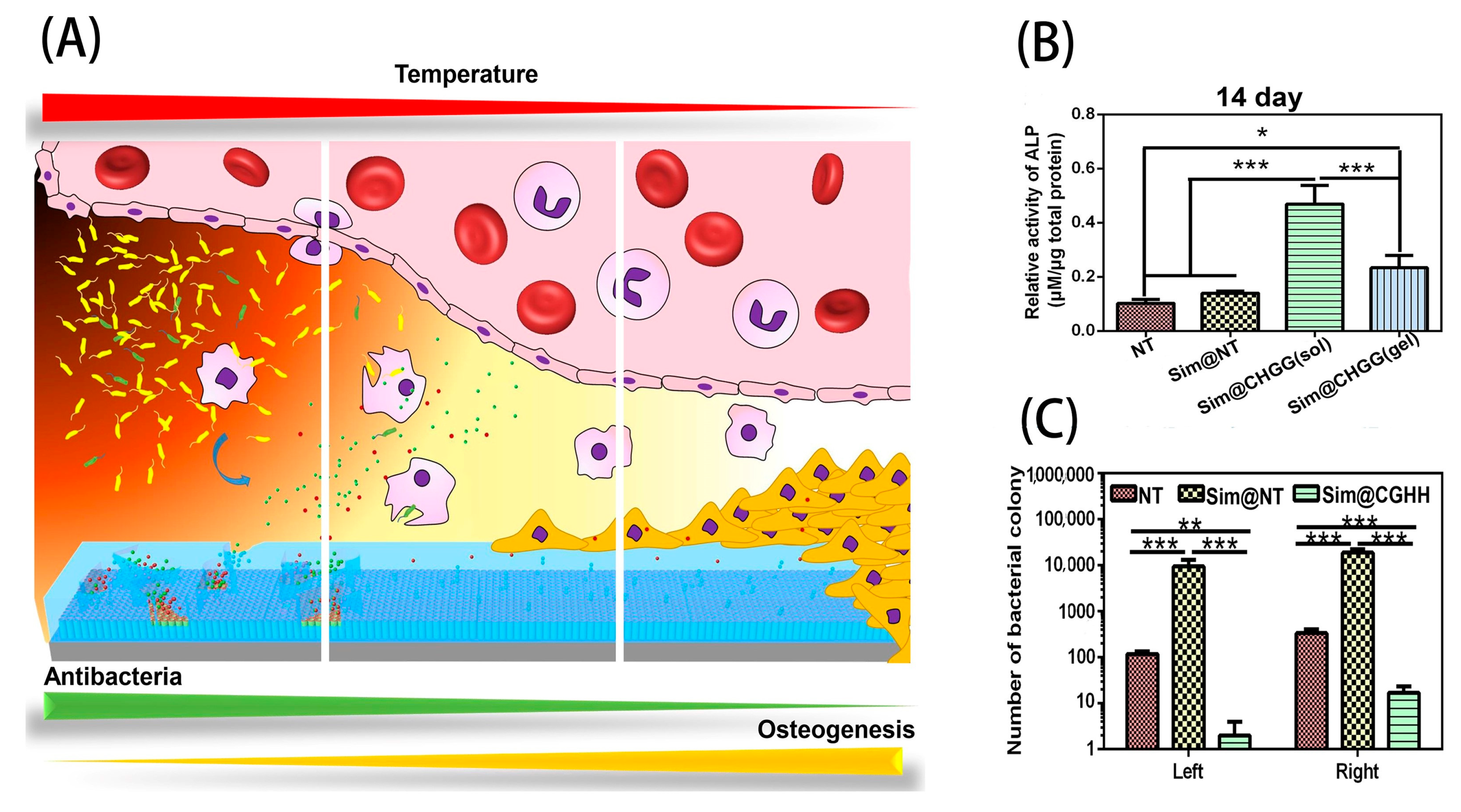
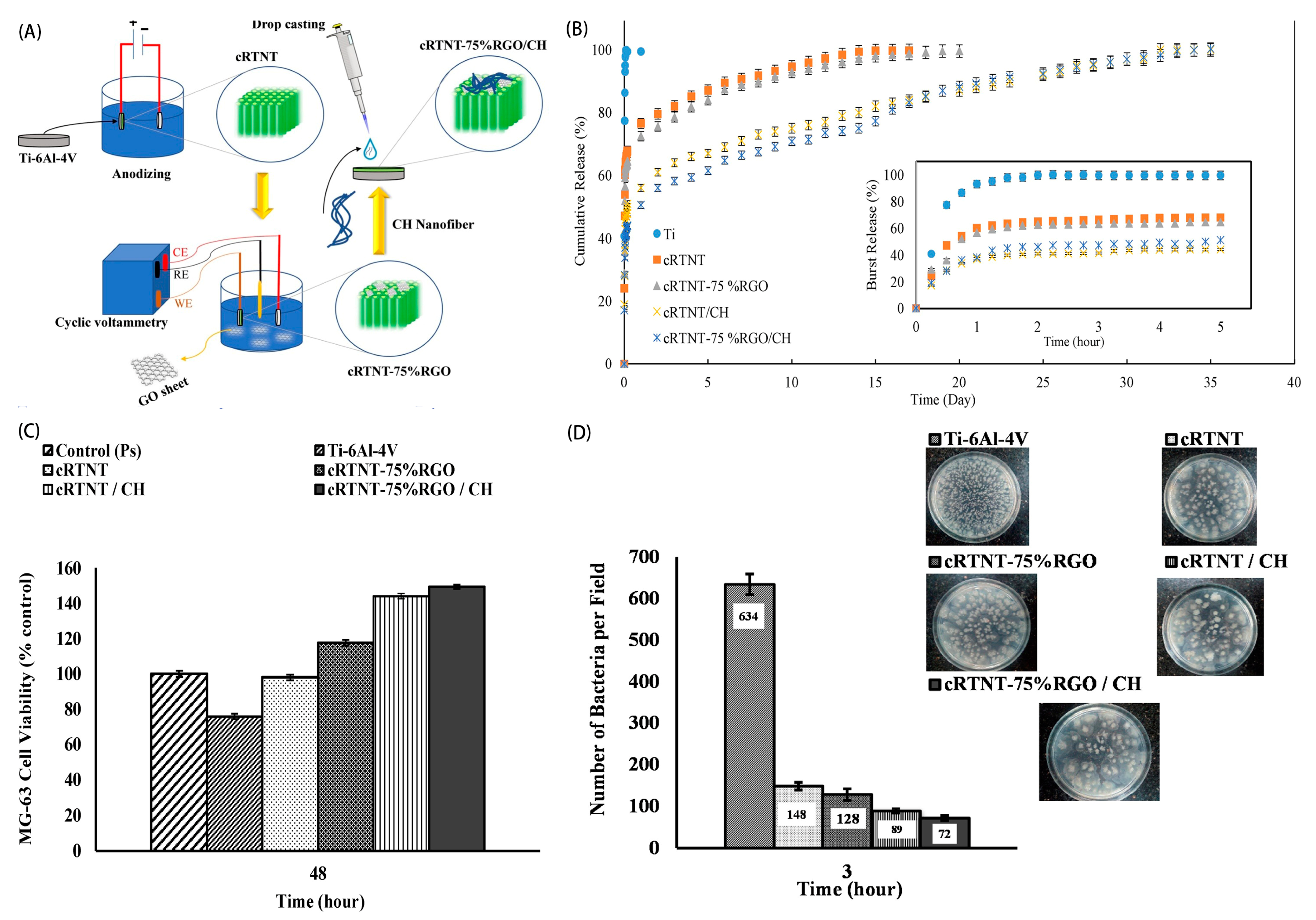
2. Surface Modification of Dental Implants
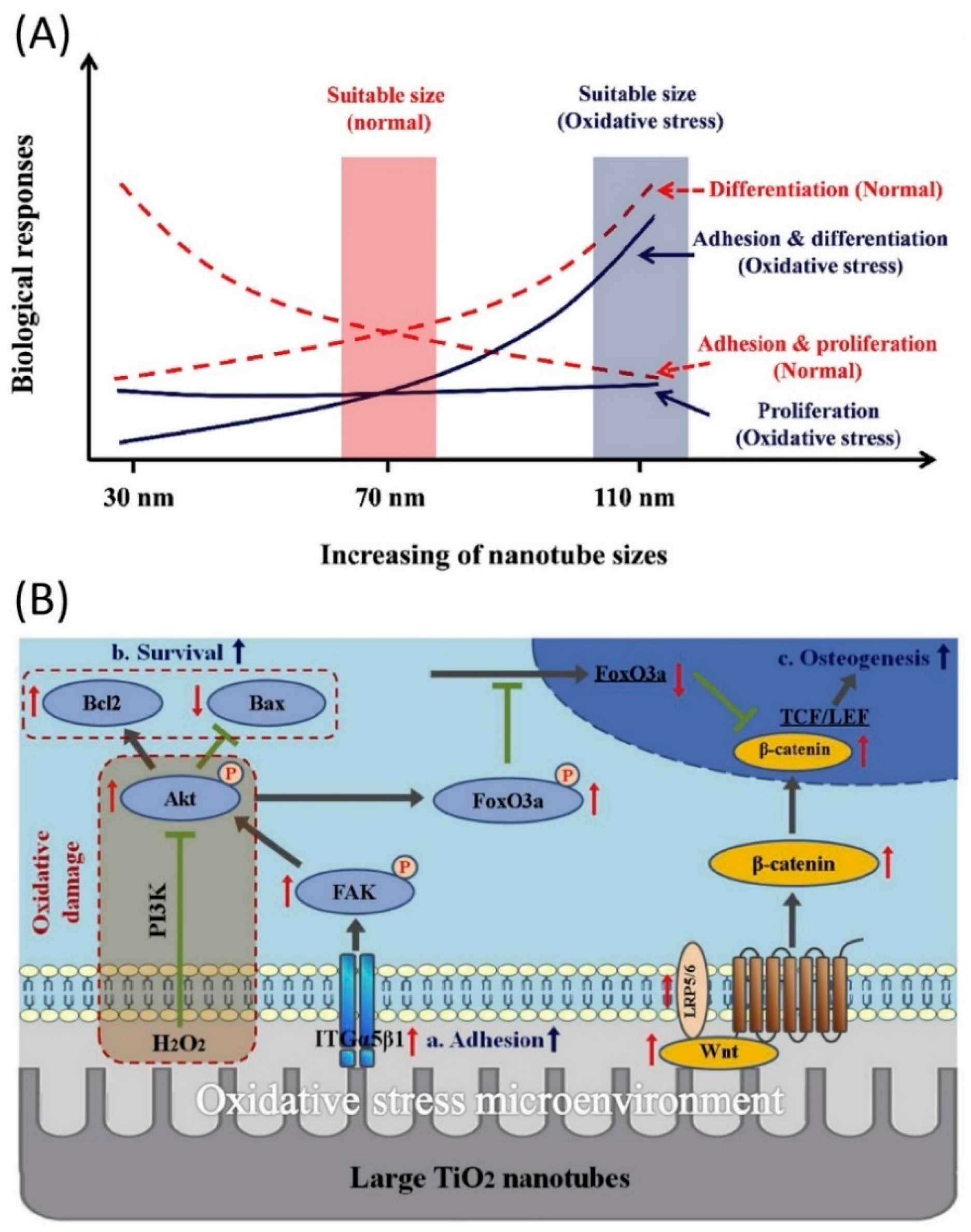
3. Applications in Tissue Engineering and Regenerative Medicine (TERM)
4. Carrier for Drug Delivery
5. Additives in Dental Materials
6. Assistance in the Diagnosis and Treatment of Oral Tumors
7. Prospective Applications and Challenges of Nano-TiO2 in Dentistry
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cuddy, M.F.; Poda, A.R.; Moser, R.D.; Weiss, C.A.; Cairns, C.; Steevens, J.A. A Weight-of-Evidence Approach to Identify Nanomaterials in Consumer Products: A Case Study of Nanoparticles in Commercial Sunscreens. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. ES&T 2012, 46, 2242–2250. [Google Scholar]
- Ding, L.; Li, J.; Huang, R.; Liu, Z.; Li, C.; Yao, S.; Wang, J.; Qi, D.; Li, N.; Pi, J. Salvianolic Acid B Protects against Myocardial Damage Caused by Nanocarrier TiO2; and Synergistic Anti-Breast Carcinoma Effect with Curcumin via Codelivery System of Folic Acid-Targeted and Polyethylene Glycol-Modified TiO2 Nanoparticles. Int. J. Nanomed. 2016, 11, 5709–5727. [Google Scholar] [CrossRef] [Green Version]
- Ai, J.W.; Liu, B.; Liu, W.D. Folic Acid-Tagged Titanium Dioxide Nanoparticles for Enhanced Anticancer Effect in Osteosarcoma Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1181–1187. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, J.; Du, L.; Li, Z.; Liu, H.; Ge, S. TiO2 Nanorod Arrays as a Photocatalytic Coating Enhanced Antifungal and Antibacterial Efficiency of Ti Substrates. Nanomedicine 2017, 12, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, S.L.; Xu, Y.; Walker, S.G.; Cho, W.; Mironava, T.; Rafailovich, M. The Impact of TiO2 Nanoparticle Exposure on Transmembrane Cholesterol Transport and Enhanced Bacterial Infectivity in Hela Cells. Acta Biomater. 2021, 135, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Lee, W.S.; Park, Y.S.; Cho, Y.K. Significantly Enhanced Antibacterial Activity of TiO2 Nanofibers with Hierarchical Nanostructures and Controlled Crystallinity. Analyst 2015, 140, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Dutta, V. Size Controlled Synthesis and Photocatalytic Activity of Anatase TiO2 Hollow Microspheres. Appl. Surf. Sci. 2012, 6, 9584–9588. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yu, W.; Zhang, J.; Zhou, Z.; Zhang, H.; Ge, H.; Wang, G.; Qin, Y. Rhodium Nanoparticles Confined in Titania Nanotubes for Efficient Hydrogen Evolution from Ammonia Borane. J. Colloid. Interface Sci. 2022, 609, 755–763. [Google Scholar] [CrossRef]
- Santos, J.S.; Fereidooni, M.; Marquez, V.; Arumugam, M.; Tahir, M.; Praserthdam, S.; Praserthdam, P. Single-Step Fabrication of Highly Stable Amorphous TiO2 Nanotubes Arrays (Am-Tnta) for Stimulating Gas-Phase Photoreduction of CO2 to Methane. Chemosphere 2022, 289, 133170. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liang, G.; Zhang, C.; Fan, L.; Yan, W.; Guo, Y.; Shuang, S.; Bi, Y.; Li, F.; Dong, C. Visible-Light-Driven Photoelectrochemical Sensing Platform Based on Bioi Nanoflowers/TiO2 Nanotubes for Detection of Atrazine in Environmental Samples. J. Hazard Mater. 2021, 409, 124894. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of Photocatalytic Bactericidal Action of Powdered Semiconductor TiO2 on Mutans Streptococci. J. Photochem. Photobiol. B 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, P.; Hao, Y.; Ding, Y.; Cai, K. Construction of Ag-Incorporated Coating on Ti Substrates for Inhibited Bacterial Growth and Enhanced Osteoblast Response. Colloids Surf. B Biointerfaces 2018, 171, 597–605. [Google Scholar] [CrossRef]
- Nesic, J.; Rtimi, S.; Laub, D.; Roglic, G.M.; Pulgarin, C.; Kiwi, J. New Evidence for TiO2 Uniform Surfaces Leading to Complete Bacterial Reduction in the Dark: Critical Issues. Colloids Surf. B Biointerfaces 2014, 123, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ma, H.; Liu, F.; Deng, J.; Ai, Y.; Zhao, X.; Mao, D.; Li, D.; Liao, B. Synthesis of Ag Ion-Implanted TiO2 Thin Films for Antibacterial Application and Photocatalytic Performance. J. Hazard Mater. 2015, 299, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Ramakrishnan, S.; Aw, M.S.; Atkins, G.J.; Findlay, D.M.; Losic, D. Biocompatible Polymer Coating of Titania Nanotube Arrays for Improved Drug Elution and Osteoblast Adhesion. Acta Biomater. 2012, 8, 449–456. [Google Scholar] [CrossRef]
- Lai, M.; Jin, Z.; Su, Z. Surface Modification of TiO2 Nanotubes with Osteogenic Growth Peptide to Enhance Osteoblast Differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 490–497. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 Nanoparticles to Escherichia Coli: Effects of Particle Size, Crystal Phase and Water Chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Zhou, Y.; Wang, C.; Li, S.; Wang, X. Toxic Effects and Molecular Mechanism of Different Types of Silver Nanoparticles to the Aquatic Crustacean Daphnia Magna. Environ. Sci. Technol. 2017, 51, 12868–12878. [Google Scholar] [CrossRef]
- Bruno, M.E.; Tasat, D.R.; Ramos, E.; Paparella, M.L.; Evelson, P.; Rebagliati, R.J.; Cabrini, R.L.; Guglielmotti, M.B.; Olmedo, D.G. Impact through Time of Different Sized Titanium Dioxide Particles on Biochemical and Histopathological Parameters. J. Biomed. Mater. Res. A 2014, 102, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, B.; Sun, J. Size- and Shape-Dependent Effects of Titanium Dioxide Nanoparticles on the Permeabilization of the Blood-Brain Barrier. J. Mater. Chem. B 2017, 5, 9558–9570. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhu, Y.; Yu, B.; Sun, Y.; Ding, X.; Xu, C.; Wu, Y.W.; Tang, Z.; Xu, F.J. Antimicrobial and Antifouling Polymeric Agents for Surface Functionalization of Medical Implants. Biomacromolecules 2018, 19, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, S.; Duan, S.; Xuliang, D.; Sun, Y.; Zhang, X.; Xu, X.; Guan, B.; Wang, C.; Hu, M.; et al. Modification of Titanium Substrates with Chimeric Peptides Comprising Antimicrobial and Titanium-Binding Motifs Connected by Linkers to Inhibit Biofilm Formation. ACS Appl. Mater. Interfaces 2016, 8, 5124–5136. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P. Antibacterial Properties of Nanostructured Cu-TiO2 Surfaces for Dental Implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z. Involvement of Fak/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of Hierarchical Micro/Nano-Topographies on the Morphology, Proliferation and Differentiation of Osteoblast-Like Cells. Colloids Surf. B Biointerfaces 2016, 145, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive Coating on Ti Alloy with High Osseointegration and Antibacterial Ag Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Bai, H.; Li, R.; Shang, J.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Wang, J.; et al. Sustained Release of Vegf to Promote Angiogenesis and Osteointegration of Three-Dimensional Printed Biomimetic Titanium Alloy Implants. Front. Bioeng. Biotechnol. 2021, 9, 757767. [Google Scholar] [CrossRef]
- Nan, J.; Zhijun, G.; Dan, S.; Yubao, L.; Yutao, Y.; Chen, C.; Li, Z.; Songsong, Z. Promoting Osseointegration of Ti Implants through Micro/Nanoscaled Hierarchical Ti Phosphate/Ti Oxide Hybrid Coating. ACS Nano 2018, 12, 7883–7891. [Google Scholar]
- André, R.S.; Zamperini, C.A.; Mima, E.G.; Longo, V.M. Antimicrobial Activity of Tio2:Ag Nanocrystalline Heterostructures: Experimental and Theoretical Insights. Chem. Phys. 2015, 459, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shen, X.; Liu, J.; Hu, Y.; Ran, Q.; Mu, C.; Cai, K. Regulation of Osteogenesis by Micro/Nano Hierarchical Titanium Surfaces through a Rock-Wnt5a Feedback Loop. Colloids Surf. B Biointerfaces 2018, 170, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of Porosity and Pore-Size Distribution in Ti6Al4V Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–392348. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Tarafder, S.; Sahasrabudhe, H.; Banerjee, D.; Bose, S. In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants. Ann. Biomed. Eng. 2017, 45, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieniawski, J.; Ziaja, W. Titanium Alloys—Advances in Properties Control; IntechOpen: London, UK, 2013; Chapter 2. [Google Scholar]
- Li, B.; Ma, J.; Wang, D.; Liu, X.; Li, H.; Zhou, L.; Liang, C.; Wang, H. Self-Adjusting Antibacterial Properties of Ag-Incorporated Nanotubes on Micro-Nanostructured Ti Surfaces. Biomater. Sci. 2019, 7, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bhatia, R.; Webster, T.J. Atomic Layer Deposition of Nano-TiO2 Thin Films with Enhanced Biocompatibility and Antimicrobial Activity for Orthopedic Implants. Int. J. Nanomed. 2017, 12, 8711–8723. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Qiao, Y.; Wang, Q.; Jin, G.; Qin, H.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X. Calcium Plasma Implanted Titanium Surface with Hierarchical Microstructure for Improving the Bone Formation. ACS Appl. Mater. Interfaces 2015, 7, 13053–130561. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The Synergistic Effect of Hierarchical Micro/Nano-Topography and Bioactive Ions for Enhanced Osseointegration. Biomaterials 2013, 34, 3184–3195. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological Actions of Silver Nanoparticles Embedded in Titanium Controlled by Micro-Galvanic Effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long-Lasting in Vivo and in Vitro Antibacterial Ability of Nanostructured Titania Coating Incorporated with Silver Nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 3488–3499. [Google Scholar] [CrossRef]
- Huang, Y.; Song, G.; Chang, X.; Wang, Z.; Zhang, X.; Han, S.; Su, Z.; Yang, H.; Yang, D.; Zhang, X. Nanostructured Ag(+)-Substituted Fluorhydroxyapatite-TiO2 Coatings for Enhanced Bactericidal Effects and Osteoinductivity of Ti for Biomedical Applications. Int. J. Nanomed. 2018, 13, 2665–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In Vitro and in Vivo Studies of Anti-Bacterial Copper-Bearing Titanium Alloy for Dental Application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.S.; Oliveira, J.R.; Milani, J.; Oliveira, L.D.; Machado, J.P.D.; Trava-Airoldi, V.J.; Lobo, A.O.; Marciano, F.R. Biomineralized Diamond-Like Carbon Films with Incorporated Titanium Dioxide Nanoparticles Improved Bioactivity Properties and Reduced Biofilm Formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunputh, U.F.; Le, H.; Lawton, K.; Besinis, A.; Tredwin, C.; Handy, R.D. Antibacterial Properties of Silver Nanoparticles Grown in Situ and Anchored to Titanium Dioxide Nanotubes on Titanium Implant against Staphylococcus Aureus. Nanotoxicology 2020, 14, 97–110. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, S.; Weng, Z.; Zhang, W.; Wan, X.; Cui, T.; Ye, J.; Liao, L.; Wang, X. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl. Mater. Interfaces 2020, 12, 54497–54506. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial Activity and Biofilm Inhibition by Surface Modified Titanium Alloy Medical Implants Following Application of Silver, Titanium Dioxide and Hydroxyapatite Nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Roguska, A.; Belcarz, A.; Zalewska, J.; Hołdyński, M.; Andrzejczuk, M.; Pisarek, M.; Ginalska, G. Metal TiO2 Nanotube Layers for the Treatment of Dental Implant Infections. ACS Appl. Mater. Interfaces 2018, 10, 17089–17099. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Valdez-Salas, B.; Curiel-Álvarez, M.; Castillo-Uribe, S.; Escamilla, A.; Nedev, N. Enhanced Antifungal Activity by Disinfected Titanium Dioxide Nanotubes Via Reduced Nano-Adhesion Bonds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 59–65. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, V.P.; Prymak, O.; Loza, K.; Epple, M.; Ennen-Roth, F.A.; Wittmar, M.; et al. Functionalization of Titania Nanotubes with Electrophoretically Deposited Silver and Calcium Phosphate Nanoparticles: Structure, Composition and Antibacterial Assay. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 97, 420–430. [Google Scholar] [CrossRef]
- Moon, K.S.; Park, Y.B.; Bae, J.M.; Choi, E.J.; Oh, S.H. Visible Light-Mediated Sustainable Antibacterial Activity and Osteogenic Functionality of Au and Pt Multi-Coated TiO2 Nanotubes. Materials 2021, 14, 5976. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Ag-Doping Regulates the Cytotoxicity of TiO2 Nanoparticles Via Oxidative Stress in Human Cancer Cells. Sci. Rep. 2017, 7, 17662. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, X. Rapid Fabrication of TiO2 Coatings with Nanoporous Composite Structure and Evaluation of Application in Artificial Implants—Sciencedirect. Surf. Coat. Technol. 2020, 381, 125094. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, H.; Lei, J.; Zhang, J.; Zhou, H.; Qi, M. Formation and in Vitro/in Vivo Performance of “Cortex-Like” Micro/Nano-Structured TiO2 Coatings on Titanium by Micro-Arc Oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 90–103. [Google Scholar] [CrossRef]
- Amin, M.S.; Randeniya, L.K.; Bendavid, A.; Martin, P.J.; Preston, E.W. Amorphous Carbonated Apatite Formation on Diamond-Like Carbon Containing Titanium Oxide. Diam. Relat. Mater. 2009, 18, 1139–1144. [Google Scholar] [CrossRef]
- Amin, M.S.; Randeniya, L.K.; Bendavid, A.; Martin, P.J.; Preston, E.W. Biomimetic Apatite Growth from Simulated Body Fluid on Various Oxide Containing Dlc Thin Films. Diam. Relat. Mater. 2011, 21, 42–49. [Google Scholar] [CrossRef]
- Esfandiari, N.; Simchi, A.; Bagheri, R. Size Tuning of Ag-Decorated Tio₂ Nanotube Arrays for Improved Bactericidal Capacity of Orthopedic Implants. J. Biomed. Mater. Res. A 2014, 102, 2625–2635. [Google Scholar] [CrossRef]
- Ahmed, F.Y.; Aly, U.F.; Abd El-Baky, R.M.; Waly, N.G. Comparative Study of Antibacterial Effects of Titanium Dioxide Nanoparticles Alone and in Combination with Antibiotics on Mdr Pseudomonas Aeruginosa Strains. Int. J. Nanomed. 2020, 15, 3393–3404. [Google Scholar]
- Zhang, P.; Zhao, Q.; Shi, M.; Yin, C.; Zhao, Z.; Shen, K.; Qiu, Y.; Xiao, Y.; Zhao, Y.; Yang, X.; et al. Fe3O4@Tio(2)-Laden Neutrophils Activate Innate Immunity Via Photosensitive Reactive Oxygen Species Release. Nano. Lett. 2020, 20, 261–271. [Google Scholar] [CrossRef]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.; Soares, P. Effect of Crystalline Phases of Titania Nanotube Arrays on Adipose Derived Stem Cell Adhesion and Proliferation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109850. [Google Scholar] [CrossRef]
- Wachesk, C.C.; Seabra, S.H.; Dos Santos, T.A.T.; Trava-Airoldi, V.J.; Lobo, A.O.; Marciano, F.R. In Vivo Biocompatibility of Diamond-Like Carbon Films Containing TiO2 Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2021, 32, 117. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Yan, W.; Chen, Z.; Shuai, X.; Wang, A.; Wang, Y. Nanotubular Topography Enhances the Bioactivity of Titanium Implants. Nanomedicine 2017, 13, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, L.; Wang, D.; Peng, F.; Zhao, X.; Liang, C.; Li, H.; Wang, H. Thermosensitive -Hydrogel-Coated Titania Nanotubes with Controlled Drug Release and Immunoregulatory Characteristics for Orthopedic Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111878. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Understanding and Augmenting the Stability of Therapeutic Nanotubes on Anodized Titanium Implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yelick, P.C.; Sharpe, P.T. Tooth Bioengineering and Regenerative Dentistry. J. Dent. Res. 2019, 98, 1173–1182. [Google Scholar] [CrossRef]
- Khoshroo, K.; Jafarzadeh Kashi, T.S.; Moztarzadeh, F.; Tahriri, M.; Jazayeri, H.W.; Tayebi, L. Development of 3d Pcl Microsphere/TiO2 Nanotube Composite Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 586–598. [Google Scholar] [CrossRef] [Green Version]
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3d-Printed Plga/TiO2 Nanocomposite Scaffolds for Bone Tissue Engineering Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rasoulianboroujeni, M.; Yazdimamaghani, M.; Khoshkenar, P.; Pothineni, V.R.; Kim, K.M.; Murray, T.A.; Rajadas, J.; Mills, D.K.; Vashaee, D.; Moharamzadeh, K.; et al. From Solvent-Free Microspheres to Bioactive Gradient Scaffolds. Nanomedicine 2017, 13, 1157–1169. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Qiu, J.; Du, Q.L.; Jia, L.; Liu, H.; Ge, S. TiO2 Nanorod Arrays Modified Ti Substrates Promote the Adhesion, Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 684–691. [Google Scholar] [CrossRef]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of Pore Size of Porous Titanium Fabricated by Vacuum Diffusion Bonding of Titanium Meshes on Cell Penetration and Bone Ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the Pore Size and Porosity of Selective Laser Melted Ti6Al4V Eli Porous Scaffold on Cell Proliferation, Osteogenesis and Bone Ingrowth. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110289. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.J.; Li, T.; Sudheesh Kumar, P.T.; Ivanovski, S. Titania Nanopores with Dual Micro-/Nano-Topography for Selective Cellular Bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; You, Y.; Ma, A.; Song, Y.; Jiao, J.; Song, L.; Shi, E.; Zhong, X.; Li, Y.; Li, C. Zn-Incorporated TiO2 Nanotube Surface Improves Osteogenesis Ability through Influencing Immunomodulatory Function of Macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias-Netipanyj, M.F.; Sopchenski, L.; Gradowski, T.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Crystallinity of TiO2 Nanotubes and Its Effects on Fibroblast Viability, Adhesion, and Proliferation. J. Mater. Sci. Mater. Med. 2020, 31, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, S.; Xue, Y.; Zhang, L.; Han, Y. A Superparamagnetic Fe3O4-TiO2 Composite Coating on Titanium by Micro-Arc Oxidation for Percutaneous Implants. J. Mater. Chem. B 2019, 7, 5265–5276. [Google Scholar] [CrossRef]
- Xu, R.; Hu, X.; Yu, X.; Wan, S.; Wu, F.; Ouyang, J.; Deng, F. Micro-/Nano-Topography of Selective Laser Melting Titanium Enhances Adhesion and Proliferation and Regulates Adhesion-Related Gene Expressions of Human Gingival Fibroblasts and Human Gingival Epithelial Cells. Int. J. Nanomed. 2018, 13, 5045–5057. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; You, L.; Wang, T.; Zhang, X.; Li, Z.; Ding, L.; Li, J.; Xiao, C.; Han, F.; Li, B. Enhanced Osseointegration of Titanium Implants by Surface Modification with Silicon-Doped Titania Nanotubes. Int. J. Nanomed. 2020, 15, 8583–8594. [Google Scholar] [CrossRef]
- Sabino, R.M.; Mondini, G.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Tanfloc/Heparin Polyelectrolyte Multilayers Improve Osteogenic Differentiation of Adipose-Derived Stem Cells on Titania Nanotube Surfaces. Carbohydr. Polym. 2021, 251, 117079. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Chan, S.M.; Li, R.; Wu, Y.; Cai, M.; Wang, A.; Wang, Y. TiO2 Nanotubes Alleviate Diabetes-Induced Osteogenetic Inhibition. Int. J. Nanomed. 2020, 15, 3523–3537. [Google Scholar] [CrossRef]
- Nishimura, K.; Shindo, S.; Movila, A.; Kayal, R.; Abdullah, A.; Savitri, I.J.; Ikeda, A.; Yamaguchi, Y.; Howait, M.; Al-Dharrab, A.; et al. Trap-Positive Osteoclast Precursors Mediate Ros/No-Dependent Bactericidal Activity via Tlr4. Free Radic. Biol. Med. 2016, 97, 330–341. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shen, X.; Luo, Z.; Hu, Y.; Li, M.; Ma, P.; Ran, Q.; Dai, L.; He, Y.; Cai, K. Osteogenesis Potential of Different Titania Nanotubes in Oxidative Stress Microenvironment. Biomaterials 2018, 167, 44–57. [Google Scholar] [CrossRef]
- Shen, X.; Ma, P.; Hu, Y.; Xu, G.; Zhou, J.; Cai, K. Mesenchymal Stem Cell Growth Behavior on Micro/Nano Hierarchical Surfaces of Titanium Substrates. Colloids Surf. B Biointerfaces 2015, 127, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem Cell Fate Dictated Solely by Altered Nanotube Dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Yu, Y.; Ma, P.; Luo, Z.; Hu, Y.; Li, M.; He, Y.; Zhang, Y.; Peng, Z.; Song, G.; et al. Titania Nanotubes Promote Osteogenesis Via Mediating Crosstalk between Macrophages and Mscs under Oxidative Stress. Colloids Surfaces B Biointerfaces 2019, 180, 39–48. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, H.; Li, Y.; Ren, M.; Xiang, J.; Zhang, Z.; Ji, P.; Yang, S. 1α,25-Dihydroxyvitamin D3-Loaded Hierarchical Titanium Scaffold Enhanced Early Osseointegration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110551. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Lewandowska, Z.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansoorianfar, M.; Khataee, A.; Riahi, Z.; Shahin, K.; Asadnia, M.; Razmjou, A.; Hojjati-Najafabadi, A.; Mei, C.; Orooji, Y.; Li, D. Scalable Fabrication of Tunable Titanium Nanotubes Via Sonoelectrochemical Process for Biomedical Applications. Ultrason. Sonochem. 2020, 64, 104783. [Google Scholar] [CrossRef]
- Hasanzadeh Kafshgari, M.; Kah, D.; Mazare, A.; Nguyen, N.T.; Distaso, M.; Peukert, W.; Goldmann, W.H.; Schmuki, P.; Fabry, B. Anodic Titanium Dioxide Nanotubes for Magnetically Guided Therapeutic Delivery. Sci. Rep. 2019, 9, 13439. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.C.; Letchmanan, K.; Chow, P.S.; Tan, R.B.H. Antibiotic Elution and Mechanical Property of TiO2 Nanotubes Functionalized Pmma-Based Bone Cements. J. Mech. Behav. Biomed. Mater. 2019, 91, 91–98. [Google Scholar] [CrossRef]
- Lin, W.T.; Tan, H.L.; Duan, Z.L.; Yue, B.; Ma, R.; He, G.; Tang, T.T. Inhibited Bacterial Biofilm Formation and Improved Osteogenic Activity on Gentamicin-Loaded Titania Nanotubes with Various Diameters. Int. J. Nanomed. 2014, 9, 1215–1230. [Google Scholar]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.J.; Losic, D. Advanced Biopolymer-Coated Drug-Releasing Titania Nanotubes (Tnts) Implants with Simultaneously Enhanced Osteoblast Adhesion and Antibacterial Properties. Colloids Surf. B Biointerfaces 2015, 130, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Geng, Z.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Wang, R.; Yang, X. Controlled Release Behaviour and Antibacterial Effects of Antibiotic-Loaded Titania Nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S. Controlling Drug Release from Titania Nanotube Arrays Using Polymer Nanocarriers and Biopolymer Coating. J. Biomater. Nanobiotechnol. 2012, 2, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Rahnamaee, S.Y.; Bagheri, R.; Heidarpour, H.; Vossoughi, M.; Golizadeh, M.; Samadikuchaksaraei, A. Nanofibrillated Chitosan Coated Highly Ordered Titania Nanotubes Array/Graphene Nanocomposite with Improved Biological Characters. Carbohydr. Polym. 2021, 254, 117465. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Ezati, M.; Mohammadnejad, J.; Houshmand, B.; Faghihi, S. Chitosan Coating of TiO2 Nanotube Arrays for Improved Metformin Release and Osteoblast Differentiation. Int. J. Nanomed. 2020, 15, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Sun, L.; Zhang, X.; Sun, Y.; Wang, J. Fabrication and Antibacterial Properties of Cefuroxime-Loaded TiO2 Nanotubes. Appl. Microbiol. Biotechnol. 2020, 104, 2947–2955. [Google Scholar] [CrossRef]
- Torres, C.C.; Campos, C.H.; Diáz, C.; Jiménez, V.A.; Vidal, F.; Guzmán, L.; Alderete, J.B. Pamam-Grafted TiO2 Nanotubes as Novel Versatile Materials for Drug Delivery Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 164–171. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, H.; Liu, Y.; Xu, L.; Wu, Z.; Hu, X.; Ma, J.; Pathak, J.L.; Liu, J.; Wu, G. Ph Dependent Silver Nanoparticles Releasing Titanium Implant: A Novel Therapeutic Approach to Control Peri-Implant Infection. Colloids Surf. B Biointerfaces 2017, 158, 127–136. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Jian, X.; Xu, J.; Gao, Z.; Song, Y.Y. Nir Light-Driven Photocatalysis on Amphiphilic TiO2 Nanotubes for Controllable Drug Release. ACS Appl. Mater. Interfaces 2020, 12, 23606–23616. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Yu, Y.; Wei, M.T.; Chang, C.C.; Ricotta, V.; Feng, K.C.; Wang, L.; Bherwani, A.K.; Ou-Yang, H.D.; Simon, M.; et al. Regulating Substrate Mechanics to Achieve Odontogenic Differentiation for Dental Pulp Stem Cells on TiO2 Filled and Unfilled Polyisoprene. Acta Biomater. 2019, 89, 6072. [Google Scholar] [CrossRef]
- Sun, J.; Forster, A.M.; Johnson, P.M.; Eidelman, N.; Quinn, G.; Schumacher, G.; Zhang, X.; Wu, W.L. Improving Performance of Dental Resins by Adding Titanium Dioxide Nanoparticles. Dent. Mater. 2011, 27, 972–982. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.; Fernández-García, M.; et al. Understanding the Antimicrobial Mechanism of Tio₂-Based Nanocomposite Films in a Pathogenic Bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, I.M.; Souza, V.S.; Hellriegel, C.; Scholten, J.D.; Collares, F.M. Ionic Liquid-Stabilized Titania Quantum Dots Applied in Adhesive Resin. J. Dent. Res. 2019, 98, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, A.; Nomura, Y.; Ochiai, T.; Sudo, T.; Nomoto, R.; Hayakawa, T.; Kanzaki, H.; Nakamura, Y.; Hanada, N. Antibacterial, Hydrophilic Effect and Mechanical Properties of Orthodontic Resin Coated with Uv-Responsive Photocatalyst. Materials 2018, 11, 889. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, G.M.F.; Bronze-Uhle, E.S.; Lisboa-Filho, P.N.; Fugolin, A.P.P.; Borges, A.F.S.; Gonzaga, C.C.; Pfeifer, C.S.; Furuse., A.Y. Effect of the Addition of Functionalized TiO2 Nanotubes and Nanoparticles on Properties of Experimental Resin Composites. Dent. Mater. 2020, 36, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.A.; de Azevedo Silva, L.J.; Ferrairo, B.M.; Erbereli, R.; Lovo, J.F.P.; Ponce Gomes, O.; Rubo, J.H.; Lisboa-Filho, P.N.; Griggs, J.A.; Fortulan, C.A.; et al. Effects of Zno/TiO2 Nanoparticle and TiO2 Nanotube Additions to Dense Polycrystalline Hydroxyapatite Bioceramic from Bovine Bones. Dent. Mater. 2020, 36, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Petersen, E.J.; Watson, S.S.; Sims, C.M.; Kassman, A.; Frukhtbeyn, S.; Skrtic, D.; Ok, M.T.; Jacobs, D.S.; Reipa, V.; et al. Biophysical Characterization of Functionalized Titania Nanoparticles and Their Application in Dental Adhesives. Acta Biomater. 2017, 53, 585–597. [Google Scholar] [CrossRef] [Green Version]
- Vojdani, M.; Bagheri, R.; Khaledi, A. Effects of Aluminum Oxide Addition on the Flexural Strength, Surface Hardness, and Roughness of Heat-Polymerized Acrylic Resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Cascione, M.; De Matteis, V.; Pellegrino, P.; Albanese, G.; De Giorgi, M.L.; Paladini, F.; Corsalini, M.; Rinaldi, R. Improvement of Pmma Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes. Nanomaterials 2021, 11, 2027. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly(methyl methacrylate) with TiO2 Nanoparticles Inclusion for Stereolitographic Complete Denture Manufacturing—The Future in Dental Care for Elderly Edentulous Patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Cristache, C.M.; Totu, E.E.; Iorgulescu, G.; Pantazi, A.; Dorobantu, D.; Nechifor, A.C.; Isildak, I.; Burlibasa, M.; Nechifor, G.; Enachescu, M. Eighteen Months Follow-Up with Patient-Centered Outcomes Assessment of Complete Dentures Manufactured Using a Hybrid Nanocomposite and Additive Cad/Cam Protocol. J. Clin. Med. 2020, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Laiteerapong, A.; Reichl, F.X.; Hickel, R.; Högg, C. Effect of Eluates from Zirconia-Modified Glass Ionomer Cements on DNA Double-Stranded Breaks in Human Gingival Fibroblast Cells. Dent. Mater. 2019, 35, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Meera Priyadarshini, B.; Neo, J.; Fawzy, A.S. Characterization of Chitosan/TiO2 Nano-Powder Modified Glass-Ionomer Cement for Restorative Dental Applications. J. Esthet. Restor. Dent. 2017, 29, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium Dioxide Nanoparticles Addition to a Conventional Glass-Ionomer Restorative: Influence on Physical and Antibacterial Properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, Antibacterial and Bond Strength Properties of Nano-Titanium-Enriched Glass Ionomer Cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jowkar, Z.; Fattah, Z.; Ghanbarian, S.; Shafiei, F. The Effects of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Dentin Pretreatments on the Microshear Bond Strength of a Conventional Glass Ionomer Cement to Dentin. Int. J. Nanomed. 2020, 15, 4755–4762. [Google Scholar] [CrossRef]
- Xue, L.; Yan, B.; Li, Y.; Tan, Y.; Luo, X.; Wang, M. Surface-Enhanced Raman Spectroscopy of Blood Serum Based on Gold Nanoparticles for Tumor Stages Detection and Histologic Grades Classification of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2018, 13, 4977–4986. [Google Scholar] [CrossRef] [Green Version]
- Girish, C.M.; Iyer, S.; Thankappan, K.; Rani, V.V.D.; Gowd, G.S.; Menon, D.; Nair, S.; Koyakutty, M. Rapid Detection of Oral Cancer Using Ag-TiO2 Nanostructured Surface-Enhanced Raman Spectroscopic Substrates. J. Mater. Chem. B 2014, 2, 989–998. [Google Scholar] [CrossRef]
- Chundayil Madathil, G.; Iyer, S.; Thankappan, K.; Gowd, G.S.; Nair, S.; Koyakutty, M. A Novel Surface Enhanced Raman Catheter for Rapid Detection, Classification, and Grading of Oral Cancer. Adv. Healthc. Mater. 2019, 8, e1801557. [Google Scholar] [CrossRef]
- Sun, N.; Liu, M.; Wang, J.; Wang, Z.; Li, X.; Jiang, B.; Pei, R. Chitosan Nanofibers for Specific Capture and Nondestructive Release of Ctcs Assisted by Pcbma Brushes. Small 2016, 12, 5090–5097. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, M.; Lin, L.; Du, L.; Shen, M.; Shi, X. Specific Capture and Release of Circulating Tumor Cells Using a Multifunctional Nanofiber-Integrated Microfluidic Chip. Nanomedicine 2019, 14, 183–199. [Google Scholar] [CrossRef]
- Sun, N.; Li, X.; Wang, Z.; Zhang, R.; Wang, J.; Wang, K.; Pei, R. A Multiscale TiO2 Nanorod Array for Ultrasensitive Capture of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2016, 8, 12638–12643. [Google Scholar] [CrossRef] [PubMed]
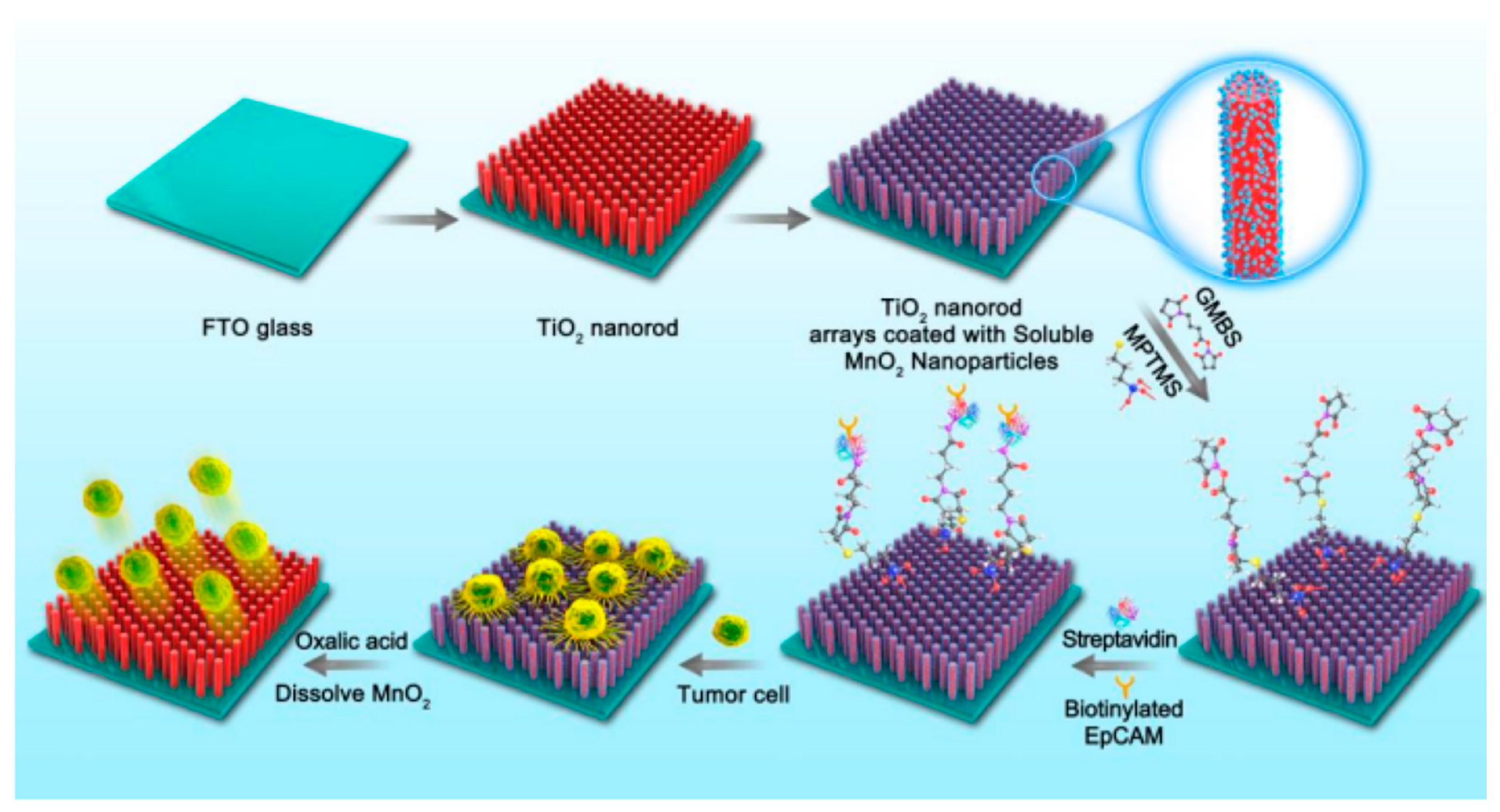
- Li, R.; Chen, F.F.; Liu, H.Q.; Wang, Z.X.; Zhang, Z.T.; Wang, Y.; Cui, H.; Liu, W.; Zhao, X.Z.; Sun, Z.J.; et al. Efficient Capture and High Activity Release of Circulating Tumor Cells by Using TiO2 Nanorod Arrays Coated with Soluble Mno(2) Nanoparticles. ACS Appl. Mater. Interfaces. 2018, 10, 16327–16334. [Google Scholar] [CrossRef]
- Maheswari, P.; Harish, S.; Ponnusamy, S.; Muthamizhchelvan, C. A Novel Strategy of Nanosized Herbal Plectranthus Amboinicus, Phyllanthus Niruri and Euphorbia Hirta Treated TiO2 Nanoparticles for Antibacterial and Anticancer Activities. Bioprocess. Biosyst. Eng. 2021, 44, 1593–1616. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.; Harish, S.; Navaneethan, M.; Muthamizhchelvan, C.; Ponnusamy, S.; Hayakawa, Y. Bio-Modified TiO2 Nanoparticles with Withania Somnifera, Eclipta Prostrata and Glycyrrhiza Glabra for Anticancer and Antibacterial Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.C.; Zhang, Y.; Tan, M.J.; Liu, Y.; Yuan, S.; Choong, C.; Tan, N.S.; Tan, T.T. Anti-Cangptl4 Ab-Conjugated N-Tio(2)/Nayf(4):Yb,Tm Nanocomposite for near Infrared-Triggered Drug Release and Enhanced Targeted Cancer Cell Ablation. Adv. Healthc. Mater. 2012, 1, 470–474. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X.; Cheng, Z.; Lian, H.; Li, C.; Lin, J. Uv-Emitting Upconversion-Based TiO2 Photosensitizing Nanoplatform: Near-Infrared Light Mediated in Vivo Photodynamic Therapy via Mitochondria-Involved Apoptosis Pathway. ACS Nano. 2015, 9, 2584–2599. [Google Scholar] [CrossRef]
- Lucky, S.S.; Idris, N.M.; Huang, K.; Kim, J.; Li, Z.; Thong, P.S.; Xu, R.; Soo, K.C.; Zhang, Y. In Vivo Biocompatibility, Biodistribution and Therapeutic Efficiency of Titania Coated Upconversion Nanoparticles for Photodynamic Therapy of Solid Oral Cancers. Theranostics 2016, 6, 1844–1865. [Google Scholar] [CrossRef]
- Yurt, F.; Ince, M.; Colak, S.G.; Ocakoglu, K.; Er, O.; Soylu, H.M.; Gunduz, C.; Avci, C.B.; Kurt, C.C. Investigation of in Vitro Pdt Activities of Zinc Phthalocyanine Immobilised TiO2 Nanoparticles. Int. J. Pharm. 2017, 524, 467–474. [Google Scholar] [CrossRef]
- Tang, R.; Zheleznyak, A.; Mixdorf, M.; Ghai, A.; Prior, J.; Black, K.C.L.; Shokeen, M.; Reed, N.; Biswas, P.; Achilefu, S. Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating Multiple Myeloma. ACS Nano. 2020, 14, 4255–4264. [Google Scholar] [CrossRef]
- Riedle, S.; Wills, J.W.; Miniter, M.; Otter, D.E.; Singh, H.; Brown, A.P.; Micklethwaite, S.; Rees, P.; Jugdaohsingh, R.; Roy, N.C.; et al. A Murine Oral-Exposure Model for Nano- and Micro-Particulates: Demonstrating Human Relevance with Food-Grade Titanium Dioxide. Small 2020, 16, e2000486. [Google Scholar] [CrossRef]
- da Silva, A.B.; Miniter, M.; Thom, W.; Hewitt, R.E.; Wills, J.; Jugdaohsingh, R.; Powell, J.J. Gastrointestinal Absorption and Toxicity of Nanoparticles and Microparticles: Myth, Reality and Pitfalls Explored through Titanium Dioxide. Curr. Opin. Toxicol. 2020, 19, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; Mas del Molino, E.; Fernández-Rosas, E.; Fernández, A.; Vázquez-Campos, S. Cell Uptake and Oral Absorption of Titanium Dioxide Nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, H.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice Than Non-Obese Mice: Gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small 2020, 16, e2001858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Y.; Liu, S.; Jia, T.; Zhou, D.; Xu, H. Foodborne TiO2 Nanoparticles Induced More Severe Hepatotoxicity in Fructose-Induced Metabolic Syndrome Mice Via Exacerbating Oxidative Stress-Mediated Intestinal Barrier Damage. Foods 2021, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Duan, Y.; Hong, M.; Zheng, L.; Fei, M.; Zhao, X.; Wang, J.; Cui, Y.; Liu, H.; Cai, J.; et al. Spleen Injury and Apoptotic Pathway in Mice Caused by Titanium Dioxide Nanoparticules. Toxicol. Lett. 2010, 195, 161–168. [Google Scholar] [CrossRef]
- Gui, S.; Zhang, Z.; Zheng, L.; Cui, Y.; Liu, X.; Li, N.; Sang, X.; Sun, Q.; Gao, G.; Cheng, Z.; et al. Molecular Mechanism of Kidney Injury of Mice Caused by Exposure to Titanium Dioxide Nanoparticles. J. Hazard. Mater. 2011, 195, 365–370. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of Oral Exposure to Titanium Dioxide Nanoparticles on Gut Microbiota and Gut-Associated Metabolism in Vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium Dioxide Nanoparticles Exacerbate Dss-Induced Colitis: Role of the Nlrp3 Inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the Role of the Gut-Liver Axis in Rats after Oral Administration of Titanium Dioxide Nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef]
- Sang, X.; Fei, M.; Sheng, L.; Zhao, X.; Yu, X.; Hong, J.; Ze, Y.; Gui, S.; Sun, Q.; Ze, X.; et al. Immunomodulatory Effects in the Spleen-Injured Mice Following Exposure to Titanium Dioxide Nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 3562–3572. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, J.S.; Kim, S.Y.; Park, M.K.; Choi, S.D.; Kim, U.J.; Park, K.; Jeong, E.J.; Nam, S.Y.; Yu, W.J. Titanium Dioxide Nanoparticles Oral Exposure to Pregnant Rats and Its Distribution. Part. Fibre Toxicol. 2019, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aijie, C.; Huimin, L.; Jia, L.; Lingling, O.; Limin, W.; Junrong, W.; Xuan, L.; Xue, H.; Longquan, S. Central Neurotoxicity Induced by the Instillation of Zno and TiO2 Nanoparticles through the Taste Nerve Pathway. Nanomedicine 2017, 12, 2453–2470. [Google Scholar] [CrossRef] [PubMed]
- Abdulnasser Harfoush, S.; Hannig, M.; Le, D.D.; Heck, S.; Leitner, M.; Omlor, A.J.; Tavernaro, I.; Kraegeloh, A.; Kautenburger, R.; Kickelbick, G.; et al. High-Dose Intranasal Application of Titanium Dioxide Nanoparticles Induces the Systemic Uptakes and Allergic Airway Inflammation in Asthmatic Mice. Respir. Res. 2020, 21, 168. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.O.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Moon, C.; Shin, I.S.; Heo, J.D.; Ko, J.W.; Kim, J.C. Titanium Dioxide Nanoparticles Exacerbate Allergic Airway Inflammation via Txnip Upregulation in a Mouse Model of Asthma. Int. J. Mol. Sci. 2021, 22, 9924. [Google Scholar] [CrossRef] [PubMed]
- Armand, L.; Tarantini, A.; Beal, D.; Biola-Clier, M.; Bobyk, L.; Sorieul, S.; Pernet-Gallay, K.; Marie-Desvergne, C.; Lynch, I.; Herlin-Boime, N.; et al. Long-Term Exposure of A549 Cells to Titanium Dioxide Nanoparticles Induces DNA Damage and Sensitizes Cells towards Genotoxic Agents. Nanotoxicology 2016, 10, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Yamada, N.; Yoshida, Y.; Yamamoto, O. Subchronic Exposure of Titanium Dioxide Nanoparticles to Hairless Rat Skin. Exp. Dermatol. 2013, 22, 278–283. [Google Scholar] [CrossRef]
- Chaudhry, Q. Opinion of the Scientific Committee on Consumer Safety (Sccs)—Revision of the Opinion on the Safety of the Use of Titanium Dioxide, Nano Form, in Cosmetic Products. Regul. Toxicol. Pharmacol. 2015, 73, 669–670. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Chen, X.; Yu, M.; Li, J.; Liu, J.; Xie, Z.; Gao, F.; Liu, Y. Applications of Titanium Dioxide Nanostructure in Stomatology. Molecules 2022, 27, 3881. https://doi.org/10.3390/molecules27123881
Liu S, Chen X, Yu M, Li J, Liu J, Xie Z, Gao F, Liu Y. Applications of Titanium Dioxide Nanostructure in Stomatology. Molecules. 2022; 27(12):3881. https://doi.org/10.3390/molecules27123881
Chicago/Turabian StyleLiu, Shuang, Xingzhu Chen, Mingyue Yu, Jianing Li, Jinyao Liu, Zunxuan Xie, Fengxiang Gao, and Yuyan Liu. 2022. "Applications of Titanium Dioxide Nanostructure in Stomatology" Molecules 27, no. 12: 3881. https://doi.org/10.3390/molecules27123881




