A Preliminary Study on the Relationship between Serum Heparan Sulfate and Cancer-Related Cognitive Impairment: The Moderating Role of Oxidative Stress in Patients with Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Population
2.2. Procedure
2.3. Measures
2.3.1. Demographic and Clinical Information
2.3.2. Cognitive Performance
2.3.3. Neuropsychological Assessment
2.3.4. Detection of Serum Biomarkers
2.4. Statistical Analyses
3. Results
3.1. Social Demographic Profile, Cognitive Performance, Clinical and Blood Biochemical Characteristics of Study Participants
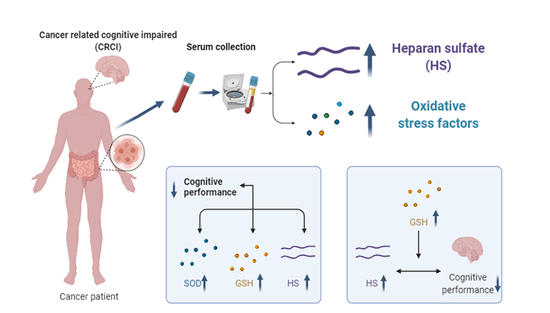
3.2. Serum Levels of HS and Oxidative Stress Factors in Participants
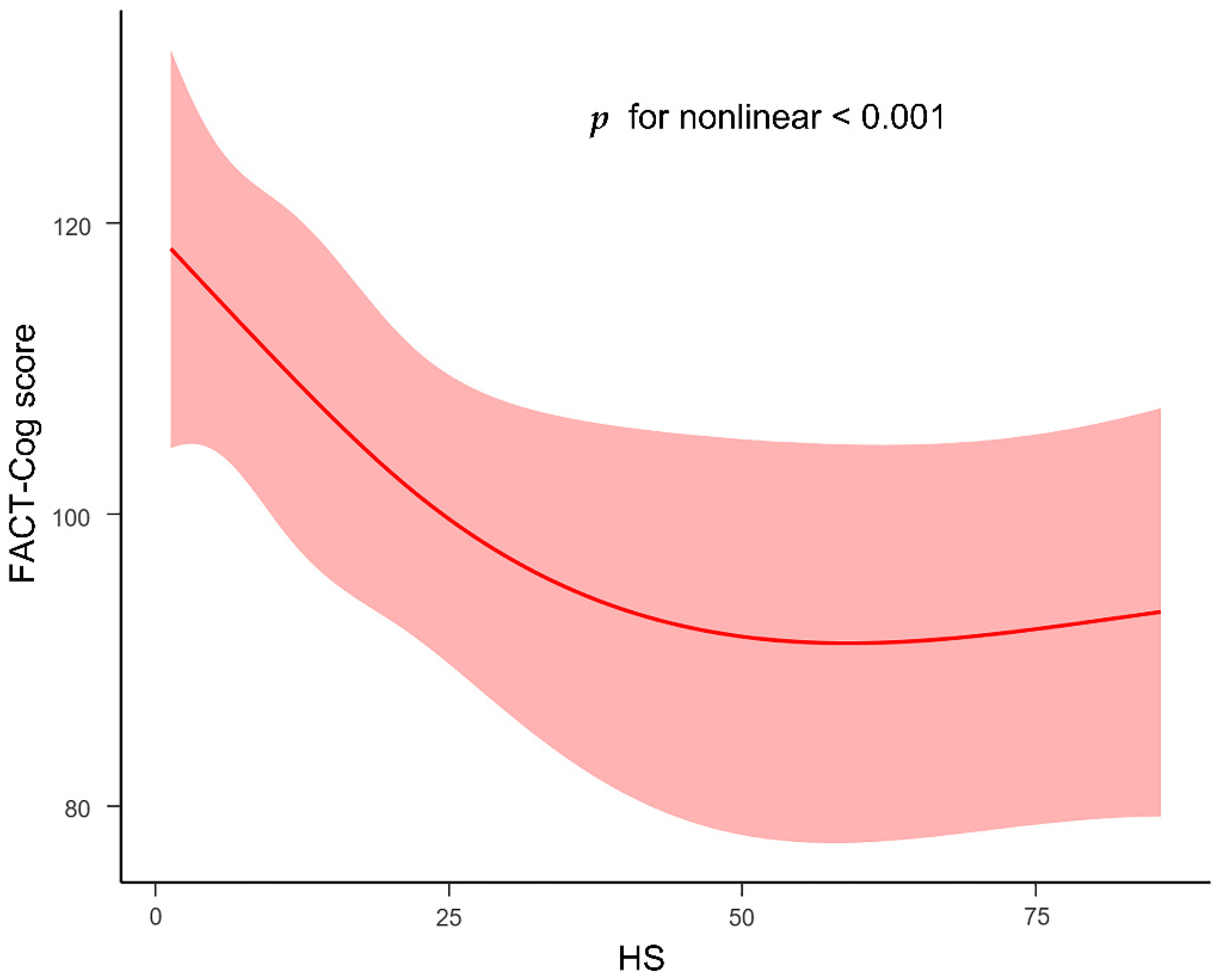
3.3. Associations among Serum HS, Oxidative Stress Factors and Cognitive Impairment
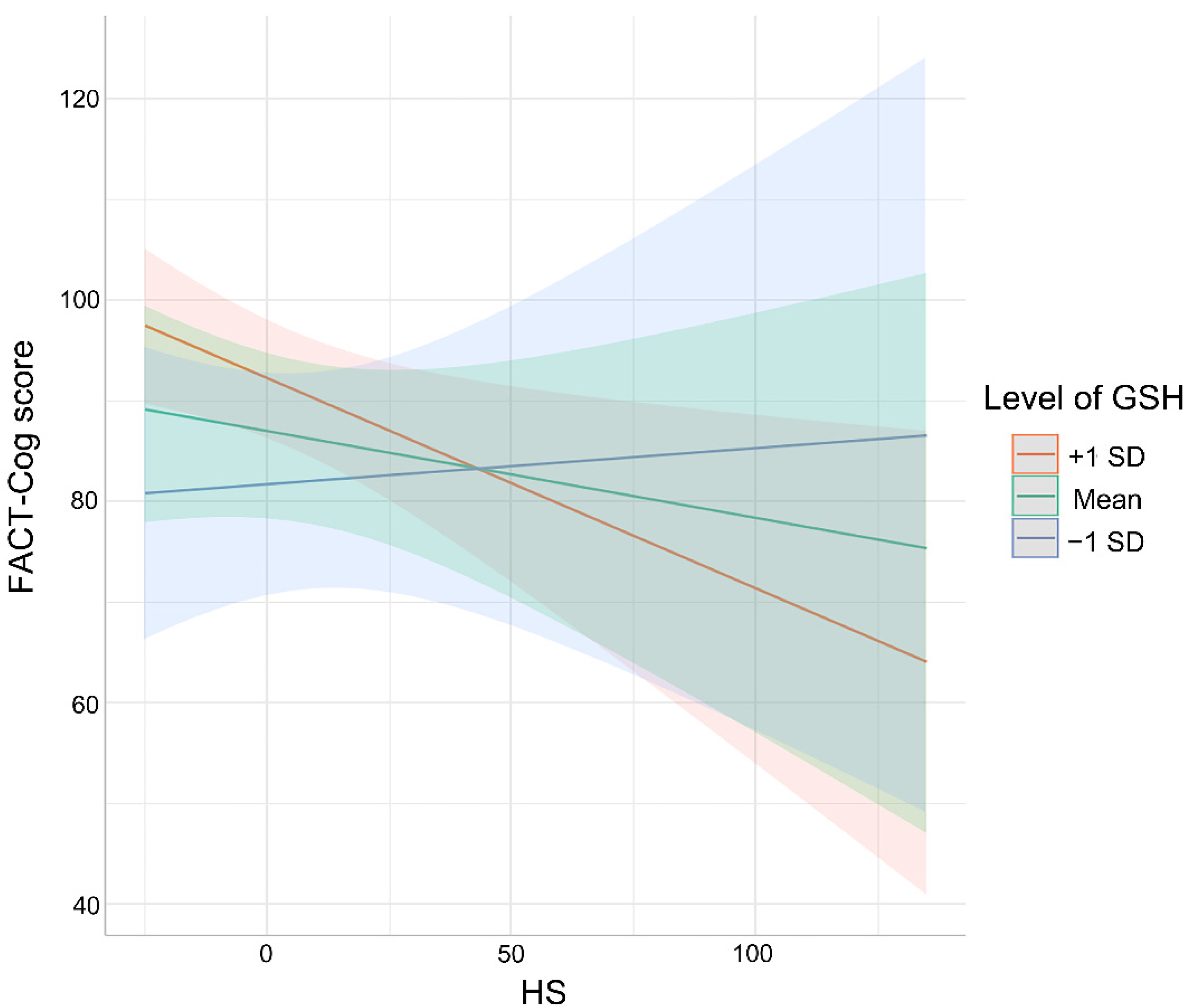
3.4. Association between Cognitive Impaired Performance and HS as Moderated by Oxidative Stress Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, E.A.; Jerzak, K.J.; Lebovic, G.; Rochon, P.A.; Elser, C.; Pritchard, K.I.; Tierney, M.C. Cognitive effects of adjuvant endocrine therapy in older women treated for early-stage breast cancer: A 1-year longitudinal study. Support. Care Cancer. 2019, 27, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Ahles, T.A.; Mohile, S.G.; Mustian, K.M.; Palesh, O.; O’Mara, A.M.; Minasian, L.M.; Williams, A.M.; et al. Longitudinal Trajectory and Characterization of Cancer-Related Cognitive Impairment in a Nationwide Cohort Study. J. Clin. Oncol. 2018, 36, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.; Dhillon, H.M.; Pond, G.R.; Rourke, S.B.; Xu, W.; Dodd, A.; Renton, C.; Park, A.; Bekele, T.; Ringash, J.; et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann. Oncol. 2014, 25, 2404–2412. [Google Scholar] [CrossRef]
- Ren, X.; Boriero, D.; Chaiswing, L.; Bondada, S.; St Clair, D.K.; Butterfield, D.A. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta. Mol. Basis. Dis. 2019, 1865, 1088–1097. [Google Scholar] [CrossRef]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Wahdan, S.; Esmat, A.; Azab, S.S. Astaxanthin Ameliorates Doxorubicin-Induced Cognitive Impairment (Chemobrain) in Experimental Rat Model: Impact on Oxidative, Inflammatory, and Apoptotic Machineries. Mol. Neurobiol. 2018, 55, 5727–5740. [Google Scholar] [CrossRef]
- Bagnall-Moreau, C.; Chaudhry, S.; Salas-Ramirez, K.; Ahles, T.; Hubbard, K. Chemotherapy-Induced Cognitive Impairment Is Associated with Increased Inflammation and Oxidative Damage in the Hippocampus. Mol. Neurobiol. 2019, 56, 7159–7172. [Google Scholar] [CrossRef]
- Williams, A.M.; Shah, R.; Shayne, M.; Huston, A.J.; Krebs, M.; Murray, N.; Thompson, B.D.; Doyle, K.; Korotkin, J.; van Wijngaarden, E.; et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol. 2018, 314, 17–23. [Google Scholar] [CrossRef]
- Ren, X.; Keeney, J.T.R.; Miriyala, S.; Noel, T.; Powell, D.K.; Chaiswing, L.; Bondada, S.; St Clair, D.K.; Butterfield, D.A. The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-α. Free Radic. Biol. Med. 2019, 134, 1–8. [Google Scholar]
- Ganz, P.A.; Bower, J.E.; Kwan, L.; Castellon, S.A.; Silverman, D.H.; Geist, C.; Breen, E.C.; Irwin, M.R.; Cole, S.W. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav. Immun. 2013, 30, S99–S108. [Google Scholar] [CrossRef] [Green Version]
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj. J. 2017, 34, 351–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatmári, T.; Mundt, F.; Kumar-Singh, A.; Möbus, L.; Ötvös, R.; Hjerpe, A.; Dobra, K. Molecular targets and signaling pathways regulated by nuclear translocation of syndecan-1. BMC Cell Biol. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, M.D.; Sanderson, R.D. Heparan sulfate in the nucleus and its control of cellular functions. Matrix. Biol. 2014, 35, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Gulberti, S.; Mao, X.; Bui, C.; Fournel-Gigleux, S. The role of heparan sulfate maturation in cancer: A focus on the 3O-sulfation and the enigmatic 3O-sulfotransferases (HS3STs). Semin. Cancer Biol. 2020, 62, 68–85. [Google Scholar] [CrossRef]
- Puangmalai, N.; Bhatt, N.; Montalbano, M.; Sengupta, U.; Gaikwad, S.; Ventura, F.; McAllen, S.; Ellsworth, A.; Garcia, S.; Kayed, R. Internalization mechanisms of brain-derived tau oligomers from patients with Alzheimer’s disease, progressive supranuclear palsy and dementia with Lewy bodies. Cell Death Dis. 2020, 11, 314. [Google Scholar] [CrossRef]
- Zhao, J.; Huvent, I.; Lippens, G.; Eliezer, D.; Zhang, A.; Li, Q.; Tessier, P.; Linhardt, R.J.; Zhang, F.; Wang, C. Glycan Determinants of Heparin-Tau Interaction. Biophys. J. 2017, 112, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Lorente-Gea, L.; García, B.; Martín, C.; Ordiales, H.; García-Suárez, O.; Piña-Batista, K.M.; Merayo-Lloves, J.; Quirós, L.M.; Fernández-Vega, I. Heparan Sulfate Proteoglycans Undergo Differential Expression Alterations in Alzheimer Disease Brains. J. Neuropathol. Exp. Neurol. 2020, 79, 474–483. [Google Scholar] [CrossRef]
- Pérez-López, N.; Martín, C.; García, B.; Solís-Hernández, M.P.; Rodríguez, D.; Alcalde, I.; Merayo, J.; Fernández-Vega, I.; Quirós, L.M. Alterations in the Expression of the Genes Responsible for the Synthesis of Heparan Sulfate in Brains With Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2021, 80, 446–456. [Google Scholar] [CrossRef]
- Clark, G.T.; Yu, Y.; Urban, C.A.; Fu, G.; Wang, C.; Zhang, F.; Linhardt, R.J.; Hurley, J.M. Circadian control of heparan sulfate levels times phagocytosis of amyloid beta aggregates. PLoS Genet. 2022, 18, e1009994. [Google Scholar] [CrossRef]
- Hippensteel, J.A.; Anderson, B.J.; Orfila, J.E.; McMurtry, S.A.; Dietz, R.M.; Su, G.; Ford, J.A.; Oshima, K.; Yang, Y.; Zhang, F.; et al. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J. Clin. Investig. 2019, 129, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.R.; Singh, I.; Azad, T.D.; Holmes, B.B.; Verghese, P.B.; Dietrich, H.H.; Diamond, M.; Bu, G.; Han, B.H.; Zipfel, G.J. Heparan sulfate proteoglycans mediate Aβ-induced oxidative stress and hypercontractility in cultured vascular smooth muscle cells. Mol. Neurodegener. 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigger, B.W.; Begley, D.J.; Virgintino, D.; Pshezhetsky, A.V. Anatomical changes and pathophysiology of the brain in mucopolysaccharidosis disorders. Mol. Genet. Metab. 2018, 125, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.R.; Jacobsen, P.B.; Booth-Jones, M.; Wagner, L.I.; Anasetti, C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J. Pain Symptom Manag. 2007, 33, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Lange, M.; Rigal, O.; Correia, H.; Giffard, B.; Beaumont, J.L.; Clisant, S.; Wagner, L. French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 3. Support. Care Cancer 2012, 20, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Lim, S.R.; Shwe, M.; Tan, Y.P.; Chan, A. Psychometric properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy-cognitive in Asian patients with breast cancer. Value Health 2013, 16, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, Y.T.; Foo, Y.L.; Shwe, M.; Tan, Y.P.; Fan, G.; Yong, W.S.; Madhukumar, P.; Ooi, W.S.; Chay, W.Y.; Dent, R.A.; et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J. Clin. Epidemiol. 2014, 67, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Xiao, F.; Gong, K.; Zhang, W.; Zhang, M.; Ruan, J.; Zhang, X.; Chen, Q.; Yu, Z. Demographically Corrected Normative Z Scores on the Neuropsychological Test Battery in Cognitively Normal Older Chinese Adults. Dement Geriatr Cogn Disord. 2020, 49, 375–383. [Google Scholar] [CrossRef]
- Wong, C.G.; Thomas, K.R.; Edmonds, E.C.; Weigand, A.J.; Bangen, K.J.; Eppig, J.S.; Jak, A.J.; Devine, S.A.; Delano-Wood, L.; Libon, D.J.; et al. Neuropsychological Criteria for Mild Cognitive Impairment in the Framingham Heart Study’s Old-Old. Dement. Geriatr. Cogn. Disord. 2018, 46, 253–265. [Google Scholar] [CrossRef]
- Ihle, A.; Zuber, S.; Gouveia, É.R.; Gouveia, B.R.; Mella, N.; Desrichard, O.; Cullati, S.; Oris, M.; Maurer, J.; Kliegel, M. Cognitive Reserve Mediates the Relation between Openness to Experience and Smaller Decline in Executive Functioning. Dement. Geriatr. Cogn. Disord. 2019, 48, 39–44. [Google Scholar] [CrossRef]
- Duff, K. Demographically corrected normative data for the Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised in an elderly sample. Appl. Neuropsychol. Adult. 2016, 23, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, H.; Kida, S.; Yoden, E.; Kinoshita, M.; Tanaka, N.; Yamamoto, R.; Koshimura, Y.; Takagi, H.; Takahashi, K.; Hirato, T.; et al. Clearance of heparan sulfate in the brain prevents neurodegeneration and neurocognitive impairment in MPS II mice. Mol. Ther. 2021, 29, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.S. How does multiple testing correction work? Nat. Biotechnol. 2009, 27, 1135–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Rourke, S.B.; Bekele, T.; Renton, C.; Dodd, A.; Zhang, H.; Beale, P.; Clarke, S.; et al. Cognitive Function in Patients With Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. J. Clin. Oncol. 2015, 33, 4085–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, H.M.; Tannock, I.F.; Pond, G.R.; Renton, C.; Rourke, S.B.; Vardy, J.L. Perceived cognitive impairment in people with colorectal cancer who do and do not receive chemotherapy. J. Cancer Surviv. 2018, 12, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Vivek, S.; Nelson, H.H.; Prizment, A.E.; Faul, J.; Crimmins, E.M.; Thyagarajan, B. Cross sectional association between cytomegalovirus seropositivity, inflammation and cognitive impairment in elderly cancer survivors. Cancer Causes Control 2022, 33, 81–90. [Google Scholar] [CrossRef]
- Chen, B.T.; Jin, T.; Patel, S.K.; Ye, N.; Sun, C.L.; Ma, H.; Rockne, R.C.; Root, J.C.; Saykin, A.J.; Ahles, T.A.; et al. Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res. Treat. 2018, 172, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Wouters, H.; Baars, J.W.; Schagen, S.B. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol. 2016, 55, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Mounier, N.M.; Abdel-Maged, A.E.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Chemotherapy-induced cognitive impairment (CICI): An overview of etiology and pathogenesis. Life Sci. 2020, 258, 118071. [Google Scholar] [CrossRef]
- Paterson, E.N.; Maxwell, A.P.; Kee, F.; Cruise, S.; Young, I.S.; McGuinness, B.; McKay, G.J. Association of renal impairment with cognitive dysfunction in the Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA). Nephrol. Dial. Transplant. 2021, 36, 1492–1499. [Google Scholar] [CrossRef]
- Stephan, Y.; Sutin, A.R.; Terracciano, A. Subjective Age and Cystatin C Among Older Adults. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2019, 74, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Cauli, O. Oxidative Stress and Cognitive Alterations Induced by Cancer Chemotherapy Drugs: A Scoping Review. Antioxidants 2021, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Q.; Zhang, L.; Zhuang, B.; Zhang, T.; Jin, S.; Sun, Y.; Xiao, S.; Zheng, B.; Fang, Y.; et al. Nutrition Impact Symptom Clusters in Patients With Head and Neck Cancer Receiving Concurrent Chemoradiotherapy. J. Pain Symptom Manage. 2021, 62, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.V.; de Barros, M.P.; Bachi, A.L.L.; Bernardi, M.M.; Kirsten, T.B.; de Fátima Monteiro Martins, M.; Rocha, P.R.D.; da Silva Rodrigues, P.; Bondan, E.F. Chemobrain in rats: Behavioral, morphological, oxidative and inflammatory effects of doxorubicin administration. Behav. Brain. Res. 2020, 378, 112233. [Google Scholar] [CrossRef]
- Sun, D.; Sun, X.; Xu, Y.; Wu, T.; Tao, L. Superoxide dismutase activity and risk of cognitive decline in older adults: Findings from the Chinese Longitudinal Healthy Longevity Survey. Exp. Gerontol. 2019, 118, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Wang, D.M.; Du, X.D.; Jia, Q.F.; Chen, D.C.; Xiu, M.; Wang, L.; Zhang, X. Elevated activity of plasma superoxide dismutase in never-treated first-episode schizophrenia patients: Associated with depressive symptoms. Schizophr. Res. 2020, 222, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Noshita, N.; Lewén, A.; Gasche, Y.; Ferrand-Drake, M.; Fujimura, M.; Morita-Fujimura, Y.; Chan, P.H. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J. Neurosci. 2002, 22, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Gibson, E.M.; Monje, M. Treating cancer therapy-related cognitive impairment. Nat. Med. 2020, 26, 1174–1175. [Google Scholar] [CrossRef]
- Zhang, X.; O’Callaghan, P.; Li, H.; Tan, Y.; Zhang, G.; Barash, U.; Wang, X.; Lannfelt, L.; Vlodavsky, I.; Lindahl, U.; et al. Heparanase overexpression impedes perivascular clearance of amyloid-β from murine brain: Relevance to Alzheimer’s disease. Acta. Neuropathol. Commun. 2021, 9, 84. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Minami, K.; Yamamoto, T.; Sonoda, H.; Yamaoka, M.; Tachibana, K.; Hirato, T.; Sato, Y. Iduronate-2-Sulfatase with Anti-human Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: A Phase 1/2 Trial. Mol. Ther. 2019, 27, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, R.J.; Chen, M.; Liu, X.Y.; Ma, K.; Xu, H.Y.; Deng, W.S.; Ye, Y.C.; Li, W.X.; Chen, X.Y.; et al. Collagen/heparan sulfate porous scaffolds loaded with neural stem cells improve neurological function in a rat model of traumatic brain injury. Neural. Regen. Res. 2021, 16, 1068–1077. [Google Scholar] [PubMed]

| Variables | Total (n = 67) | Cognitive Impaired Group (n = 32) | Cognitive Normal Group (n = 35) | p | Adjusted p |
|---|---|---|---|---|---|
| Age, years | 60 (54, 66.5) | 62.5 (57, 69) | 57 (53, 66) | 0.08 | 0.252 |
| BMI, kg/m2 | 22.06 (20.36, 25.08) | 22.65 (20.97, 25.11) | 21.85 (20.34, 24.69) | 0.38 | 0.544 |
| Gender, n (%) | 0.76 | 0.824 | |||
| Male | 40 (60) | 18 (56) | 22 (63) | ||
| Female | 27 (40) | 14 (44) | 13 (37) | ||
| Education, n (%) years | 0.48 | 0.615 | |||
| 6 | 17 (25) | 10 (31) | 7 (20) | ||
| 9 | 22 (33) | 11 (34) | 11 (31) | ||
| 12 | 17 (25) | 5 (16) | 12 (34) | ||
| 15 | 6 (9) | 3 (9) | 3 (9) | ||
| 16 | 5 (7) | 3 (9) | 2 (6) | ||
| Cancer staging, n (%) | 0.23 | 0.482 | |||
| 1 | 2 (3) | 1 (3) | 1 (3) | ||
| 2 | 9 (13) | 7 (22) | 2 (6) | ||
| 3 | 32(47.8) | 13 (41) | 19(54) | ||
| 4 | 24 (36) | 11 (34) | 13 (37) | ||
| HVLT-R | 0.69 ± 1.18 | 0.52 ± 1.31 | 0.86 ± 1.04 | 0.25 | 0.482 |
| TMT A | −0.62 (−1.17, 0.12) | −0.4 (−1.19, 0.88) | −0.64 (−1.14, −0.01) | 0.37 | 0.544 |
| WAIS-III | −1.13 (−1.59, −0.12) | −1.16 (−1.59, −0.63) | −0.48 (−1.59, −0.02) | 0.38 | 0.544 |
| FACT-Cog | 102 (77, 115.5) | 74 (69, 90.25) | 114 (104, 127.5) | <0.001 | <0.001 |
| Variables | Total (n = 67) | Cognitive Impaired Group (n = 32) | Cognitive Normal Group (n = 35) | p | Adjusted p |
|---|---|---|---|---|---|
| TC, mmol/L | 4.24 ± 0.65 | 4.50 ± 0.64 | 4.00 ± 0.57 | <0.01 | <0.01 |
| TG, mmol/L | 1.76 (1.39, 2.51) | 1.98 (1.7, 2.26) | 1.65 (1.18, 8.61) | 0.47 | 0.616 |
| CRP, mg/L | 1.70 (1.45, 2.83) | 2.85 (2.61, 3.28) | 1.48 (1.38, 1.53) | <0.01 | <0.001 |
| CA125, U/mL | 13.16 (7.64, 28.3) | 17.48 (9.18, 56.61) | 10.67 (6.85, 15.25) | <0.01 | 0.036 |
| CA199, U/mL | 11.82 (8.11, 24.45) | 12.07 (7.84, 83.09) | 11.77 (8.34, 18.7) | 0.593 | 0.696 |
| TBIL, μmol/L | 9.30 (7.65, 13.17) | 10.25 (7.65, 13.47) | 8.99 (7.85, 12) | 0.7 | 0.789 |
| ALT, U/L | 18.39 (15.5, 29.5) | 19.27 (12.5, 30) | 18.35 (16.12, 25) | 0.92 | 0.482 |
| AST, U/L | 21.00 (18.55, 29) | 24.00 (18.75, 34) | 20.00 (18.55, 23.56) | 0.2 | 0.961 |
| Albumin, g/L | 41.67 ± 4.02 | 42.20 ± 4.77 | 41.18 ± 3.18 | 0.31 | 0.544 |
| BUN, mmol/L | 4.88 (4.31, 5.67) | 4.69 (3.78, 5.48) | 5.00 (4.52, 5.87) | 0.23 | 0.482 |
| Cr, μmol/L | 69.76 ± 15.25 | 69.70 ± 13.66 | 69.82 ± 16.77 | 0.97 | 0.97 |
| CysC, mg/L | 0.98 ± 0.22 | 1.04 ± 0.22 | 0.92 ± 0.21 | 0.03 | 0.101 |
| HCY, μmol/L | 12.16 (11.82, 12.72) | 12.01 (11.5, 12.81) | 12.23 (11.94, 12.64) | 0.33 | 0.544 |
| Variables | Total (n = 67) | Cognitive Impaired Group (n = 32) | Cognitive Normal Group (n = 35) | p | Adjusted p |
|---|---|---|---|---|---|
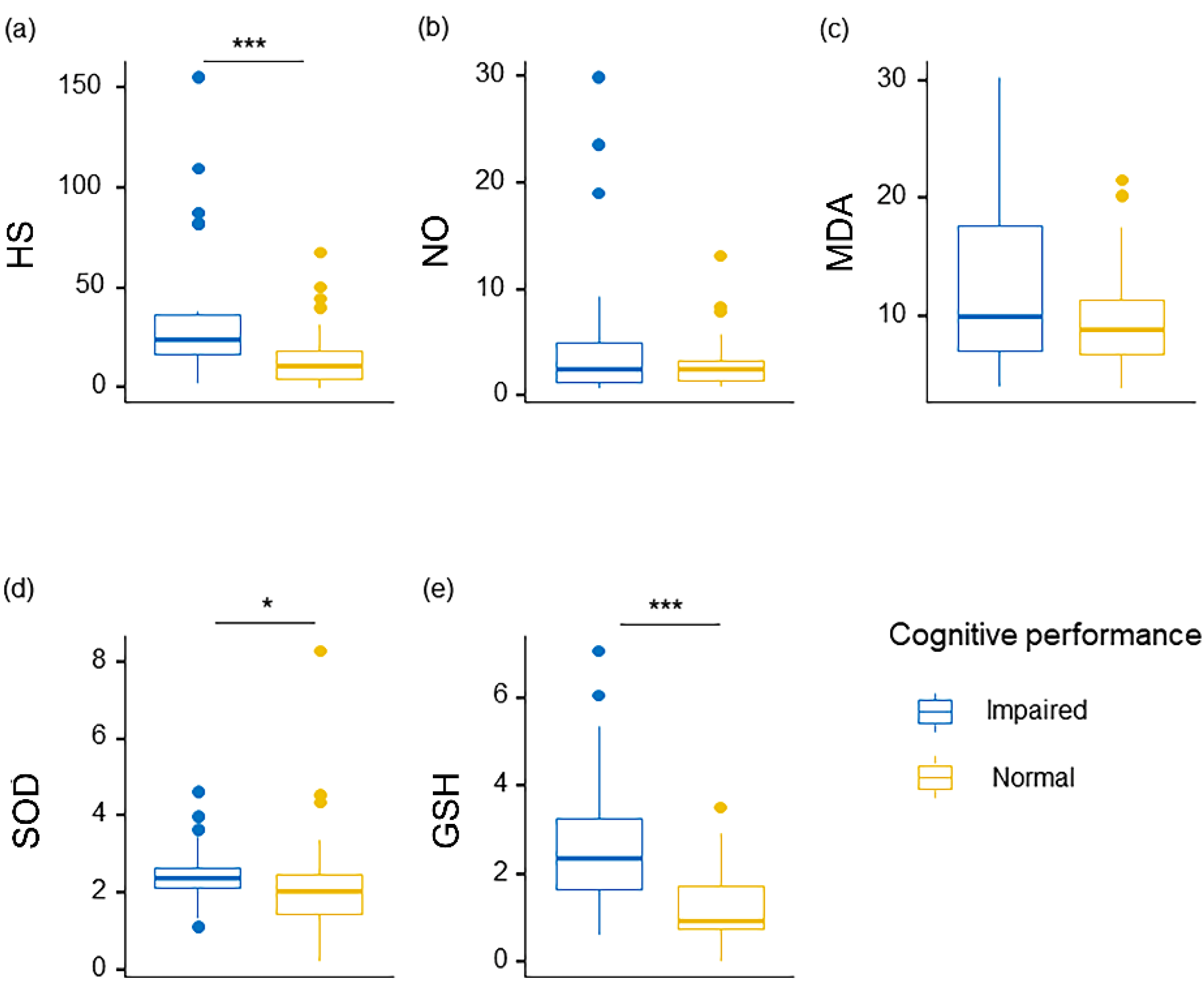
| HS, μg/mL | 17.31 (5.39, 31.43) | 24.50 (16.48, 36.37) | 10.93 (4.26, 18.17) | <0.001 | <0.001 |
| GSH, μmol/L | 1.63 (0.96, 2.85) | 2.35 (1.67, 3.25) | 0.96 (0.76, 1.74) | <0.001 | <0.001 |
| SOD, U/mL | 2.32 (1.71, 2.61) | 2.37 (2.12, 2.66) | 2.07 (1.43, 2.46) | 0.026 | 0.100 |
| MDA, μmol/L | 9.42 (6.92, 12.73) | 10.01 (7.06, 17.63) | 8.84 (6.81, 11.35) | 0.231 | 0.482 |
| NO, μmol/L | 2.54 (1.33, 4.25) | 2.46 (1.27, 4.93) | 2.56 (1.43, 3.21) | 0.589 | 0.696 |
| Point Estimate | CI | Std. Error | p | |
|---|---|---|---|---|
| Crude | −0.28298 | −0.4666478 −0.09931496 | 0.09196 | 0.00306 |
| Model 1 | −0.26614 | −0.4506025 −0.08168605 | 0.09228 | 0.00539 |
| Model 2 | −0.23739 | −0.4186311 −0.05614362 | 0.09061 | 0.0111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, T.; Zhu, M.; Sun, J.; Zhou, Z.; Chen, J.; Teng, L. A Preliminary Study on the Relationship between Serum Heparan Sulfate and Cancer-Related Cognitive Impairment: The Moderating Role of Oxidative Stress in Patients with Colorectal Cancer. Curr. Oncol. 2022, 29, 2681-2694. https://doi.org/10.3390/curroncol29040219
Wang D, Wang T, Zhu M, Sun J, Zhou Z, Chen J, Teng L. A Preliminary Study on the Relationship between Serum Heparan Sulfate and Cancer-Related Cognitive Impairment: The Moderating Role of Oxidative Stress in Patients with Colorectal Cancer. Current Oncology. 2022; 29(4):2681-2694. https://doi.org/10.3390/curroncol29040219
Chicago/Turabian StyleWang, Danhui, Teng Wang, Min Zhu, Jun Sun, Zhou Zhou, Jinghua Chen, and Liping Teng. 2022. "A Preliminary Study on the Relationship between Serum Heparan Sulfate and Cancer-Related Cognitive Impairment: The Moderating Role of Oxidative Stress in Patients with Colorectal Cancer" Current Oncology 29, no. 4: 2681-2694. https://doi.org/10.3390/curroncol29040219
APA StyleWang, D., Wang, T., Zhu, M., Sun, J., Zhou, Z., Chen, J., & Teng, L. (2022). A Preliminary Study on the Relationship between Serum Heparan Sulfate and Cancer-Related Cognitive Impairment: The Moderating Role of Oxidative Stress in Patients with Colorectal Cancer. Current Oncology, 29(4), 2681-2694. https://doi.org/10.3390/curroncol29040219