Cutaneous Malignancies in Tattoos, a Case Series of Six Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Literature Research and Evaluation of the Data
3. Results
3.1. Cases with the Formation of Melanoma in Tattooed Skin
3.2. Cases with the Formation of Basal Cell Carcinoma in Tattooed Skin
4. Discussion
4.1. Melanoma and Black Tattoo Ink; Difficulties in Defining Etiopathogenesis
4.2. Basal Cell Carcinoma in Tattoo Ink and Toxicity of Injected Pigments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anforderungen der Hygiene beim Tätowieren, (2002, überarbeitet 2017). Available online: https://www.awmf.org/uploads/tx_szleitlinien/029-024l_S1_Anforderungen-Hygiene-Taetowieren_2019-07.pdf (accessed on 24 January 2020).
- Ricci, F.; Paradisi, A.; Maier, S.A.; Kovacs, M.; Podda, M.; Peris, K.; Abeni, D. Melanoma and tattoos: A case report and review of the literature. Eur. J. Dermatol. 2017, 28, 50–55. [Google Scholar]
- Laumann, A.E.; Derick, A.J. Tattoos and body piercings in the United States: A national data set. J. Am. Acad. Dermatol. 2006, 55, 413–421. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften—Ständige Kommission LA-RL. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik, Therapie und Nachsorge des Melanoms; Lang-Version 3.2. 2019:280. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Melanom/Melanom_Version_3/LL_Melanom_Langversion_3.3.pdf (accessed on 24 January 2020).
- Nolan, K.A.; Kling, M.; Birge, M.; Kling, A.; Fishman, S.; Phelps, R. Melanoma arising in a tattoo: Case report and review of the literature. Cutis 2013, 92, 227–230. [Google Scholar]
- Statista Research Department. Weltweite Umfrage zum Tragen von Tattoos 2018 Statista Research Department: Statista Research Department; 2018. Available online: https://de.statista.com/statistik/daten/studie/864407/umfrage/weltweite-umfrage-zum-tragen-von-tattoos/ (accessed on 24 January 2021).
- Kluger, N. Cutaneous complications related to permanent decorative tattooing. Expert Rev. Clin. Immunol. 2010, 6, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, K.H.; Sepehri, M.; Serup, J. Tattooist-Associated Tattoo Complications: “Overworked Tattoo”, “Pigment Overload” and Infections Producing Early and Late Adverse Events. Dermatology 2020, 236, 208–215. [Google Scholar]
- Kluger, N.; Phan, A.; Debarbieux, S.; Balme, B.; Thomas, L. Skin Cancers Arising in Tattoos: Coincidental or Not? Dermatology 2008, 217, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.N.; Craig, J.R. Melanoma in a tattoo of the breast. J. Surg. Oncol. 1984, 25, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Huisman, S.; Van Der Bent, S.A.S.; Wolkerstorfer, A.; Rustemeyer, T. Granulomatous tattoo reactions in permanent makeup of the eyebrows. J. Cosmet. Dermatol. 2019, 18, 212–214. [Google Scholar] [CrossRef]
- Caucanas, M.; El Hayderi, L.; Lebas, E.; Richert, B.; Dezfoulian, B.; Nikkels, A.F. Dermatological complications of temporary and indelible tattoos. Ann. Dermatol. Venereol. 2011, 138, 161–162. [Google Scholar] [CrossRef]
- Van der Bent, S.A.; Wolkerstorfer, A.; Rustemeyer, T. Cutaneous adverse reactions to tattoos. Ned. Tijdschr. Geneeskd. 2016, 160, A9808. [Google Scholar] [PubMed]
- Kluger, N.; Koljonen, V. Tattoos, inks, and cancer. Lancet Oncol. 2012, 13, e161–e168. [Google Scholar] [CrossRef]
- Paradisi, A.; Capizzi, R.; De Simone, C.; Fossati, B.; Proietti, I.; Amerio, P.L. Malignant melanoma in a tattoo: Case report and review of the literature. Melanoma Res. 2006, 16, 375–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, W.E.; Richwine, E.E.; Sheehan, D.J. Pseudolymphomatous and lichenoid reaction to a red tattoo: A case report. Cutis 2011, 87, 89–92. [Google Scholar] [PubMed]
- Kirsch, N. Malignant melanoma developing in a tattoo. Int. J. Dermatol. 1972, 11, 16–20. [Google Scholar] [CrossRef] [PubMed]
- van der Bent, S.A.S.; de Winter, R.W.; Wolkerstorfer, A.; Rustemeyer, T. Red tattoo reactions, a prospective cohort on clinical aspects. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e384–e386. [Google Scholar] [PubMed]
- Huisman, S.; van der Bent, S.A.; Maijer, K.I.; Tio, D.C.; Rustemeyer, T. Cutaneous non-allergic complications in tattoos: An overview of the literature. La Presse Med. 2020, 49, 104049. [Google Scholar] [CrossRef]
- Cherkaoui El Baraka, F.; Kluger, N.; Ollivier, I.; Bourgoin, R.; Grossin, M.; Zeboulon, C.; Phan, C.; Sin, C.; Mahé, E. Melanoma within tattoos: Two cases and a systematic literature review. Ann. Dermatol. Venereol. 2020, 147, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, T.; Arzberger, E.; Scarfì, F.; Puches, R.F.; Hofmann-Wellenhof, R.; Zalaudek, I. A dangerous fruit of Belladonna. J. Am. Acad. Dermatol. 2016, 75, e93–e94. [Google Scholar] [CrossRef]
- Kluger, N.; Saarinen, K. Melanoma on a tattoo. La Presse Med. 2015, 44, 473–475. [Google Scholar] [CrossRef]
- Anthony, E.P.; Godbolt, A.; Tang, F.; McMeniman, E.K. Malignant melanoma disguised in a tattoo. Australas. J. Dermatol. 2015, 56, 232–233. [Google Scholar] [CrossRef] [PubMed]
- JJoyce, C.W.; Duff, G.; McKenna, D.; Regan, P.J. Malignant Melanoma Arising in Red Tattoo Ink. Arch. Plast. Surg. 2015, 42, 475–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchernev, G.; Chokoeva, A.A. Melanoma in a Chinese dragon tattoo. Lancet 2015. [Google Scholar] [CrossRef]
- Caccavale, S.; Moscarella, E.; Salvatores, G.D.F.; Piccolo, V.; Russo, T.; Argenziano, G. When a melanoma is uncovered by a tattoo. Int. J. Dermatol. 2016, 55, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Kluger, N.; Koskenmies, S.; Jeskanen, L.; Övermark, M.; Saksela, O. Melanoma on Tattoos: Two Finnish Cases. Acta Derm. Venereol. 2014, 94, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Körner, R.; Pföhler, C.; Vogt, T.; Müller, C.S.L. Histopathology of body art revisited—analysis and discussion of 19 cases. J. Dtsch. Dermatol. Ges. 2013, 11, 1073–1080. [Google Scholar] [CrossRef]
- Pohl, L.; Kaiser, K.; Raulin, C. Pitfalls and recommendations in cases of laser removal of decorative tattoos with pigmented lesions: Case report and review of the literature. JAMA Dermatol. 2013, 149, 1087–1089. [Google Scholar] [CrossRef] [Green Version]
- Varga, E.; Korom, I.; Varga, J.; Kohán, J.; Kemény, L.; Oláh, J. Melanoma and melanocytic nevi in decorative tattoos: Three case reports. J. Cutan. Pathol. 2011, 38, 994–998. [Google Scholar] [CrossRef]
- Jaigirdar, A.A.; Yeh, M.W.; Sharifi, E.; Browne, L.W.; Leong, S.P. Coexisting Tattoo Pigment and Metastatic Melanoma in the Same Sentinel Lymph Node. J. Cutan. Med. Surg. 2009, 13, 321–325. [Google Scholar] [CrossRef]
- Singh, R.S.; Diwan, A.H.; Prieto, V.G. Potential diagnostic pitfalls in melanoma arising in a cutaneous tattoo. Histopathology 2007, 51, 283–285. [Google Scholar] [CrossRef]
- Shariff, Z.; Tehrani, H.; Jagadeesan, J.; Hardwicke, J. Artwork: To be studied. Dermatol. Online J. 2006, 12, 21. [Google Scholar] [CrossRef]
- Stinco, G.; De Francesco, V.; Frattasio, A.; Quinkenstein, E.; Patrone, P. Malignant Melanoma in a Tattoo. Dermatology 2003, 206, 345–346. [Google Scholar] [CrossRef]
- Khan, I.U.; Moiemen, N.S.; Firth, J.; Frame, J.D. Malignant melanoma disguised by a tattoo. Br. J. Plast. Surg. 1999, 52, 598. [Google Scholar] [CrossRef] [PubMed]
- Soroush, V.; Gurevitch, A.W.; Peng, S.K. Malignant melanoma in a tattoo: Case report and review of the literature. Cutis 1997, 59, 111–112. [Google Scholar] [PubMed]
- Kircik, L.; Armus, S.; van den Broek, H. Malignant melanoma in a tattoo. Int. J. Dermatol. 1993, 32, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Bartal, A.H.; Cohen, Y.; Robinson, E. Malignant melanoma arising at tattoo sites used for radiotherapy field marking. Br. J. Radiol. 1980, 53, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Wolfort, F.C.; Hoopes, J.E.; Filtze, H.S.; Cochran, T.C. Superficial melanoma in a tattoo. Br. J. Plast. Surg. 1974, 27, 303–304. [Google Scholar] [CrossRef]
- Allen, A.C. The Skin: A Clinicopathologic Treatise; The CV Mosby Company: St. Louis, MO, USA, 1954. [Google Scholar]
- Sharlit, H. Melanoma caused by indelible pencil. Arch. Dermatol. 1938, 37, 301–306. [Google Scholar] [CrossRef]
- Edmonds, N.; Cropley, T.; Noland, M.; Flowers, R. Basal cell carcinoma arising in a tattoo. Clin. Dermatol. Rev. 2020, 4, 167–169. [Google Scholar]
- Abudu, B.; Erickson, C.P.; Calame, A.; Cohen, P.R. Basal Cell Carcinoma Originating in a Tattoo: Case Report and Review of an Uncommon Complication in Tattoo Recipients. Dermatol. Pract. Concept. 2019, 9, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messmer, E.M.; Mohring-Bengisu, C.; Miller, C. Lidline Tattoo Associated with Basal Cell Carcinoma of the Lid Margin—Coincidence or Association? Klin. Monbl. Augenheilkd. 2018, 235, 785–788. [Google Scholar]
- Omidian, M.; Emad-Mostofi, N. Basal cell carcinoma arising from traditional tattoo. Arch. Iran. Med. 2009, 12, 198. [Google Scholar] [PubMed]
- Lee, J.-S.; Park, J.; Kim, S.-M.; Yun, S.-K.; Kim, H.-U. Basal Cell Carcinoma Arising in a Tattooed Eyebrow. Ann. Dermatol. 2009, 21, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnie, A.J.; Kulkarni, K.; Varma, S. Basal cell carcinoma arising in a tattoo. Clin. Exp. Dermatol. 2006, 31, 820–821. [Google Scholar] [CrossRef] [PubMed]
- Doumat, F.; Kaise, W.; Barbaud, A.; Schmutz, J. Basal Cell Carcinoma in a Tattoo. Dermatology 2004, 208, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Wiener, D.A.; Scher, R.K. Basal cell carcinoma arising in a tattoo. Cutis 1987, 39, 125–126. [Google Scholar] [PubMed]
- Earley, M. Basal cell carcinoma arising in tattoos: A clinical report of two cases. Br. J. Plast. Surg. 1983, 36, 258–259. [Google Scholar] [CrossRef]
- Shrout, M.; DeCoster, R.; Wermeling, R.; Vasconez, H.C. Risk Factors for Squamous Cell Carcinoma: A Case for Red Pigment in Tattoos. Am. Surg. 2019, 85, e77–e78. [Google Scholar] [CrossRef] [PubMed]
- Paprottka, F.J.; Krezdorn, N.; Narwan, M.; Turk, M.; Sorg, H.; Noah, E.M.; Hebebrand, D. Trendy Tattoos—Maybe a Serious Health Risk? Aesthetic Plast. Surg. 2017, 42, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Sherif, S.; Blakeway, E.; Fenn, C.; German, A.; Laws, P. A Case of Squamous Cell Carcinoma Developing Within a Red-Ink Tattoo. J. Cutan. Med. Surg. 2016, 21, 61–63. [Google Scholar] [CrossRef]
- Maxim, E.; Higgins, H.; D’Souza, L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int. J. Women’s Dermatol. 2017, 3, 228–230. [Google Scholar] [CrossRef]
- Schmitz, I.; Prymak, O.; Epple, M.; Ernert, C.; Tannapfel, A. Squamous cell carcinoma in association with a red tattoo. J. Dtsch. Dermatol. Ges. 2016, 14, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.T.; Karagas, M.R.; Samie, F.H. Eruptive Squamous Cell Carcinomas of the Keratoacanthoma Type Arising in a Cosmetic Lip Tattoo. Dermatol. Surg. 2015, 41, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Paprottka, F.J.; Bontikous, S.; Lohmeyer, J.A.; Hebebrand, D. Squamous-cell Carcinoma Arises in Red Parts of Multicolored Tattoo within Months. Plast. Reconstr. Surg.-Glob. Open 2014, 2, e114. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.P.; Dentlinger, R.B.; Forystek, A.M.; Stevens, T.; Huerter, C. Poorly Differentiated Squamous Cell Carcinoma Arising in Tattooed Skin. Case Rep. Med. 2010, 2010, 431813. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.; Yamauchi, P.S. Rapidly growing squamous cell carcinoma from permanent makeup tattoo. J. Am. Acad. Dermatol. 2009, 60, 1073–1074. [Google Scholar] [CrossRef]
- Goldenberg, G.; Patel, S.; Patel, M.J.; Williford, P.; Sangueza, O. Eruptive squamous cell carcinomas, keratoacanthoma type, arising in a multicolor tattoo. J. Cutan. Pathol. 2007, 35, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Pitarch, G.; Martínez-Menchón, T.; Martínez-Aparicio, A.; Sánchez-Carazo, J.L.; Muñoz, D.; Fortea, J.M. Squamous cell carcinoma over tattoos. J. Am. Acad. Dermatol. 2007, 56, 1072–1073. [Google Scholar] [CrossRef]
- McQuarrie, D.G. Squamous-cell carcinoma arising in a tattoo. Minn. Med. 1966, 49, 799–801. [Google Scholar] [PubMed]
- Colboc, H.; Bazin, D.; Moguelet, P.; Reguer, S.; Amode, R.; Jouanneau, C.; Lucas, I.; Deschamps, L.; Descamps, V.; Kluger, N. Chemical composition and distribution of tattoo inks within tattoo-associated keratoacanthoma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e313–e315. [Google Scholar] [CrossRef] [PubMed]
- Giornale Italiano di Dermatologia e Venereologia 2019 Sep 30. Available online: https://www.minervamedica.it/en/journals/Ital-J-Dermatol-Venereol/article.php?cod=R23Y9999N00A19093001&acquista=1 (accessed on 24 January 2020).
- Kluger, N.; Douvin, D.; Dupuis-Fourdan, F.; Doumecq-Lacoste, J.M.; Descamps, V. Keratoacanthomas on recent tattoos: Two cases. Ann. Dermatol. Venereol. 2017, 144, 776–783. [Google Scholar] [CrossRef]
- Fraga, G.R.; Prossick, T.A. Tattoo-associated keratoacanthomas: A series of 8 patients with 11 keratoacanthomas. J. Cutan. Pathol. 2010, 37, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gon Ados, S.; Minelli, L.; Meissner, M.C. Keratoacanthoma in a tattoo. Dermatol. Online J. 2009, 15, 9. [Google Scholar] [PubMed]
- Kluger, N.; Minier-Thoumin, C.; Plantier, F. Keratoacanthoma occurring within the red dye of a tattoo. J. Cutan. Pathol. 2008, 35, 504–507. [Google Scholar] [CrossRef] [PubMed]
- De Antoni, E.; Brambullo, T.; Pescarini, E.; Salmaso, R.; Bassetto, F.; Vindigni, V. Dermatofibrosarcoma Protuberans on Tattooed Skin: A Case Report. Adv. Skin Wound Care 2020, 33, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lastrucci, I.; Gunnella, S.; Pileri, A.; Maio, V.; Grandi, V. Dermatofibrosarcoma protuberans secondary to a decorative tattoo: An Isotattootopic Response? Indian J. Dermatol. 2018, 63, 439. [Google Scholar] [CrossRef] [PubMed]
- Lobato-Berezo, A.; Churruca-Grijelmo, M.; Martínez-Pérez, M.; Imbernón-Moya, A.; Vargas-Laguna, M.E.; Fernández-Cogolludo, E.; Aguilar-Martínez, A.; Gallego-Valdés, M. Ángel Dermatofibroma Arising within a Black Tattoo. Case Rep. Dermatol. Med. 2014, 2014, 745304. [Google Scholar] [PubMed] [Green Version]
- Reddy, K.K.; Hanke, C.W.; Tierney, E.P. Malignancy arising within cutaneous tattoos: Case of dermatofibrosarcoma protuberans and review of literature. J. Drugs Dermatol. 2011, 10, 837–842. [Google Scholar]
- Baker, P.A.; O’Dowd, G.J.; Khan, I.U. Dermatofibrosarcoma Protuberans Arising in a Decorative Tattoo. Sarcoma 2005, 9, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Souza, E.S.; Rocha Bde, O.; Batista Eda, S.; Oliveira, R.F.; Farre, L.; Bittencourt, A.L. T-cell-predominant lymphoid hyperplasia in a tattoo. An. Bras. Dermatol. 2014, 89, 1019–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulonguet, I.; Garçon, N.; Rivet, J.; Cavelier-Balloy, B. Nodule developing over a tattoo: Challenge. Cutaneous lymphoid hyperplasia (pseudolymphoma). Am. J. Dermatopathol. 2014, 36, 101–102. [Google Scholar] [CrossRef]
- Bouchy, C.G.; Kerdraon, R.; Kluger, N.; Armingaud, P.; Wakosa, A.; Estève, E. Cutaneous lymphoid hyperplasia (pseudolymphoma) on the red dye of a tattoo. Ann. Pathol. 2013, 33, 273–277. [Google Scholar]
- Pasolini, G.; Ghidini, P.; Arisi, M.; Pedretti, A.; Ungari, M.; Pinton, P.C. Pseudolymphoma tattoo-induced. Dermatol. Rep. 2011, 3, e47. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Romero, L. Cutaneous Lymphoid Hyperplasia (Pseudolymphoma) in a Tattoo After Far Infrared Light. Dermatol. Surg. 2009, 35, 1434–1438. [Google Scholar] [CrossRef]
- Mahalingam, M.; Kim, E.; Bhawan, J. Morphea-Like Tattoo Reaction. Am. J. Dermatopathol. 2002, 24, 392–395. [Google Scholar] [CrossRef] [PubMed]
- West, C.C.; Morritt, A.N.; Pedelty, L.; Lam, D.G. Cutaneous leiomyosarcoma arising in a tattoo—A tumour with no humour. J. Plast. Reconstr. Aesthetic Surg. 2009, 2, e79–e80. [Google Scholar] [CrossRef] [PubMed]
- Laux, P.; Tralau, T.; Tentschert, J.; Blume, A.; Al Dahouk, S.; Bäumler, W.; Bernstein, E.; Bocca, B.; Alimonti, A.; Colebrook, H.; et al. A medical-toxicological view of tattooing. Lancet 2016, 387, 395–402. [Google Scholar] [CrossRef]
- Humphries, A.; Lister, T.S.; Wright, P.A.; Hughes, M.P. Determination of the thermal and physical properties of black tattoo ink using compound analysis. Lasers Med. Sci. 2013, 28, 1107–1112. [Google Scholar] [CrossRef]
- Carlsen, K.H.; Sepehri, M. Tattoo Complaints and Complications: Diagnosis and Clinical Spectrum. Curr. Probl. Dermatol. 2015, 48, 48–60. [Google Scholar]
- Beute, T.C.; Miller, C.H.; Timko, A.L.; Ross, E.V. In vitro spectral analysis of tattoo pigments. Dermatol. Surg. 2008, 34, 508–515. [Google Scholar]
- Forte, G.; Petrucci, F.; Cristaudo, A.; Bocca, B. Market survey on toxic metals contained in tattoo inks. Sci. Total Environ. 2009, 407, 5997–6002. [Google Scholar] [CrossRef] [PubMed]
- Bil, W.; Van Der Bent, S.A.S.; Spiekstra, S.; Nazmi, K.; Rustemeyer, T.; Gibbs, S. Comparison of the skin sensitization potential of 3 red and 2 black tattoo inks using interleukin-18 as a biomarker in a reconstructed human skin model. Contact Dermat. 2018, 79, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Hauri, U.; Hohl, C. Photostability and Breakdown Products of Pigments Currently Used in Tattoo Inks. Curr. Probl. Dermatol. 2015, 48, 164–169. [Google Scholar] [PubMed]
- Kluger, N. Tattoo Reactions Associated with Targeted Therapies and Immune Checkpoint Inhibitors for Advanced Cancers: A Brief Review. Dermatology 2019, 235, 522–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, C.A.; Twigg, P.C.; Baker, R.; Tobin, D.J. Tattoo ink nanoparticles in skin tissue and fibroblasts. Beilstein J. Nanotechnol. 2015, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiver, I.; Hutzler, C.; Andree, S.; Laux, P.; Luch, A. Identification and hazard prediction of tattoo pigments by means of pyrolysis—gas chromatography/mass spectrometry. Arch. Toxicol. 2016, 90, 1639–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, E.T.K.; Pedersen, E. Chemical Substances in Tattoo Ink; Danish Environmental Protection Agency Danish Environmental Protection Agency: Copenhagen, Danish, 2012. [Google Scholar]
- Hering, H.; Zoschke, C.; Kühn, M.; Gadicherla, A.K.; Weindl, G.; Luch, A.; Schreiver, I. TatS: A novel in vitro tattooed human skin model for improved pigment toxicology research. Arch. Toxicol. 2020, 94, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, M.; Lerche, C.M.; Carlsen, K.H.; Serup, J. Search for Internal Cancers in Mice Tattooed with Inks of High Contents of Potential Carcinogens: A One-Year Autopsy Study of Red and Black Tattoo Inks Banned in the Market. Dermatology 2017, 233, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerche, C.M.; Heerfordt, I.M.; Serup, J.; Poulsen, T.; Wulf, H.C. Red tattoos, ultraviolet radiation and skin cancer in mice. Exp. Dermatol. 2017, 26, 1091–1096. [Google Scholar] [CrossRef]
- Lehner, K.; Santarelli, F.; Vasold, R.; König, B.; Landthaler, M.; Bäumler, W. Black tattoo inks are a source of problematic substances such as dibutyl phthalate. Contact Dermat. 2011, 65, 231–238. [Google Scholar] [CrossRef]
- Gallagher, R.P.; MacArthur, A.C.; Lee, T.K.; Leblanc, A.; Elwood, J.M.; Borugian, M.; Abanto, Z.; Spinelli, J.J.; Weber, J.-P. Plasma levels of polychlorinated biphenyls and risk of cutaneous malignant melanoma: A preliminary study. Int. J. Cancer 2010, 128, 1872–1880. [Google Scholar] [CrossRef]
- Leijs, M.M.; Esser, A.; Amann, P.M.; Schettgen, T.; Gube, M.; Merk, H.F.; Kraus, T.; Baron, J.M. Hyperpigmentation and higher incidence of cutaneous malignancies in moderate-high PCB- and dioxin exposed individuals. Environ Res. 2018, 164, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.P.; Kelly, P.J.; Jardine, M.; Perkovic, V.; Cass, A.; Craig, J.; Eris, J.; Webster, A. Long-Term Cancer Risk of Immunosuppressive Regimens after Kidney Transplantation. J. Am. Soc. Nephrol. 2010, 21, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Sauer, E.; Gauer, B.; Nascimento, S.; Nardi, J.; Göethel, G.; Costa, B.; Correia, D.; Matte, U.; Charão, M.; Arbo, M.; et al. The role of B7 costimulation in benzene immunotoxicity and its potential association with cancer risk. Environ. Res. 2018, 166, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Malignes Melanom: Schwarzer Hautkreb. Available online: https://www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/krebsarten/hautkrebs/malignes-melanom-schwarzer-hautkrebs.html (accessed on 21 January 2021).
- Vasold, R.; Naarmann, N.; Ulrich, H.; Fischer, D.; Konig, B.; Landthaler, M.; Bäumler, W. Tattoo pigments are cleaved by laser light-the chemical analysis in vitro provide evidence for hazardous compounds. Photochem. Photobiol. 2004, 80, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wezel, K. Untersuchung des Verhaltens von Tätowiertinten und Pigmenten unter Lichteinfluss. Master’s Thesis, University of Giessen, Giessen, Germany, 2013. [Google Scholar]
- Gaugler, S. Analysis of bioactive compounds in tattoo inks before and after irradiation with sunlight using HPTLC and in situ detection with vibrio fischeri. Master’s Thesis, University of Hohenheim, Stuttgart, Germany, 2011. [Google Scholar]
- Hering, H.; Sung, A.Y.; Röder, N.; Hutzler, C.; Berlien, H.-P.; Laux, P.; Luch, A.; Schreiver, I. Laser Irradiation of Organic Tattoo Pigments Releases Carcinogens with 3,3′-Dichlorobenzidine Inducing DNA Strand Breaks in Human Skin Cells. J. Investig. Dermatol. 2018, 138, 2687–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiver, I.; Luch, A. At the dark end of the rainbow: Data gaps in tattoo toxicology. Arch. Toxicol. 2016, 90, 1763–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, D.T.; Zens, M.S.; Marmarelis, E.L.; Gilbert-Diamond, D.; Karagas, M.R. Cosmetic Tattooing and Early Onset Basal Cell Carcinoma: A Population-based Case–Control Study from New Hampshire. Epidemiology 2020, 31, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G. Italian Melanoma Intergroup for the Italian Melanoma Intergroup (IMI); Colombino, M.; Casula, M.; Manca, A.; Mandalà, M.; Cossu, A. Molecular Pathways in Melanomagenesis: What We Learned from Next-Generation Sequencing Approaches. Curr. Oncol. Rep. 2018, 20, 86. [Google Scholar] [CrossRef] [Green Version]

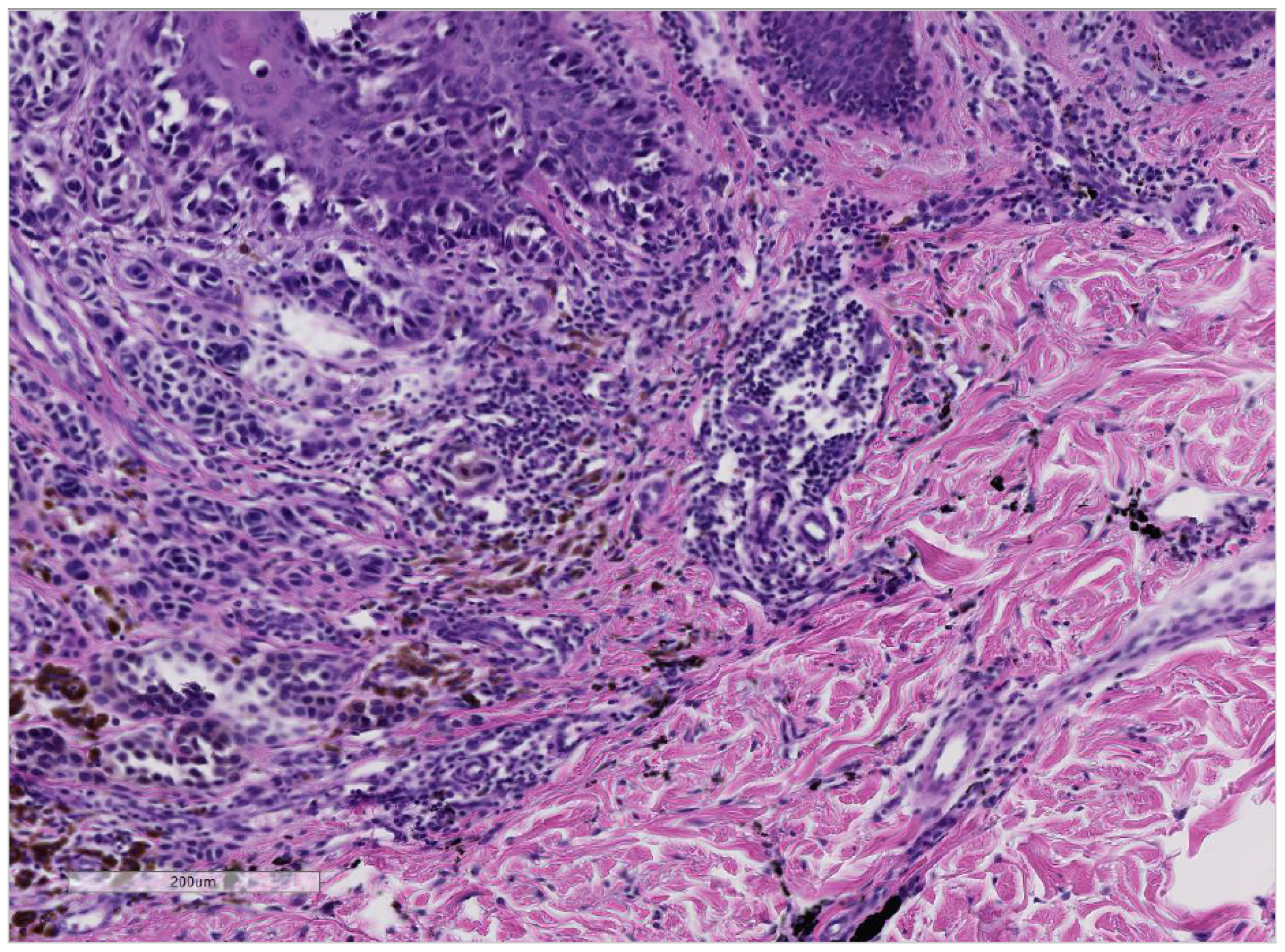






| Tumor-Type / Cases | Gender | Median Age | Location | Occurence | Color | Date & Reference |
|---|---|---|---|---|---|---|
| Malignant Melanoma 36 Cases | 32 male / 3 female / 1 NA | 45 years (range: 9 years–82 years, 2 NA) | Back | 7 (19%) | 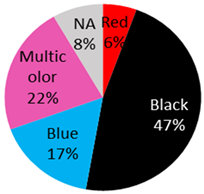 | 2021 (current study), 2019: [20], 2018: [2], 2016: [21], 2015: [22,23,24,25,26], 2014: [27], 2013: [5,28,29], 2011: [30] 2009: [31], 2008: [9], 2007: [32], 2006: [15,33], 2003: [34], 1999: [35], 1997: [36], 1993: [37], 1984: [10], 1980: [38], 1974: [39], 1972: [17] 1967: [40], 1938: [41] |
| Shoulder | 1 (3%) | |||||
| Arm | 17 (47%) | |||||
| Leg | 3 (8%) | |||||
| Chest | 3 (8%) | |||||
| Abdomen | 1 (3%) | |||||
| Scapula | 2 (6%) | |||||
| Forehead | 1 (3%) | |||||
| NA | 1 (3%) | |||||
| Basal-Cell-Carcinoma 18 Cases | 13 male / 5 female | 54 years (range: 28 years–76 years) | Arm | 3 (17%) | 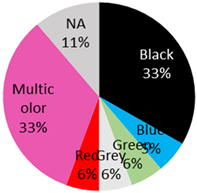 | 2021 (current study), 2020 [42], 2019: [43], 2018: [44], 2012: [14], 2009: [45,46], 2006: [47], 2004: [48], 1987: [49], 1983 [50] |
| Back | 5 (28%) | |||||
| Eyebrow | 1 (6%) | |||||
| Hand | 1 (6%) | |||||
| Lip | 2 (11%) | |||||
| Scapula | 1 (6%) | |||||
| Shoulder | 3 (17%) | |||||
| Temple | 2 (11%) | |||||
| Squamous cell carcinoma 13 Cases | 5 male, 6 female, 2 NA) | 47 years (range:24 years–79 years) | Arm | 4 (31%) | 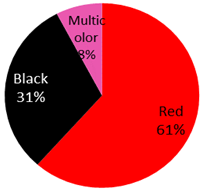 | 2019: [51], 2018: [52], 2017: [53,54], 2016: [55], 2015: [56], 2014: [57], 2010: [58], 2009: [59], 2008: [60], 2007: [61], 1966: [62] |
| Lip | 2 (15%) | |||||
| Leg | 3 (23%) | |||||
| Back | 1 (8%) | |||||
| NA | 3 (23%) | |||||
| Keratoacantoma 16 Cases | 7 male, 5 female, 4 NA | 54 years (range 36 years–72 years) | Scapula | 1 (6%) |  | 2020: [63], 2019: [64,65], 2017: [65], 2010: [66], 2009: [67], 2008: [68] |
| Leg | 3 (19%) | |||||
| Arm | 8 (50%) | |||||
| Shin | 2 (13%) | |||||
| Ankle | 2 (13%) | |||||
| Dermatofibrosarcoma protuberans 5 Cases | 3 male, 2 female | 31 years (range: 21 years–37 years, 1NA) | Arm | 1 (20%) | 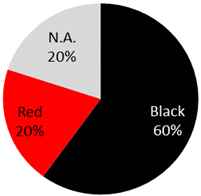 | 2020: [69], 2018: [70], 2014: [71], 2011: [72], 2005: [73] |
| Back | 2 (40%) | |||||
| Thigh | 1 (20%) | |||||
| N.A. | 1 (20%) | |||||
| Cutaneous lymphoid Hyperplasia 6 Cases | 3 male, 3 female | 35 years (range: 25 years–67 years) | Leg | 2 (33%) | 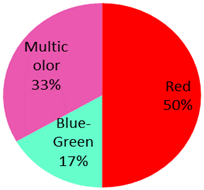 | 2014: [74,75], 2013: [76] 2011: [77] 2009: [78] 2002: [79] |
| Shoulder | 1 (40%) | |||||
| Back | 1 (17%) | |||||
| Ankle | 1 (17%) | |||||
| N.A. | 1 (17%) | |||||
| Cutaneous leiomyosarcoma 1 Case | male | 41 years | Arm | 1 (100%) | Black | 2009: [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leijs, M.; Schaefer, H.; Rübben, A.; Cacchi, C.; Rustemeyer, T.; van der Bent, S. Cutaneous Malignancies in Tattoos, a Case Series of Six Patients. Curr. Oncol. 2021, 28, 4721-4737. https://doi.org/10.3390/curroncol28060398
Leijs M, Schaefer H, Rübben A, Cacchi C, Rustemeyer T, van der Bent S. Cutaneous Malignancies in Tattoos, a Case Series of Six Patients. Current Oncology. 2021; 28(6):4721-4737. https://doi.org/10.3390/curroncol28060398
Chicago/Turabian StyleLeijs, Marike, Hannah Schaefer, Albert Rübben, Claudio Cacchi, Thomas Rustemeyer, and Sebastiaan van der Bent. 2021. "Cutaneous Malignancies in Tattoos, a Case Series of Six Patients" Current Oncology 28, no. 6: 4721-4737. https://doi.org/10.3390/curroncol28060398
APA StyleLeijs, M., Schaefer, H., Rübben, A., Cacchi, C., Rustemeyer, T., & van der Bent, S. (2021). Cutaneous Malignancies in Tattoos, a Case Series of Six Patients. Current Oncology, 28(6), 4721-4737. https://doi.org/10.3390/curroncol28060398





