An Example of Personalized Treatment in HR+ HER2+ Long Survivor Breast Cancer Patient (Case Report)
Abstract
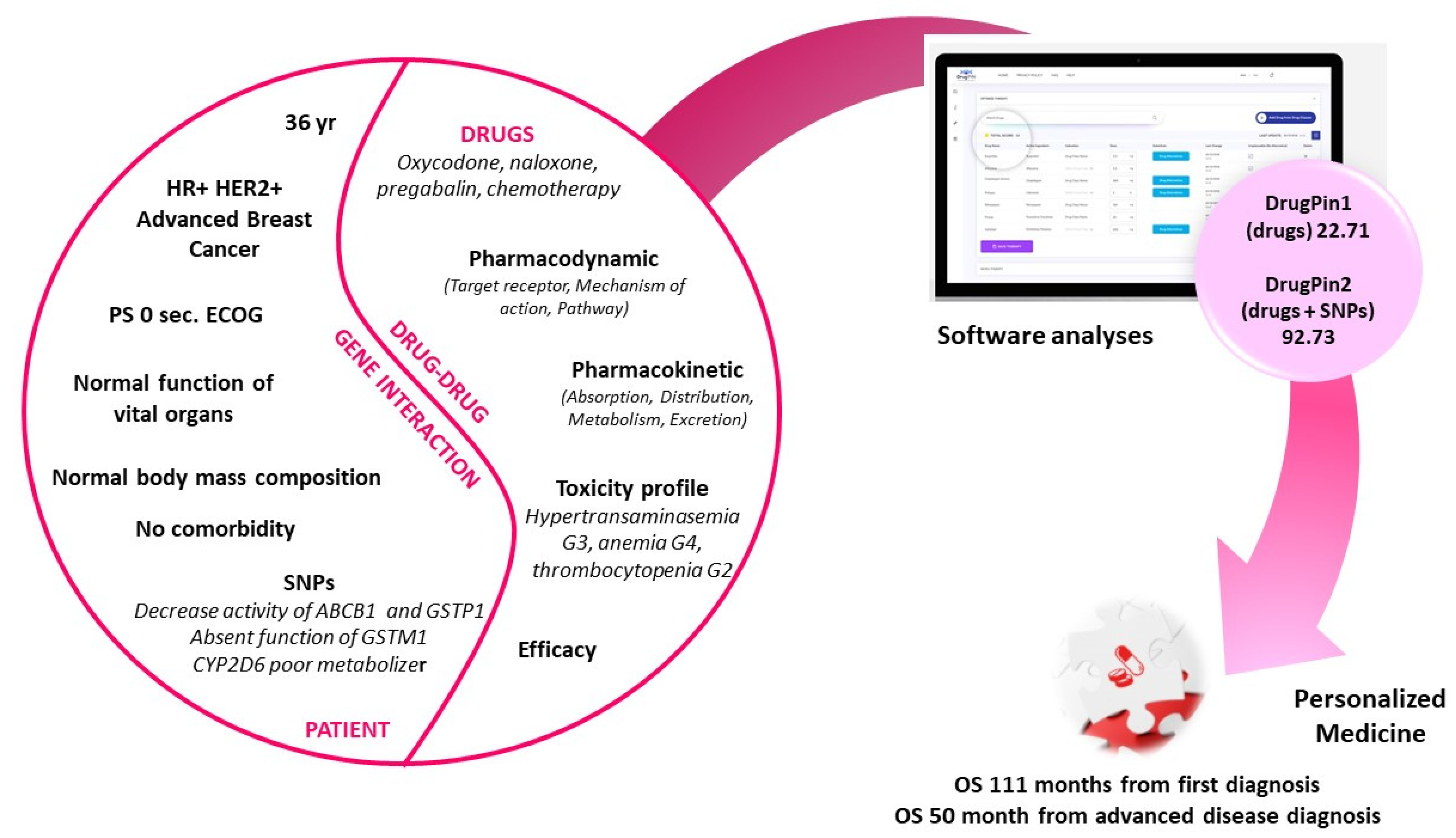
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberto, M.; Rossi, A.; Panebianco, M.; Pomes, L.; Arrivi, G.; Ierinò, D.; Simmaco, M.; Marchetti, P.; Mazzuca, F. Drug–Drug Interactions and Pharmacogenomic Evaluation in Colorectal Cancer Patients: The New Drug-PIN® System Comprehensive Approach. Pharmaceuticals 2021, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, C.; Sheehan, N.L. Understanding and preventing drug–drug and drug–gene interactions. Expert Rev. Clin. Pharmacol. 2014, 7, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.M.; Danahey, K.; Knoebel, R.W.; Ratain, M.J.; Meltzer, D.O.; O’Donnell, P.H. Analysis of comprehensive pharmacogenomic profiling to impact in-hospital prescribing. Pharm. Genom. 2019, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Verbeurgt, P.; Mamiya, T.; Oesterheld, J. How common are drug and gene interactions? Prevalence in a sample of 1143 patients with CYP2C9, CYP2C19 and CYP2D6 genotyping. Pharmacogenomics 2014, 15, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.E.; McLeod, H.L. Pharmacogenomics—Drug Disposition, Drug Targets, and Side Effects. N. Engl. J. Med. 2003, 348, 538–549. [Google Scholar] [CrossRef] [Green Version]
- Erichsen, H.C.; Chanock, S.J. SNPs in cancer research and treatment. Br. J. Cancer 2004, 90, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Palmirotta, R.; Carella, C.; Silvestris, E.; Cives, M.; Stucci, S.L.; Tucci, M.; Lovero, D.; Silvestris, F. SNPs in predicting clinical efficacy and toxicity of chemotherapy: Walking through the quicksand. Oncotarget 2018, 9, 25355–25382. [Google Scholar] [CrossRef]
- Angelini, S.; Botticelli, A.; Onesti, C.E.; Giusti, R.; Sini, V.; Durante, V.; Strigari, L.; Gentile, G.; Cerbelli, B.; Pellegrini, P.; et al. Pharmacogenetic Approach to Toxicity in Breast Cancer Patients Treated with Taxanes. Anticancer Res. 2017, 37, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, P.; Tulsyan, S.; Agarwal, G.; Lal, P.; Mittal, R.; Mittal, B. Influence of ABCB1 genetic variants in breast cancer treatment outcomes. Cancer Epidemiol. 2013, 37, 754–761. [Google Scholar] [CrossRef]
- Córdoba, E.E.; Abba, M.C.; Lacunza, E.; Fernandez, E.E.; Güerci, A.M. Polymorphic Variants in Oxidative Stress Genes and Acute Toxicity in Breast Cancer Patients Receiving Radiotherapy. Cancer Res. Treat. 2016, 48, 948–954. [Google Scholar] [CrossRef]
- Etienne-Grimaldi, M.-C.; Boyer, J.-C.; Beroud, C.; Mbatchi, L.; Van Kuilenburg, A.; Bobin-Dubigeon, C.; Thomas, F.; Chatelut, E.; Merlin, J.-L.; Pinguet, F.; et al. New advances in DPYD genotype and risk of severe toxicity under capecitabine. PLoS ONE 2017, 12, e0175998. [Google Scholar] [CrossRef] [PubMed]
- Pascual, T.; Apellániz-Ruiz, M.; Pernaut, C.; Cueto-Felgueroso, C.; Villalba, P.; Álvarez, C.; Manso, L.; Inglada-Pérez, L.; Robledo, M.; Rodriguez-Antona, C.; et al. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS ONE 2017, 12, e0180192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boso, V.; Herrero, M.J.; Santaballa, A.; Palomar, L.; Megias, J.E.; De La Cueva, H.; Rojas, L.; Marqués, M.R.; Poveda, J.L.; Montalar, J.; et al. SNPs and taxane toxicity in breast cancer patients. Pharmacogenomics 2014, 15, 1845–1858. [Google Scholar] [CrossRef]
- Sini, V.; Botticelli, A.; Lunardi, G.; Gori, S.; Marchetti, P. Pharmacogenetics and aromatase inhibitor induced side effects in breast cancer patients. Pharmacogenomics 2017, 18, 821–830. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Trubetskoy, V.; Nurhussein-Patterson, A.; Hall, J.P.; Nath, A.; Huo, D.; Fleming, G.F.; Ingle, J.N.; Abramson, V.G.; Morrow, P.K.; et al. Clinical evaluation of germline polymorphisms associated with capecitabine toxicity in breast cancer: TBCRC-015. Breast Cancer Res. Treat. 2020, 181, 623–633. [Google Scholar] [CrossRef]
- Jabir, R.S.; Naidu, R.; Annuar, M.A.B.A.; Ho, G.F.; Munisamy, M.; Stanslas, J. Pharmacogenetics of taxanes: Impact of gene polymorphisms of drug transporters on pharmacokinetics and toxicity. Pharmacogenomics 2012, 13, 1979–1988. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, R.; Raj, G.M.; Kayal, S.; Ramesh, A.; Shewade, D.G. Influence of ABCB1 C3435T and C1236T gene polymorphisms on tumour response to docetaxel-based neo-adjuvant chemotherapy in locally advanced breast cancer patients of South India. J. Clin. Pharm. Ther. 2019, 44, 188–196. [Google Scholar] [CrossRef]
- Zhong, J.; Guo, Z.; Fan, L.; Zhao, X.; Zhao, B.; Cao, Z.; Cheng, L.; Shi, Y.; Li, X.; Zhang, Y.; et al. ABCB1 polymorphism predicts the toxicity and clinical outcome of lung cancer patients with taxane-based chemotherapy. Thorac. Cancer 2019, 10, 2088–2095. [Google Scholar] [CrossRef]
- Mir, O.; Alexandre, J.; Tran, A.; Durand, J.-P.; Pons, G.; Treluyer, J.-M.; Goldwasser, F. Relationship between GSTP1 Ile105Val polymorphism and docetaxel-induced peripheral neuropathy: Clinical evidence of a role of oxidative stress in taxane toxicity. Ann. Oncol. 2009, 20, 736–740. [Google Scholar] [CrossRef]
- Tran, A.; Jullien, V.; Alexandre, J.; Rabillon, F.; Girre, V.; Pons, G.; Goldwasser, F.; Rey, E.; Diéras, V.; Treluyer, J. Pharmacokinetics and toxicity of docetaxel: Role of CYP3A, MDR1, and GST polymorphisms. Clin. Pharmacol. Ther. 2006, 79, 570–580. [Google Scholar] [CrossRef]
- Sugishita, M.; Imai, T.; Kikumori, T.; Mitsuma, A.; Shimokata, T.; Shibata, T.; Morita, S.; Inada-Inoue, M.; Sawaki, M.; Hasegawa, Y.; et al. Pharmacogenetic association between GSTP1 genetic polymorphism and febrile neutropenia in Japanese patients with early breast cancer. Breast Cancer 2014, 23, 195–201. [Google Scholar] [CrossRef]
- Zhang, B.L.; Tong, S.U.; Zhang, B.N.; Zheng, S.; Ning, L.Ü.; Xu, B.H.; Xiang, W.A.; Chen, G.J.; Yu, D.K.; Lin, D.X. Polymorphisms of GSTP1 is associated with differences of chemotherapy response and toxicity in breast cancer. Chin. Med. J. 2011, 124, 199–204. [Google Scholar] [PubMed]
- Yoshihama, T.; Fukunaga, K.; Hirasawa, A.; Nomura, H.; Akahane, T.; Kataoka, F.; Yamagami, W.; Aoki, D.; Mushiroda, T. GSTP1 rs1695 is associated with both hematological toxicity and prognosis of ovarian cancer treated with paclitaxel plus carboplatin combination chemotherapy: A comprehensive analysis using targeted resequencing of 100 pharmacogenes. Oncotarget 2018, 9, 29789–29800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnunen, M.; Piirainen, P.; Kokki, H.; Lammi, P.; Kokki, M. Updated Clinical Pharmacokinetics and Pharmacodynamics of Oxycodone. Clin. Pharmacokinet. 2019, 58, 705–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiskanen, T.; Olkkola, K.T.; Kalso, E. Effects of blocking CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone*. Clin. Pharmacol. Ther. 1998, 64, 603–611. [Google Scholar] [CrossRef]
- Susce, M.T.; Murray-Carmichael, E.; De Leon, J. Response to hydrocodone, codeine and oxycodone in a CYP2D6 poor metabolizer. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1356–1358. [Google Scholar] [CrossRef]
- Foster, A.; Mobley, E.; Wang, Z. Complicated Pain Management in a CYP450 2D6 Poor Metabolizer. Pain Pract. 2007, 7, 352–356. [Google Scholar] [CrossRef]
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.; Balleine, R.L.; Blair, E.Y.; McLachlan, A.J.; Ackland, S.P.; Garg, M.B.; Evans, S.; Farlow, D.; Collins, M.; Rivory, L.P.; et al. Predictors of Vinorelbine Pharmacokinetics and Pharmacodynamics in Patients With Cancer. J. Clin. Oncol. 2006, 24, 2448–2455. [Google Scholar] [CrossRef]
- Gusella, M.; Pasini, F.; Caruso, D.; Barile, C.; Modena, Y.; Fraccon, A.P.; Bertolaso, L.; Menon, D.; Crepaldi, G.; Bononi, A.; et al. Clinical outcomes of oral metronomic vinorelbine in advanced non-small cell lung cancer: Correlations with pharmacokinetics and MDR1 polymorphisms. Cancer Chemother. Pharmacol. 2019, 83, 493–500. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panebianco, M.; Taurelli Salimbeni, B.; Roberto, M.; Marchetti, P. An Example of Personalized Treatment in HR+ HER2+ Long Survivor Breast Cancer Patient (Case Report). Curr. Oncol. 2021, 28, 1980-1987. https://doi.org/10.3390/curroncol28030184
Panebianco M, Taurelli Salimbeni B, Roberto M, Marchetti P. An Example of Personalized Treatment in HR+ HER2+ Long Survivor Breast Cancer Patient (Case Report). Current Oncology. 2021; 28(3):1980-1987. https://doi.org/10.3390/curroncol28030184
Chicago/Turabian StylePanebianco, Martina, Beatrice Taurelli Salimbeni, Michela Roberto, and Paolo Marchetti. 2021. "An Example of Personalized Treatment in HR+ HER2+ Long Survivor Breast Cancer Patient (Case Report)" Current Oncology 28, no. 3: 1980-1987. https://doi.org/10.3390/curroncol28030184




