Primary Care Continuity and Wait Times to Receiving Breast Cancer Chemotherapy: A Population-Based Retrospective Cohort Study Using CanIMPACT Data
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Variables and Data Sources
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Data Source | Data Elements |
|---|---|
| Ontario Cancer Registry (OCR) | Date of breast cancer diagnosis, age at diagnosis, sex, other cancer diagnoses, cancer stage |
| Registered Persons Database (RPDB) | Postal code at time of diagnosis, health region |
| 2006 Statistics Canada Census and Postal Code Conversion File Plus, version 5C | Rurality, neighborhood income quintile |
| Immigration Refugee and Citizenship Canada permanent resident (IRCC-PR) database | Immigration status |
| Ontario Health Insurance Plan (OHIP) | Number of PCP visits (billed encounters) total and per provider, reasons for visits, diagnostic codes, chemotherapy receipt, start of adjuvant chemotherapy |
| ICES Physician Database | Physician specialty |
| Client Agency Program Enrolment database (CAPE) and Corporate Provider Database | Primary care enrolment model |
| Canadian Institute for Health Information: Discharge Abstract Database (DAD) and Same-Day Surgery (SDS) database | Diagnosis codes, surgery receipt |
| Cancer Activity Level Reporting (ALR) database | Date of radiotherapy receipt |
Appendix B
| Total n = 12,781 | Primary Care Interval in Days (from First Contact to First Oncology Visit) | ||||||
|---|---|---|---|---|---|---|---|
| Screened n = 2916 (22.8%) | Symptomatic n = 9865 (77.2%) | ||||||
| Median (IQR) | 90th Percentile | Kruskal–Wallis p-value * | Median (IQR) | 90th Percentile | Kruskal–Wallis p-value * | ||
| Total | 34 (21, 50) | 72 | 34 (17, 62) | 111 | |||
| Age (Categorical) | <0.0001 | <0.0001 | |||||
| <40 years | 1102 (8.6%) | 20 (18, 34) | 51 | 29 (14, 56) | 101 | ||
| 40–49 years | 3481 (27.2%) | 21 (9, 42) | 85 | 35 (19, 64) | 117 | ||
| 50–59 years | 4225 (33.1%) | 34 (21, 51) | 74 | 35 (16, 64) | 113 | ||
| 60–69 years | 3045 (23.8%) | 35 (22, 50) | 70 | 34 (16, 63) | 108 | ||
| 70–74 years | 607 (4.7%) | 35 (20, 50) | 67 | 34 (17, 59) | 99 | ||
| >74 years | 321 (2.5%) | 37 (21, 60) | 79 | 28 (14, 55) | 83 | ||
| Urban/Rural Residence | <0.0001 | 0.0078 | |||||
| Urban | 11,189 (87.5%) | 34 (20, 49) | 70 | 34 (17, 63) | 110 | ||
| Rural | 699 (5.5%) | 36 (21, 50) | 74 | 35 (17, 62) | 113 | ||
| Rural—remote | 596 (4.7%) | 41 (25, 60) | 84 | 32 (15, 56) | 106 | ||
| Rural—very remote | 292–297 (2.3%) | 43 (26, 70) | 91 | 28 (12, 58) | 113 | ||
| Rural—unknown | ≤5 | ** | ** | ** | ** | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
| Immigration Status | 0.4322 | 0.9899 | |||||
| Long-term residents | 11,075 (86.7%) | 34 (21, 50) | 71 | 34 (17, 62) | 111 | ||
| Immigrants | 1706 (13.3%) | 33 (17, 54) | 76 | 34 (17, 63) | 109 | ||
| Immigrant Region of Origin | 0.2853 | 0.0783 | |||||
| East Asia and Pacific | 544 (4.3%) | 33 (17, 62) | 94 | 33 (15, 63) | 112 | ||
| Eastern Europe and Central Asia | 286 (2.2%) | 27 (9, 51) | 56 | 33 (16, 62) | 112 | ||
| Latin America and Caribbean | 239 (1.9%) | 36 (21, 53) | 84 | 41 (19, 72) | 107 | ||
| Middle East and North Africa | 145 (1.1%) | 41 (16, 51) | 62 | 42 (19, 75) | 121 | ||
| South Asia | 270 (2.1%) | 30 (15, 44) | 78 | 28 (15, 53) | 95 | ||
| Sub-Saharan Africa | 87 (0.7%) | 41 (23, 55) | 69 | 37 (18, 69) | 90 | ||
| USA/New Zealand/Australia | 37 (0.3%) | 36 (28, 76) | 105 | 36 (15, 60) | 117 | ||
| Western Europe | 98 (0.8%) | 38 (24, 67) | 113 | 29 (14, 49) | 87 | ||
| Neighbourhood Income Quintile | 0.7635 | 0.7172 | |||||
| 1 (lowest) | 2020 (15.8%) | 35 (21, 54) | 75 | 34 (17, 62) | 105 | ||
| 2 | 2384 (18.7%) | 34 (21, 52) | 71 | 34 (17, 63) | 106 | ||
| 3 | 2523 (19.7%) | 35 (21, 51) | 72 | 34 (17, 63) | 108 | ||
| 4 | 2819 (22.1%) | 35 (21, 49) | 70 | 34 (16, 59) | 107 | ||
| 5 (highest) | 2994 (23.4%) | 34 (21, 49) | 70 | 34 (17, 64) | 122 | ||
| Unknown | 41 (0.3%) | 42 (25, 89) | 102 | 42 (25, 63) | 112 | ||
| Comorbidity Burden | 0.9419 | <0.0001 | |||||
| 0–5 ADGs | 7287 (57.0%) | 35 (21, 50) | 71 | 33 (16, 59) | 105 | ||
| 6–9 ADGs | 4425 (34.6%) | 34 (21, 51) | 73 | 36 (18, 66) | 115 | ||
| 10+ ADGs | 1069 (8.4%) | 35 (21, 53) | 71 | 34 (17, 63) | 120 | ||
| History of Mental Health Visits | 0.6662 | 0.0007 | |||||
| Yes | 4127 (32.3%) | 34 (21, 51) | 72 | 35 (18, 65) | 115 | ||
| No | 8654 (67.7%) | 35 (21, 50) | 72 | 33 (16, 61) | 108 | ||
| Stage | <0.0001 | <0.0001 | |||||
| Stage I | 2839 (22.2%) | 37 (23, 54) | 76 | 39 (21, 71) | 122 | ||
| Stage II | 7311 (57.2%) | 33 (20, 49) | 71 | 34 (17, 61) | 106 | ||
| Stage III | 2631 (20.6%) | 32 (19, 48) | 69 | 29 (14, 56) | 107 | ||
| Primary Care Practice Model | 0.5489 | 0.0078 | |||||
| Straight FFS | 1887 (14.8%) | 36 (20, 52) | 71 | 32 (15, 62) | 103 | ||
| Enhanced FFS | 6281 (49.1%) | 35 (21, 51) | 71 | 35 (17, 62) | 108 | ||
| Capitation | 2235 (17.5%) | 35 (21, 50) | 73 | 35 (17, 68) | 119 | ||
| Team-based capitation | 2206 (17.3%) | 33 (20, 50) | 72 | 33 (17, 61) | 115 | ||
| Other | 172 (1.3%) | 35 (25, 48) | 63 | 27 (12, 49) | 77 | ||
| Primary Care Enrolment Status | 0.4377 | 0.0256 | |||||
| Rostered | 10,900 (85.3%) | 34 (21, 50) | 72 | 34 (17, 62) | 112 | ||
| Not rostered | 1881 (14.7%) | 36 (20, 52) | 71 | 32 (15, 62) | 103 | ||
| Health Region | <0.0001 | <0.0001 | |||||
| 1 Erie St. Clair | 713 (5.6%) | 35 (21, 49) | 71 | 40 (21, 68) | 116 | ||
| 2 South West | 992 (7.8%) | 44 (27, 67) | 89 | 40 (20, 69) | 119 | ||
| 3 Waterloo Wellington | 654 (5.1%) | 33 (20, 44) | 57 | 27 (14, 51) | 105 | ||
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 29 (15, 43) | 57 | 32 (16, 55) | 99 | ||
| 5 Central West | 543 (4.2%) | 39 (25, 50) | 63 | 33 (17, 52) | 90 | ||
| 6 Mississauga Halton | 750 (5.9%) | 31 (17, 49) | 78 | 35 (16, 65) | 116 | ||
| 7 Toronto Central | 1061 (8.3%) | 34 (19, 55) | 76 | 34 (16, 67) | 120 | ||
| 8 Central | 1784 (14.0%) | 29 (17, 47) | 69 | 31 (15, 61) | 114 | ||
| 9 Central East | 1710 (13.4%) | 29 (18, 41) | 52 | 32 (15, 58) | 104 | ||
| 10 South East | 520 (4.1%) | 40 (25, 61) | 80 | 31 (17, 56) | 103 | ||
| 11 Champlain | 1335 (10.4%) | 44 (30, 57) | 70 | 46 (28, 74) | 127 | ||
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 27 (17, 43) | 68 | 27 (15, 58) | 110 | ||
| 13 North East | 478 (3.7%) | 32 (21, 43) | 63 | 27 (12, 53) | 91 | ||
| 14 North West | 252 (2.0%) | 56 (37, 77) | 95 | 35 (14, 63) | 107 | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
Appendix C
| Total n = 12,781 | Surgery-to-Adjuvant-Chemotherapy Interval in Days | |||
|---|---|---|---|---|
| Median (IQR) | 90th percentile | Kruskal–Wallis p-value * | ||
| Total | 58 (46, 74) | 93 | ||
| Age (Categorical) | <0.0001 | |||
| <40 years | 1102 (8.6%) | 52 (41, 68) | 87 | |
| 40–49 years | 3481 (27.2%) | 56 (44, 71) | 91 | |
| 50–59 years | 4225 (33.1%) | 58 (46, 74) | 93 | |
| 60–69 years | 3045 (23.8%) | 60 (48, 76) | 96 | |
| 70–74 years | 607 (4.7%) | 62 (49, 81) | 98 | |
| >74 years | 321 (2.5%) | 65 (49, 81) | 105 | |
| Urban/Rural Residence | <0.0001 | |||
| Urban | 11,189 (87.5%) | 57 (45, 73) | 92 | |
| Rural | 699 (5.5%) | 62 (48, 77) | 94 | |
| Rural—remote | 596 (4.7%) | 63 (50, 79) | 98 | |
| Rural—very remote | 292–297 (2.3%) | 66 (49, 86) | 110 | |
| Rural—unknown | ≤5 | ** | ** | |
| Unknown | ≤5 | ** | ** | |
| Immigration Status | 0.2876 | |||
| Long-term residents | 11,075 (86.7%) | 58 (46, 74) | 93 | |
| Immigrants | 1706 (13.3%) | 57 (44, 75) | 96 | |
| Immigrant Region of Origin | 0.1119 | |||
| East Asia and Pacific | 544 (4.3%) | 57 (43, 77) | 98 | |
| Eastern Europe and Central Asia | 286 (2.2%) | 56 (45, 70) | 88 | |
| Latin America and Caribbean | 239 (1.9%) | 59 (46, 77) | 107 | |
| Middle East and North Africa | 145 (1.1%) | 59 (43, 76) | 91 | |
| South Asia | 270 (2.1%) | 60 (46, 78) | 102 | |
| Sub-Saharan Africa | 87 (0.7%) | 53 (43, 70) | 99 | |
| USA/New Zealand/Australia | 37 (0.3%) | 52 (38, 72) | 91 | |
| Western Europe | 98 (0.8%) | 55 (42, 67) | 87 | |
| Neighbourhood Income Quintile | 0.0456 | |||
| 1 (lowest) | 2020 (15.8%) | 57 (45, 75) | 93 | |
| 2 | 2384 (18.7%) | 58 (46, 75) | 94 | |
| 3 | 2523 (19.7%) | 58 (46, 76) | 96 | |
| 4 | 2819 (22.1%) | 58 (45, 73) | 92 | |
| 5 (highest) | 2994 (23.4%) | 57 (45, 72) | 91 | |
| Unknown | 41 (0.3%) | 62 (48, 85) | 104 | |
| Comorbidity Burden | 0.0561 | |||
| 0–5 ADGs | 7287 (57.0%) | 57 (46, 73) | 92 | |
| 6–9 ADGs | 4425 (34.6%) | 58 (46, 74) | 94 | |
| 10+ ADGs | 1069 (8.4%) | 59 (45, 78) | 99 | |
| History of Mental Health Visits | 0.0595 | |||
| Yes | 4127 (32.3%) | 58 (46, 75) | 94 | |
| No | 8654 (67.7%) | 57 (45, 74) | 92 | |
| Stage | <0.0001 | |||
| Stage I | 2839 (22.2%) | 60 (48, 76) | 98 | |
| Stage II | 7311 (57.2%) | 58 (47, 75) | 93 | |
| Stage III | 2631 (20.6%) | 53 (41, 69) | 87 | |
| Primary Care Practice Model | 0.1546 | |||
| Straight FFS | 1887 (14.8%) | 57 (45, 74) | 96 | |
| Enhanced FFS | 6281 (49.1%) | 58 (45, 74) | 94 | |
| Capitation | 2235 (17.5%) | 57 (45, 73) | 92 | |
| Team-based capitation | 2206 (17.3%) | 59 (47, 73) | 91 | |
| Other | 172 (1.3%) | 56 (42, 75) | 97 | |
| Primary Care Enrolment Status | 0.5820 | |||
| Rostered | 10,900 (85.3%) | 58 (46, 74) | 93 | |
| Not rostered | 1881 (14.7%) | 57 (45, 74) | 96 | |
| Health Region | <0.0001 | |||
| 1 Erie St. Clair | 713 (5.6%) | 50 (40, 69) | 86 | |
| 2 South West | 992 (7.8%) | 63 (52, 79) | 98 | |
| 3 Waterloo Wellington | 654 (5.1%) | 52 (41, 68) | 83 | |
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 56 (45, 70) | 86 | |
| 5 Central West | 543 (4.2%) | 58 (44, 74) | 98 | |
| 6 Mississauga Halton | 750 (5.9%) | 55 (43, 71) | 92 | |
| 7 Toronto Central | 1061 (8.3%) | 57 (45, 71) | 96 | |
| 8 Central | 1784 (14.0%) | 56 (43, 73) | 93 | |
| 9 Central East | 1710 (13.4%) | 57 (45, 74) | 94 | |
| 10 South East | 520 (4.1%) | 59 (48, 73) | 91 | |
| 11 Champlain | 1335 (10.4%) | 65 (53, 79) | 96 | |
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 63 (51, 78) | 93 | |
| 13 North East | 478 (3.7%) | 61 (45, 77) | 107 | |
| 14 North West | 252 (2.0%) | 52 (36, 72) | 97 | |
| Unknown | ≤5 | ** | ** | |
References
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/canadian-cancer-statistics.html (accessed on 24 June 2021).
- Statistics Canada. Table 2: Count and Percentage of Surgically Treated Female Breast Tumours, by American Joint Committee on Cancer Stage at Diagnosis, by Province and Territory, Canada (except Quebec), 2010 to 2012 Combined. 2018. Available online: https://www150.statcan.gc.ca/n1/pub/82-003-x/2018008/article/00001/tbl/tbl02-eng.htm (accessed on 27 July 2021).
- Powis, M.; Groome, P.; Biswanger, N.; Kendell, C.; Decker, K.M.; Grunfeld, E.; McBride, M.L.; Urquhart, R.; Winget, M.; Porter, G.A.; et al. Cross-Canada Differences in Early-Stage Breast Cancer Treatment and Acute-Care Use. Curr. Oncol. 2019, 26, 624–639. [Google Scholar] [CrossRef] [Green Version]
- Raphael, M.J.; Biagi, J.J.; Kong, W.; Mates, M.; Booth, C.M.; MacKillop, W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2016, 160, 17–28. [Google Scholar] [CrossRef]
- Zhan, Q.-H.; Fu, J.-Q.; Fu, F.-M.; Zhang, J.; Wang, C. Survival and time to initiation of adjuvant chemotherapy among breast cancer patients: A systematic review and meta-analysis. Oncotarget 2017, 9, 2739–2751. [Google Scholar] [CrossRef] [Green Version]
- Tørring, M.L.; Falborg, A.Z.; Jensen, H.; Neal, R.D.; Weller, D.; Reguilon, I.; Menon, U.; Vedsted, P.; Almberg, S.S.; Anandan, C.; et al. Advanced-stage cancer and time to diagnosis: An International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur. J. Cancer Care 2019, 28, e13100. [Google Scholar] [CrossRef] [PubMed]
- Tørring, M.L.; Frydenberg, M.; Hansen, R.P.; Olesen, F.; Vedsted, P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care. Eur. J. Cancer 2013, 49, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112 (Suppl. 1), S92–S107. [Google Scholar] [CrossRef] [Green Version]
- Ermiah, E.; Abdalla, F.; Buhmeida, A.; Larbesh, E.; Pyrhönen, S.; Collan, Y. Diagnosis delay in Libyan female breast cancer. BMC Res. Notes 2012, 5, 452. [Google Scholar] [CrossRef] [Green Version]
- Huo, Q.; Cai, C.; Zhang, Y.; Kong, X.; Jiang, L.; Ma, T.; Zhang, N.; Yang, Q. Delay in Diagnosis and Treatment of Symptomatic Breast Cancer in China. Ann. Surg. Oncol. 2015, 22, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.A.; Westcombe, A.M.; Love, S.B.; Littlejohns, P.; Ramirez, A.J. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 1999, 353, 1119–1126. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Clarke, C.A.; Lichtensztajn, D.Y.; Giordano, S.H. Delayed Initiation of Adjuvant Chemotherapy Among Patients with Breast Cancer. JAMA Oncol. 2016, 2, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Mujar, M.; Dahlui, M.; Yip, C.H.; Taib, N.A. Delays in time to primary treatment after a diagnosis of breast cancer: Does it impact survival? Prev. Med. 2013, 56, 222–224. [Google Scholar] [CrossRef]
- Cabral, A.L.L.V.; Giatti, L.; Casale, C.; Cherchiglia, M.L. Social vulnerability and breast cancer: Differentials in the interval between diagnosis and treatment of women with different sociodemographic profiles. Cienc. Saude Coletiva 2019, 24, 613–622. [Google Scholar]
- Saint-Jacques, N.; Younis, T.; Dewar, R.; Rayson, D. Wait times for breast cancer care. Br. J. Cancer 2007, 96, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Khorana, A.A.; Tullio, K.; Elson, P.; Pennell, N.A.; Grobmyer, S.R.; Kalady, M.F.; Raymond, D.; Abraham, J.; Klein, E.A.; Walsh, R.M.; et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS ONE 2019, 14, e0213209. [Google Scholar]
- Ramirez, A.J.; Westcombe, A.M.; Burgess, C.C.; Sutton, S.; Littlejohns, P.; Richards, M.A. Factors predicting delayed presentation of symptomatic breast cancer: A systematic review. Lancet 1999, 353, 1127–1131. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Mao, F.; Guan, J.; Wang, X.; Zhang, Y.; Zhang, X.; Shen, S.; Sun, Q. The influence on survival of delay in the treatment initiation of screening detected non-symptomatic breast cancer. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Ye, F.; Zhao, B.; Tang, H.; Wang, J.; Xiao, X.; Xie, X. Risk factors for delay of adjuvant chemotherapy in non-metastatic breast cancer patients: A systematic review and meta-analysis involving 186982 patients. PLoS ONE 2017, 12, e0173862. [Google Scholar] [CrossRef] [PubMed]
- Losk, K.; Freedman, R.A.; Lin, N.U.; Golshan, M.; Pochebit, S.M.; Lester, S.C.; Natsuhara, K.; Camuso, K.; King, T.A.; Bunnell, C.A.; et al. Implementation of Surgeon-Initiated Gene Expression Profile Testing (Onco type DX) Among Patients with Early-Stage Breast Cancer to Reduce Delays in Chemotherapy Initiation. J. Oncol. Pract. 2017, 13, e815–e820. [Google Scholar] [CrossRef]
- Blackmore, K.M.; Weerasinghe, A.; Holloway, C.M.B.; Majpruz, V.; Mirea, L.; O’Malley, F.P.; Harris, C.P.; Hendry, A.; Hey, A.; Kornecki, A.; et al. Comparison of wait times across the breast cancer treatment pathway among screened women undergoing organized breast assessment versus usual care. Can. J. Public Health 2019, 110, 595–605. [Google Scholar] [CrossRef]
- McKevitt, E.C.; Dingee, C.K.; Leung, S.-P.; Brown, C.J.; Van Laeken, N.Y.; Lee, R.; Kuusk, U. Reduced Time to Breast Cancer Diagnosis with Coordination of Radiological and Clinical Care. Cureus 2017, 9, e1919. [Google Scholar] [CrossRef] [Green Version]
- Plotogea, A.; Chiarelli, A.M.; Mirea, L.; Prummel, M.V.; Chong, N.; Shumak, R.S.; O’Malley, F.P.; Holloway, C.M.B.; the Breast Screening Study Group. Factors associated with wait times across the breast cancer treatment pathway in Ontario. SpringerPlus 2013, 2, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics Canada. Immigration and Ethnocultural Diversity in Canada. 2018. Available online: https://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm (accessed on 12 July 2019).
- Lofters, A.K.; CanIMPACT for the CanIMPACT Team; McBride, M.L.; Li, D.; Whitehead, M.; Moineddin, R.; Jiang, L.; Grunfeld, E.; Groome, P.A. Disparities in breast cancer diagnosis for immigrant women in Ontario and BC: Results from the CanIMPACT study. BMC Cancer 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Ginsburg, O.; Fischer, H.D.; Austin, P.C.; Creatore, M.I.; Narod, S.A.; Rochon, P.A. A Population-Based Cross-Sectional Study Comparing Breast Cancer Stage at Diagnosis between Immigrant and Canadian-Born Women in Ontario. Breast J. 2017, 23, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, L.; Bondy, S.J.; Chen, Z.; Maaten, S. Physician Care of Cancer Patients. In Primary Care in Ontario: ICES Atlas; Jaakkimainen, L., Upshur, R.E.G., Klein-Geltink, J.E., Leong, A., Maaten, S., Schultz, S.E., Eds.; The Institute for Clinical Evaluative Sciences (ICES): Toronto, ON, Canada, 2006; pp. 161–174. [Google Scholar]
- Roetzheim, R.G.; Ferrante, J.M.; Lee, J.-H.; Chen, R.; Love-Jackson, K.M.; Gonzalez, E.C.; Fisher, K.J.; McCarthy, E.P. Influence of Primary Care on Breast Cancer Outcomes Among Medicare Beneficiaries. Ann. Fam. Med. 2012, 10, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, J.M.; Lee, J.H.; McCarthy, E.P.; Fisher, K.J.; Chen, R.; Gonzalez, E.C.; Love-Jackson, K.; Roetzheim, R.G. Primary care utilization and colorectal cancer incidence and mortality among Medicare beneficiaries: A population-based, case-control study. Ann. Intern. Med. 2013, 159, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.E.; Doebbeling, C.C. Beyond the Traditional Prognostic Indicators: The Impact of Primary Care Utilization on Cancer Survival. J. Clin. Oncol. 2007, 25, 5793–5799. [Google Scholar] [CrossRef]
- Arnold, L.D.; McGilvray, M.M.O.; Cooper, J.K.; James, A.S. Inadequate Cancer Screening: Lack of Provider Continuity is a Greater Obstacle than Medical Mistrust. J. Health Care Poor Underserved 2017, 28, 362–377. [Google Scholar] [CrossRef] [Green Version]
- Canadian Institute for Health Information. Continuity of Care with Family Medicine Physicians: Why It Matters. Available online: https://secure.cihi.ca/estore/productFamily.htm?locale=en&pf=PFC2865 (accessed on 24 June 2021).
- Mathews, M.; Ryan, D.; Bulman, D. What does satisfaction with wait times mean to cancer patients? BMC Cancer 2015, 15, 1017. [Google Scholar] [CrossRef] [Green Version]
- Provost, S.; Pineault, R.; Tousignant, P.; Roberge, D.; Tremblay, D.; Breton, M.; Benhadj, L.; Diop, M.; Fournier, M.; Brousselle, A. Does the primary care experience influence the cancer diagnostic process? Int. J. Fam. Med. 2015, 2015, 176812. [Google Scholar] [CrossRef]
- Parsonage, R.K.; Hiscock, J.; Law, R.-J.; Neal, R.D. Patient perspectives on delays in diagnosis and treatment of cancer: A qualitative analysis of free-text data. Br. J. Gen. Pract. 2017, 67, e49–e56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICES. Working with ICES Data. 2019. Available online: https://www.ices.on.ca/Data-and-Privacy/ICES-data/Working-with-ICES-Data (accessed on 4 July 2019).
- Grunfeld, E. It takes a team: CanIMPACT: Canadian Team to Improve Community-Based Cancer Care along the Continuum. Can. Fam. Physician Med. Fam. Can. 2016, 62, 781–782. [Google Scholar]
- Kendell, C.; Decker, K.M.; Groome, P.A.; McBride, M.L.; Jiang, L.; Krzyzanowska, M.; Porter, G.; Turner, D.; Urquhart, R.; Winget, M.; et al. Use of Physician Services during the Survivorship Phase: A Multi-Province Study of Women Diagnosed with Breast Cancer. Curr. Oncol. 2017, 24, 81–89. [Google Scholar] [CrossRef]
- McBride, M.L.; CanIMPACT for the CanIMPACT Team; Groome, P.A.; Decker, K.; Kendell, C.; Jiang, L.; Whitehead, M.; Li, D.; Grunfeld, E. Adherence to quality breast cancer survivorship care in four Canadian provinces: A CanIMPACT retrospective cohort study. BMC Cancer 2019, 19, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, M.; Lebenbaum, M.; Lam, K.; Chong, N.; Azimaee, M.; Iron, K.; Manuel, D.; Guttmann, A. Describing the linkages of the immigration, refugees and citizenship Canada permanent resident data and vital statistics death registry to Ontario’s administrative health database. BMC Med. Inform. Decis. Mak. 2016, 16, 135. [Google Scholar] [CrossRef] [Green Version]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.E.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef]
- Weller, D.; Vedsted, P.; Anandan, C.; Zalounina, A.; Fourkala, E.O.; Desai, R.; Liston, W.; Jensen, H.; Barisic, A.; Gavin, A.; et al. An investigation of routes to cancer diagnosis in 10 international jurisdictions, as part of the International Cancer Benchmarking Partnership: Survey development and implementation. BMJ Open 2016, 6, e009641. [Google Scholar] [CrossRef] [Green Version]
- Defusing the Confusion: Concepts and Measures of Continuity of Healthcare. Available online: https://www.worldcat.org/title/defusing-the-confusion-concepts-and-measures-of-continuity-of-health-care/oclc/50248087 (accessed on 20 October 2021).
- Breslau, N.; Reeb, K.G. Continuity of care in a university-based practice. J. Med. Educ. 1975, 50, 965–969. [Google Scholar] [CrossRef]
- Rodriguez, H.P.; Marshall, R.E.; Rogers, W.H.; Safran, D.G. Primary Care Physician Visit Continuity: A Comparison of Patient-reported and Administratively Derived Measures. J. Gen. Intern. Med. 2008, 23, 1499–1502. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Lofters, A.; Moineddin, R.; Decker, K.; Groome, P.; Kendell, C.; Krzyzanowska, M.; Li, D.; McBride, M.L.; Mittmann, N.; et al. Primary care physician use across the breast cancer care continuum: CanIMPACT study using Canadian administrative data. Can. Fam. Physician 2016, 62, e589–e598. [Google Scholar] [PubMed]
- Silver, S.A.; Bota, S.E.; McArthur, E.; Clemens, K.K.; Harel, Z.; Naylor, K.L.; Sood, M.M.; Garg, A.X.; Wald, R. Association of Primary Care Involvement with Death or Hospitalizations for Patients Starting Dialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 521–529. [Google Scholar] [CrossRef]
- Starfield, B.; Weiner, J.; Mumford, L.; Steinwachs, D. Ambulatory care groups: A categorization of diagnoses for research and management. Health Serv. Res. 1991, 26, 53–74. [Google Scholar]
- Steele, L.S.; Glazier, R.H.; Lin, E.; Evans, M. Using Administrative Data to Measure Ambulatory Mental Health Service Provision in Primary Care. Med. Care 2004, 42, 960–965. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS 9.4. Available online: https://www.sas.com/content/dam/SAS/en_ca/User%20Group%20Presentations/Hamilton-User-Group/OrrLawrence-NewFeaturesSAS%209.4-Spring2014.pdf (accessed on 24 June 2021).
- Weller, D.; Menon, U.; Falborg, A.Z.; Jensen, H.; Barisic, A.; Knudsen, A.K.; Bergin, R.; Brewster, D.; Cairnduff, V.; Gavin, A.T.; et al. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP). BMJ Open 2018, 8, e023870. [Google Scholar] [CrossRef]
- Menon, U.; Vedsted, P.; Falborg, A.Z.; Jensen, H.; Harrison, S.; Reguilon, I.; Barisic, A.; Bergin, R.; Brewster, D.H.; Butler, J.; et al. Time intervals and routes to diagnosis for lung cancer in 10 jurisdictions: Cross-sectional study findings from the International Cancer Benchmarking Partnership (ICBP). BMJ Open 2019, 9, e025895. [Google Scholar] [CrossRef]
- Ontario Breast Screening Program (OBSP) Guidelines Summary. Available online: https://better-program.ca/fr/evidence/resource/ontario-breast-screening-program-obsp-guidelines-summary/ (accessed on 24 June 2021).
- Barisic, A.; Kish, M.; Gilbert, J.; Mittmann, N.; Moineddin, R.; Sisler, J.; Vedsted, P.; Grunfeld, E. Family physician access to and wait times for cancer diagnostic investigations: Regional differences among 3 provinces. Can. Fam. Physician Med. Fam. Can. 2016, 62, e599–e607. [Google Scholar]
- Velikova, G.; Booth, L.; Johnston, C.; Forman, D.; Selby, P. Breast cancer outcomes in South Asian population of West Yorkshire. Br. J. Cancer 2004, 90, 1926–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thøgersen, H.; Møller, B.; Åsli, L.M.; Bhargava, S.; Kvåle, R.; Fjellbirkeland, L.; Robsahm, T.E.; Aaserud, S.; Babigumira, R.; Larsen, I.K. Waiting times and treatment following cancer diagnosis: Comparison between immigrants and the Norwegian host population. Acta Oncol. 2020, 59, 376–383. [Google Scholar] [CrossRef]
- Wang, A.M.Q.; Yung, E.M.; Nitti, N.; Shakya, Y.; Alamgir, A.K.M.; Lofters, A.K. Breast and Colorectal Cancer Screening Barriers Among Immigrants and Refugees: A Mixed-Methods Study at Three Community Health Centres in Toronto, Canada. J. Immigr. Minor. Health 2019, 21, 473–482. [Google Scholar] [CrossRef]
- Vahabi, M.; Lofters, A.; Kumar, M.; Glazier, R.H. Breast cancer screening disparities among immigrant women by world region of origin: A population-based study in Ontario, Canada. Cancer Med. 2016, 5, 1670–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarrant, C.; Dixon-Woods, M.; Colman, A.M.; Stokes, T. Continuity and Trust in Primary Care: A Qualitative Study Informed by Game Theory. Ann. Fam. Med. 2010, 8, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Redaniel, M.T.; Martin, R.M.; Ridd, M.J.; Wade, J.; Jeffreys, M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: Historical cohort study using the Clinical Practice Research Datalink. PLoS ONE 2015, 10, e0126608. [Google Scholar] [CrossRef] [Green Version]
- Lukács, G.; Kovacs, A.; Csanádi, M.; Moizs, M.; Repa, I.; Kaló, Z.; Vokó, Z.; Pitter, J.G. Benefits of Timely Care in Pancreatic Cancer: A Systematic Review to Navigate Through the Contradictory Evidence. Cancer Manag. Res. 2019, 11, 9849–9861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, C.; Whitehead, M.; Eisen, A.; Holloway, C.M.B.; Groome, P.A. Breast cancer diagnosis and treatment wait times in specialized diagnostic units compared with usual care: A population-based study. Curr. Oncol. 2020, 27, 377–385. [Google Scholar] [CrossRef] [PubMed]
- McPhail, S.; Elliss-Brookes, L.; Shelton, J.; Ives, A.; Greenslade, M.; Vernon, S.W.; Morris, E.J.A.; Richards, M. Emergency presentation of cancer and short-term mortality. Br. J. Cancer 2013, 109, 2027–2034. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Marchildon, G.P.; Hutchison, B. Primary care in Ontario, Canada: New proposals after 15 years of reform. Health Policy 2016, 120, 732–738. [Google Scholar] [CrossRef] [Green Version]
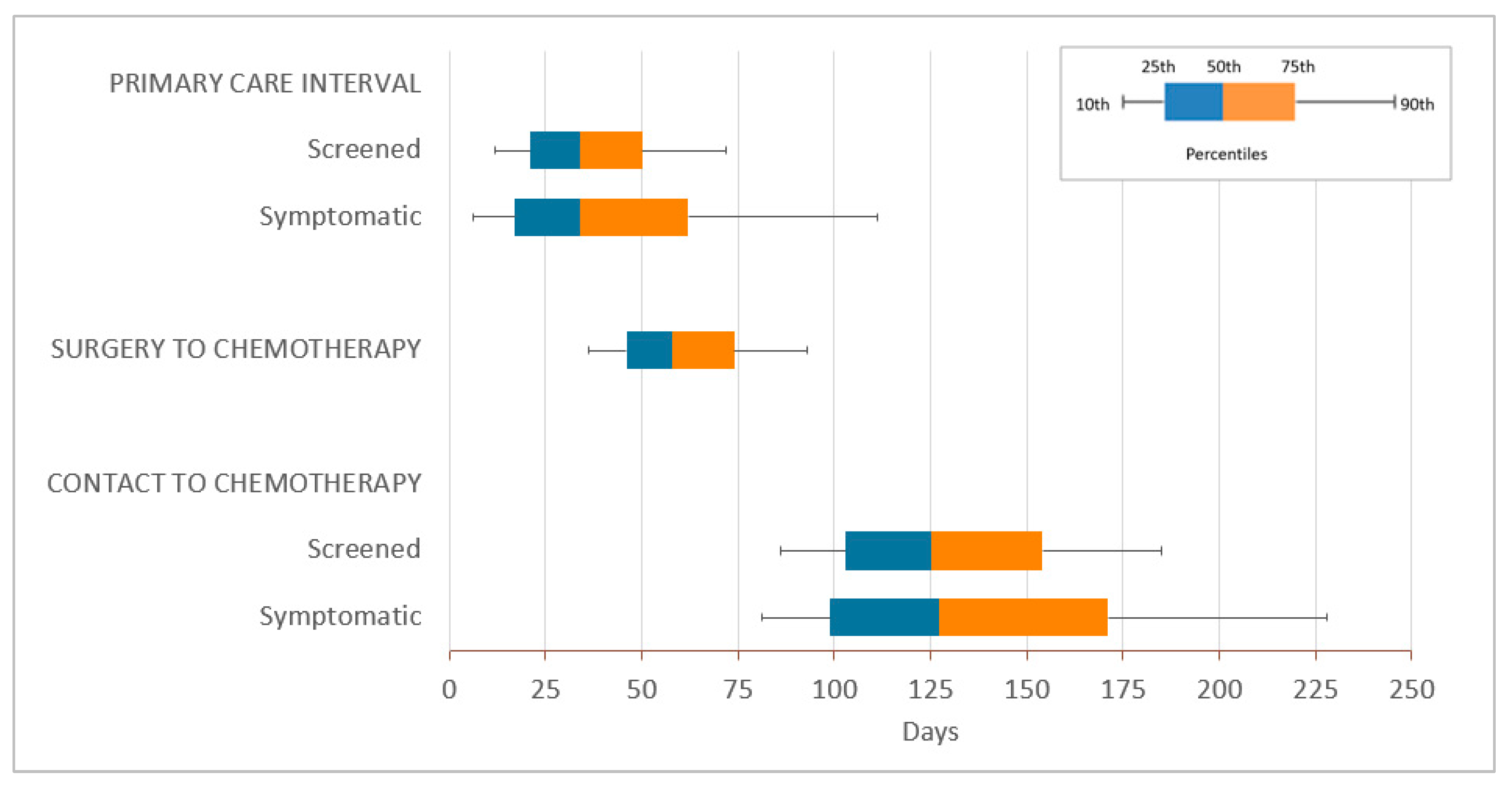



| Total n = 12,781 | PCP Continuity | p-Value | ||||
|---|---|---|---|---|---|---|
| 0 Visits | 1–2 Visits | UPC ≤ 0.75 (Low) | UPC > 0.75 (High) | |||
| Total | 800 (100%) | 1536 (100%) | 3914 (100%) | 6531 (100%) | ||
| Age (Categorical) | ||||||
| <40 years | 1102 (8.6%) | 69 (8.6%) | 142 (9.2%) | 457 (11.7%) | 434 (6.6%) | <0.001 |
| 40–49 years | 3481 (27.2%) | 226 (28.3%) | 499 (32.5%) | 1237 (31.6%) | 1519 (23.3%) | |
| 50–59 years | 4225 (33.1%) | 302 (37.8%) | 533 (34.7%) | 1251 (32.0%) | 2139 (32.8%) | |
| 60–69 years | 3045 (23.8%) | 176 (22.0%) | 309 (20.1%) | 779 (19.9%) | 1781 (27.3%) | |
| 70–74 years | 607 (4.7%) | 15 (1.9%) | 37 (2.4%) | 126 (3.2%) | 429 (6.6%) | |
| >74 years | 321 (2.5%) | 12 (1.5%) | 16 (1.0%) | 64 (1.6%) | 229 (3.5%) | |
| Urban/Rural Residence | ||||||
| Urban | 11,189 (87.5%) | 664 (83.0%) | 1283 (83.5%) | 3549 (90.7%) | 5693 (87.2%) | <0.001 |
| Rural | 699 (5.5%) | 45 (5.6%) | 108 (7.0%) | 149 (3.8%) | 397 (6.1%) | |
| Rural—remote | 596 (4.7%) | 62 (7.8%) | 94 (6.1%) | 119 (3.0%) | 321 (4.9%) | |
| Rural—very remote | 292–297 (2.3%) | 25–30 (3.1–3.8%) | 50–55 (3.3–3.6%) | 93–98 (2.4–2.5%) | 115–120 (1.8%) | |
| Rural—unknown | * | * | * | * | * | |
| Unknown | * | * | * | * | * | |
| Immigration Status | ||||||
| Long-term residents | 11,075 (86.7%) | 681 (85.1%) | 1373 (89.4%) | 3281 (83.8%) | 5740 (87.9%) | <0.001 |
| Immigrants | 1706 (13.3%) | 119 (14.9%) | 163 (10.6%) | 633 (16.2%) | 791 (12.1%) | |
| Immigrant Region of Origin | ||||||
| East Asia and Pacific | 544 (4.3%) | 34 (4.3%) | 51 (3.3%) | 191 (4.9%) | 268 (4.1%) | <0.001 |
| Eastern Europe and Central Asia | 286 (2.2%) | 29 (3.6%) | 43 (2.8%) | 96 (2.5%) | 118 (1.8%) | |
| Latin America and Caribbean | 239 (1.9%) | 13 (1.6%) | 16 (1.0%) | 94 (2.4%) | 116 (1.8%) | |
| Middle East and North Africa | 145 (1.1%) | 16 (2.0%) | 6 (0.4%) | 55 (1.4%) | 68 (1.0%) | |
| South Asia | 270 (2.1%) | 12 (1.5%) | 16 (1.0%) | 111 (2.8%) | 131 (2.0%) | |
| Sub-Saharan Africa | 87 (0.7%) | 3–7 (0.4–0.9%) | 6–10 (0.4–0.7%) | 44 (1.1%) | 30 (0.5%) | |
| USA/New Zealand/Australia | 37 (0.3%) | * | 5–9 (0.3–0.6%) | 14 (0.4%) | 12 (0.2%) | |
| Western Europe | 98 (0.8%) | 6 (0.8%) | 16 (1.0%) | 28 (0.7%) | 48 (0.7%) | |
| Neighbourhood Income Quintile | ||||||
| 1 (lowest) | 2020 (15.8%) | 150 (18.8%) | 227 (14.8%) | 597 (15.3%) | 1046 (16.0%) | <0.001 |
| 2 | 2384 (18.7%) | 191 (23.9%) | 276 (18.0%) | 696 (17.8%) | 1221 (18.7%) | |
| 3 | 2523 (19.7%) | 140–144 (17.5–18.0%) | 274–278 (17.8–18.1%) | 807 (20.6%) | 1298 (19.9%) | |
| 4 | 2819 (22.1%) | 153 (19.1%) | 351 (22.9%) | 873 (22.3%) | 1442 (22.1%) | |
| 5 (highest) | 2994 (23.4%) | 160 (20.0%) | 401 (26.1%) | 928 (23.7%) | 1505 (23.0%) | |
| Unknown | 41 (0.3%) | * | * | 13 (0.3%) | 19 (0.3%) | |
| Comorbidity Burden | ||||||
| 0–5 ADGs | 7287 (57.0%) | 788 (98.5%) | 1472 (95.8%) | 1773 (45.3%) | 3254 (49.8%) | <0.001 |
| 6–9 ADGs | 4425 (34.6%) | 10–14 (1.3–1.8%) | 55–59 (3.6–3.8%) | 1661 (42.4%) | 2695 (41.3%) | |
| 10+ ADGs | 1069 (8.4%) | * | * | 480 (12.3%) | 582 (8.9%) | |
| History of Mental Health Visits | ||||||
| Yes | 4127 (32.3%) | 18 (2.3%) | 149 (9.7%) | 1486 (38.0%) | 2474 (37.9%) | <0.001 |
| Cancer Detection Method | ||||||
| Screening | 2916 (22.8%) | 164 (20.5%) | 328 (21.4%) | 776 (19.8%) | 1648 (25.2%) | <0.001 |
| Symptomatic | 9865 (77.2%) | 636 (79.5%) | 1208 (78.6%) | 3138 (80.2%) | 4883 (74.8%) | |
| Stage | ||||||
| Stage I | 2839 (22.2%) | 140 (17.5%) | 328 (21.4%) | 886 (22.6%) | 1485 (22.7%) | 0.017 |
| Stage II | 7311 (57.2%) | 470 (58.8%) | 889 (57.9%) | 2251 (57.5%) | 3701 (56.7%) | |
| Stage III | 2631 (20.6%) | 190 (23.8%) | 319 (20.8%) | 777 (19.9%) | 1345 (20.6%) | |
| Primary Care Practice Model | ||||||
| Straight FFS | 1887 (14.8%) | 301 (37.6%) | 277 (18.0%) | 542 (13.8%) | 767 (11.7%) | <0.001 |
| Enhanced FFS | 6281 (49.1%) | 228 (28.5%) | 553 (36.0%) | 2036 (52.0%) | 3464 (53.0%) | |
| Capitation | 2235 (17.5%) | 110 (13.8%) | 303 (19.7%) | 654 (16.7%) | 1168 (17.9%) | |
| Team-based capitation | 2206 (17.3%) | 123 (15.4%) | 369 (24.0%) | 642 (16.4%) | 1072 (16.4%) | |
| Other | 172 (1.3%) | 38 (4.8%) | 34 (2.2%) | 40 (1.0%) | 60 (0.9%) | |
| Health Region | ||||||
| 1 Erie St. Clair | 713 (5.6%) | 47 (5.9%) | 88 (5.7%) | 221 (5.6%) | 357 (5.5%) | <0.001 |
| 2 South West | 992 (7.8%) | 55 (6.9%) | 145 (9.4%) | 242 (6.2%) | 550 (8.4%) | |
| 3 Waterloo Wellington | 654 (5.1%) | 59 (7.4%) | 125 (8.1%) | 140 (3.6%) | 330 (5.1%) | |
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 101 (12.6%) | 198 (12.9%) | 413 (10.6%) | 756 (11.6%) | |
| 5 Central West | 543 (4.2%) | 25 (3.1%) | 30 (2.0%) | 197 (5.0%) | 291 (4.5%) | |
| 6 Mississauga Halton | 750 (5.9%) | 47 (5.9%) | 67 (4.4%) | 280 (7.2%) | 356 (5.5%) | |
| 7 Toronto Central | 1061 (8.3%) | 65 (8.1%) | 121 (7.9%) | 357 (9.1%) | 518 (7.9%) | |
| 8 Central | 1784 (14.0%) | 72 (9.0%) | 152 (9.9%) | 626 (16.0%) | 934 (14.3%) | |
| 9 Central East | 1710 (13.4%) | 90 (11.3%) | 177 (11.5%) | 495 (12.6%) | 948 (14.5%) | |
| 10 South East | 520 (4.1%) | 49 (6.1%) | 81 (5.3%) | 125 (3.2%) | 265 (4.1%) | |
| 11 Champlain | 1335 (10.4%) | 108 (13.5%) | 183 (11.9%) | 444 (11.3%) | 600 (9.2%) | |
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 12–16 (1.5–2.0%) | 70–74 (4.6–4.8%) | 165–169 (4.2–4.3%) | 266–270 (4.1%) | |
| 13 North East | 478 (3.7%) | 44 (5.5%) | 64 (4.2%) | 129 (3.3%) | 241 (3.7%) | |
| 14 North West | 252 (2.0%) | 24 (3.0%) | 34 (2.2%) | 78 (2.0%) | 116 (1.8%) | |
| Unknown | * | * | * | * | * | |
| Total n = 12,781 | Contact-to-Adjuvant-Chemotherapy Interval in Days | ||||||
|---|---|---|---|---|---|---|---|
| Screened n = 2916 (22.8%) | Symptomatic n = 9865 (77.2%) | ||||||
| Median (IQR) | 90th Percentile | p-Value * | Median (IQR) | 90th Percentile | p-Value * | ||
| Total | 125 (103, 154) | 185 | 127 (99, 171) | 228 | |||
| Age (Categorical) | <0.0001 | <0.0001 | |||||
| <40 years | 1102 (8.6%) | 107 (85, 124) | 189 | 115 (90, 155) | 205 | ||
| 40–49 years | 3481 (27.2%) | 115 (93, 147) | 178 | 126 (99, 170) | 228 | ||
| 50–59 years | 4225 (33.1%) | 124 (103, 154) | 187 | 128 (101, 175) | 233 | ||
| 60–69 years | 3045 (23.8%) | 126 (105, 155) | 184 | 132 (103, 176) | 231 | ||
| 70–74 years | 607 (4.7%) | 125 (104, 158) | 185 | 138 (108, 179) | 224 | ||
| >74 years | 321 (2.5%) | 137 (118, 162) | 187 | 134 (104, 175) | 221 | ||
| Urban/Rural Residence | <0.0001 | 0.4999 | |||||
| Urban | 11,189 (87.5%) | 123 (102, 153) | 182 | 127 (99, 170) | 227 | ||
| Rural | 699 (5.5%) | 127 (110, 159) | 189 | 125 (102, 175) | 223 | ||
| Rural—remote | 596 (4.7%) | 134 (110, 164) | 194 | 127 (98, 173) | 225 | ||
| Rural—very remote | 292–297 (2.3%) | 153 (122, 184) | 231 | 132 (104, 182) | 259 | ||
| Rural—unknown | ≤5 | ** | ** | ** | ** | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
| Immigration Status | 0.1425 | 0.0008 | |||||
| Long-term residents | 11,075 (86.7%) | 125 (103, 154) | 184 | 126 (99, 170) | 227 | ||
| Immigrants | 1706 (13.3%) | 129 (104, 161) | 194 | 133 (104, 175) | 231 | ||
| Immigrant Region of Origin | 0.9288 | 0.0085 | |||||
| East Asia and Pacific | 544 (4.3%) | 135 (106, 161) | 191 | 138 (104, 175) | 231 | ||
| Eastern Europe and Central Asia | 286 (2.2%) | 135 (102, 167) | 191 | 127 (100, 173) | 230 | ||
| Latin America and Caribbean | 239 (1.9%) | 129 (116, 154) | 258 | 141 (108, 179) | 241 | ||
| Middle East and North Africa | 145 (1.1%) | 124 (104, 147) | 191 | 134 (108, 181) | 218 | ||
| South Asia | 270 (2.1%) | 126 (98, 160) | 194 | 134 (109, 169) | 217 | ||
| Sub-Saharan Africa | 87 (0.7%) | 137 (103, 155) | 163 | 139 (106, 180) | 225 | ||
| USA/New Zealand/Australia | 37 (0.3%) | 119 (103, 148) | 162 | 119 (100, 178) | 231 | ||
| Western Europe | 98 (0.8%) | 123 (105, 176) | 203 | 111 (94, 144) | 231 | ||
| Neighbourhood Income Quintile | 0.1196 | 0.1620 | |||||
| 1 (lowest) | 2020 (15.8%) | 128 (106, 160) | 188 | 130 (100, 175) | 226 | ||
| 2 | 2384 (18.7%) | 125 (104, 155) | 181 | 128 (100, 170) | 231 | ||
| 3 | 2523 (19.7%) | 125 (104, 155) | 183 | 127 (101, 174) | 225 | ||
| 4 | 2819 (22.1%) | 127 (103, 153) | 186 | 126 (99, 168) | 226 | ||
| 5 (highest) | 2994 (23.4%) | 122 (100, 151) | 184 | 125 (98, 170) | 231 | ||
| Unknown | 41 (0.3%) | 170 (119, 226) | 247 | 143 (102, 182) | 234 | ||
| Comorbidity Burden | 0.7763 | <0.0001 | |||||
| 0–5 ADGs | 7287 (57.0%) | 124 (104, 153) | 183 | 123 (98, 166) | 219 | ||
| 6–9 ADGs | 4425 (34.6%) | 126 (103, 155) | 189 | 133 (103, 178) | 238 | ||
| 10+ ADGs | 1069 (8.4%) | 126 (104, 158) | 182 | 135 (104, 183) | 245 | ||
| History of Mental Health Visits | 0.9609 | <0.0001 | |||||
| Yes | 4127 (32.3%) | 124 (102, 155) | 191 | 132 (103, 176) | 233 | ||
| No | 8654 (67.7%) | 126 (104, 154) | 183 | 125 (98, 169) | 225 | ||
| Stage | 0.0010 | <0.0001 | |||||
| Stage I | 2839 (22.2%) | 128 (105, 158) | 188 | 136 (105, 185) | 242 | ||
| Stage II | 7311 (57.2%) | 125 (103, 154) | 184 | 127 (100, 169) | 225 | ||
| Stage III | 2631 (20.6%) | 119 (100, 146) | 182 | 119 (93, 162) | 219 | ||
| Primary Care Model | 0.0373 | 0.0012 | |||||
| Straight FFS | 1887 (14.8%) | 127 (104, 152) | 182 | 126 (100, 169) | 221 | ||
| Enhanced FFS | 6281 (49.1%) | 127 (104, 159) | 190 | 128 (100, 172) | 230 | ||
| Capitation | 2235 (17.5%) | 121 (102, 153) | 180 | 127 (100, 175) | 233 | ||
| Team-based capitation | 2206 (17.3%) | 121 (101, 149) | 182 | 122 (97, 166) | 228 | ||
| Other | 172 (1.3%) | 126 (108, 157) | 190 | 117 (91, 155) | 203 | ||
| Primary Care Enrolment Status | 0.7247 | 0.6580 | |||||
| Rostered | 10,900 (85.3%) | 125 (103, 155) | 185 | 127 (99, 171) | 230 | ||
| Not rostered | 1881 (14.7%) | 127 (104, 152) | 183 | 127 (100, 169) | 221 | ||
| Health Region | <0.0001 | <0.0001 | |||||
| 1 Erie St. Clair | 713 (5.6%) | 118 (99, 142) | 179 | 120 (92, 157) | 208 | ||
| 2 South West | 992 (7.8%) | 138 (113, 167) | 200 | 133 (103, 172) | 227 | ||
| 3 Waterloo Wellington | 654 (5.1%) | 119 (98, 141) | 167 | 112 (91, 150) | 207 | ||
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 118 (100, 140) | 170 | 116 (96, 155) | 213 | ||
| 5 Central West | 543 (4.2%) | 120 (99, 150) | 182 | 126 (99, 171) | 223 | ||
| 6 Mississauga Halton | 750 (5.9%) | 120 (96, 154) | 196 | 124 (96, 173) | 234 | ||
| 7 Toronto Central | 1061 (8.3%) | 126 (106, 155) | 184 | 134 (105, 185) | 247 | ||
| 8 Central | 1784 (14.0%) | 124 (101, 154) | 188 | 128 (101, 174) | 231 | ||
| 9 Central East | 1710 (13.4%) | 114 (95, 146) | 179 | 127 (98, 171) | 220 | ||
| 10 South East | 520 (4.1%) | 126 (106, 159) | 183 | 120 (99, 157) | 217 | ||
| 11 Champlain | 1335 (10.4%) | 144 (121, 169) | 189 | 148 (120, 189) | 249 | ||
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 126 (103, 162) | 176 | 122 (102, 176) | 237 | ||
| 13 North East | 478 (3.7%) | 118 (98, 147) | 190 | 117 (88, 160) | 216 | ||
| 14 North West | 252 (2.0%) | 143 (108, 161) | 198 | 128 (92, 173) | 231 | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, R.L.; Lofters, A.; Moineddin, R.; Krzyzanowska, M.; Grunfeld, E. Primary Care Continuity and Wait Times to Receiving Breast Cancer Chemotherapy: A Population-Based Retrospective Cohort Study Using CanIMPACT Data. Curr. Oncol. 2021, 28, 4786-4804. https://doi.org/10.3390/curroncol28060405
Walsh RL, Lofters A, Moineddin R, Krzyzanowska M, Grunfeld E. Primary Care Continuity and Wait Times to Receiving Breast Cancer Chemotherapy: A Population-Based Retrospective Cohort Study Using CanIMPACT Data. Current Oncology. 2021; 28(6):4786-4804. https://doi.org/10.3390/curroncol28060405
Chicago/Turabian StyleWalsh, Rachel Lin, Aisha Lofters, Rahim Moineddin, Monika Krzyzanowska, and Eva Grunfeld. 2021. "Primary Care Continuity and Wait Times to Receiving Breast Cancer Chemotherapy: A Population-Based Retrospective Cohort Study Using CanIMPACT Data" Current Oncology 28, no. 6: 4786-4804. https://doi.org/10.3390/curroncol28060405
APA StyleWalsh, R. L., Lofters, A., Moineddin, R., Krzyzanowska, M., & Grunfeld, E. (2021). Primary Care Continuity and Wait Times to Receiving Breast Cancer Chemotherapy: A Population-Based Retrospective Cohort Study Using CanIMPACT Data. Current Oncology, 28(6), 4786-4804. https://doi.org/10.3390/curroncol28060405





