Serology-Based Model for Personalized Epithelial Ovarian Cancer Risk Evaluation
Abstract
:1. Introduction
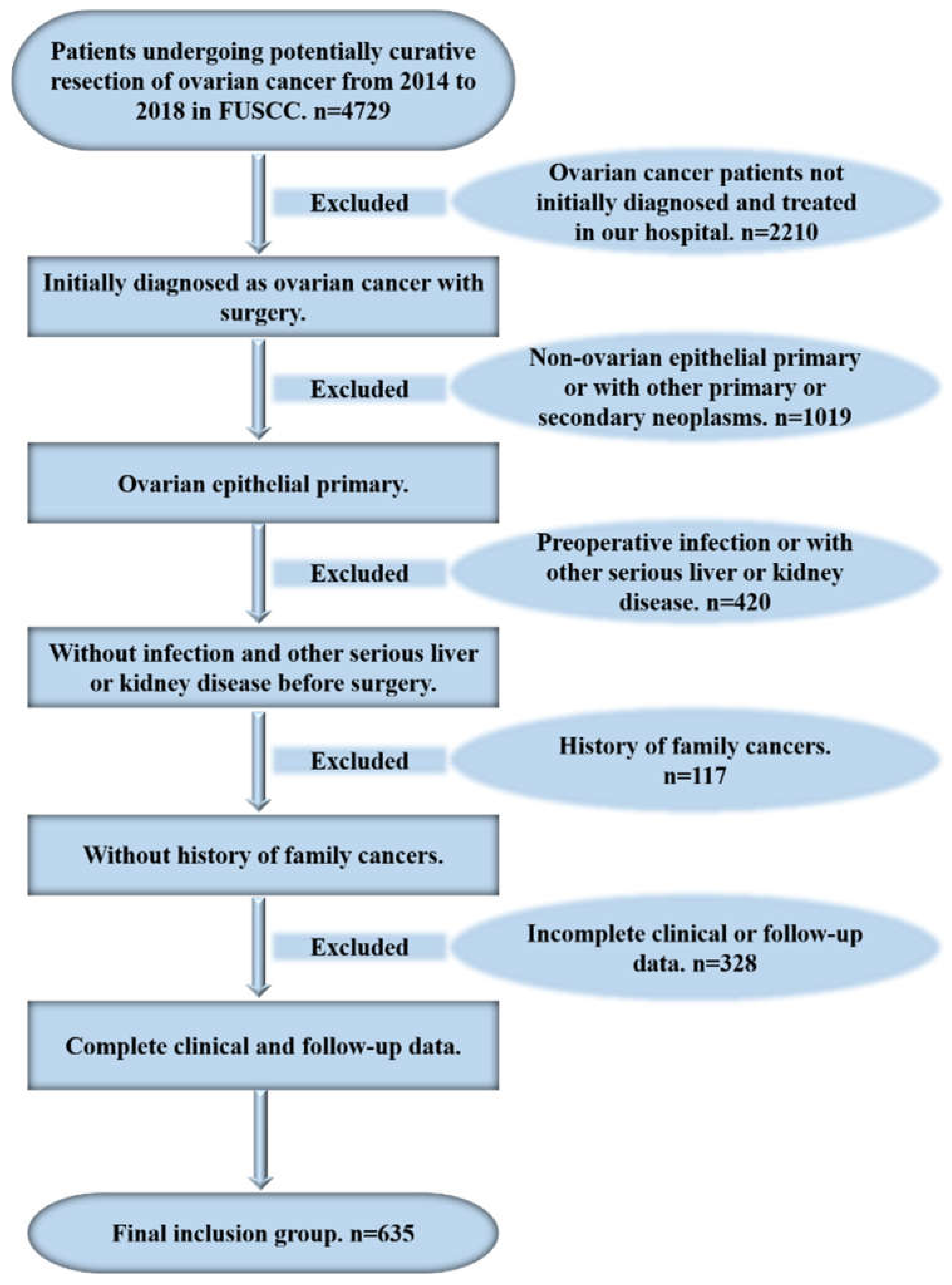
2. Patients and Methods
2.1. Patients
2.2. Data Collection
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Demographic Parameters
3.2. Clinical and Pathological Parameters Based on the RMS before Surgery
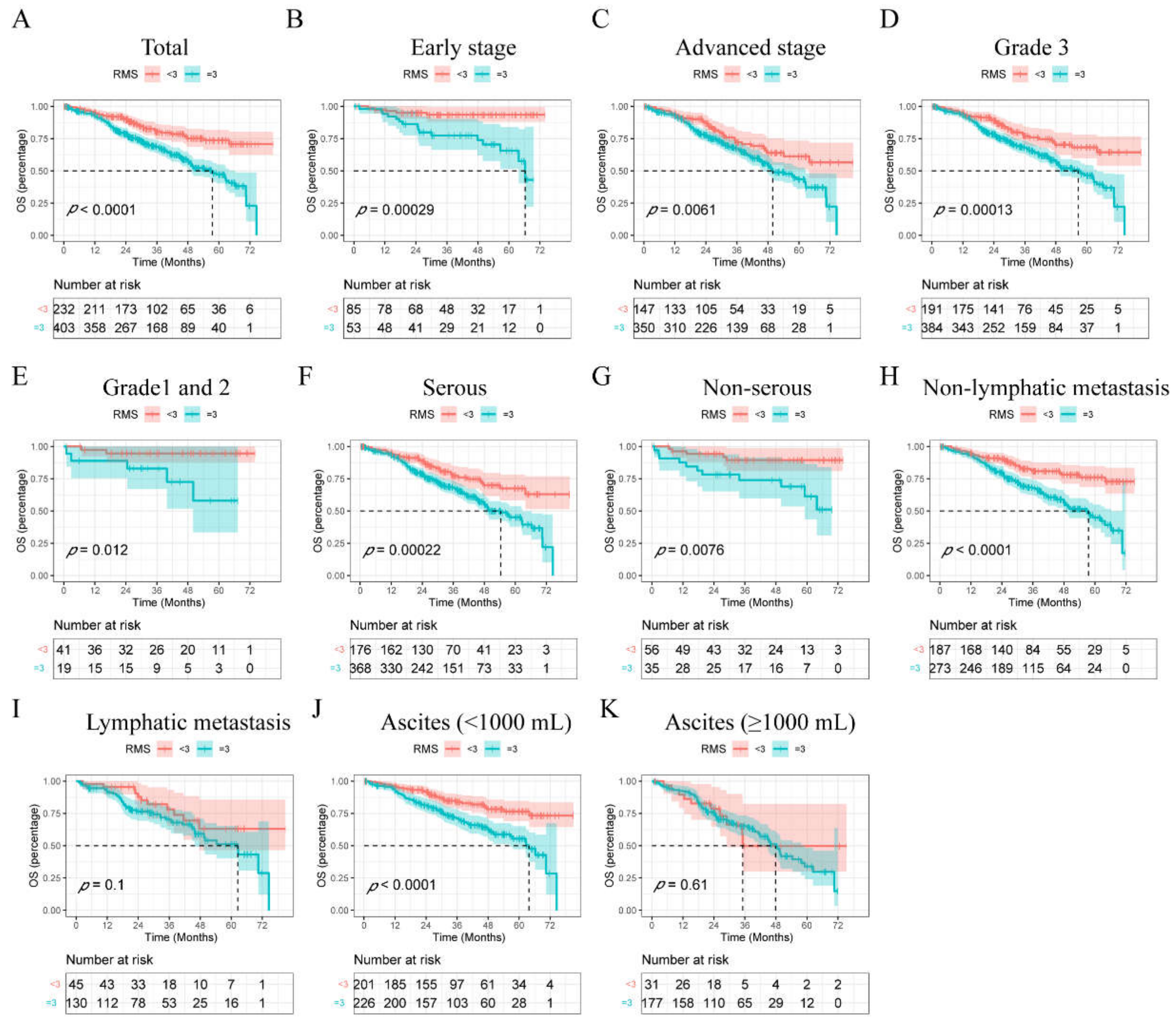
3.3. Survival Rates Postoperatively Based on the RMS
3.4. Univariable and Multivariable Cox Regression Analyses of Parameters Related to OS in EOC Patients Undergoing Curative Resection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih Ie, M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, Z.; Menon, U. Ovarian cancer screening: Current status and future directions. Best Pr. Res. Clin Obs. Gynaecol. 2020, 65, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 59–78. [Google Scholar] [CrossRef] [PubMed]
- Muraji, M.; Sudo, T.; Iwasaki, S.; Ueno, S.; Wakahashi, S.; Yamaguchi, S.; Fujiwara, K.; Nishimura, R. Histopathology predicts clinical outcome in advanced epithelial ovarian cancer patients treated with neoadjuvant chemotherapy and debulking surgery. Gynecol. Oncol. 2013, 131, 531–534. [Google Scholar] [CrossRef]
- Pectasides, D.; Fountzilas, G.; Aravantinos, G.; Bamias, A.; Kalofonos, H.P.; Skarlos, D.; Briasoulis, E.; Konstantara, A.; Economopoulos, T.; Dimopoulos, M.A. Epithelial ovarian carcinoma in younger vs older women: Is age an independent prognostic factor? The Hellenic Oncology Cooperative Group experience. Int. J. Gynecol. Cancer 2007, 17, 1003–1010. [Google Scholar] [CrossRef]
- Chang, S.J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Millstein, J.; Budden, T.; Goode, E.L.; Anglesio, M.S.; Talhouk, A.; Intermaggio, M.P.; Leong, H.S.; Chen, S.; Elatre, W.; Gilks, B.; et al. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1240–1250. [Google Scholar] [CrossRef]
- Fang, F.; Cardenas, H.; Huang, H.; Jiang, G.; Perkins, S.M.; Zhang, C.; Keer, H.N.; Liu, Y.; Nephew, K.P.; Matei, D. Genomic and Epigenomic Signatures in Ovarian Cancer Associated with Resensitization to Platinum Drugs. Cancer Res. 2018, 78, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Hu, Y.; Sun, S.; Chen, L.; Schnaubelt, M.; Clark, D.; Ao, M.; Zhang, Z.; Chan, D.; Qian, J.; et al. Glycoproteomics-based signatures for tumor subtyping and clinical outcome prediction of high-grade serous ovarian cancer. Nat. Commun. 2020, 11, 6139. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, X.; Wu, X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: A potential role in the coagulation cascade, diagnosis and prognosis. Int. J. Oncol. 2019, 54, 1719–1733. [Google Scholar] [CrossRef] [Green Version]
- Salminen, L.; Braicu, E.I.; Lääperi, M.; Jylhä, A.; Oksa, S.; Hietanen, S.; Sehouli, J.; Kulbe, H.; Bois, A.D.; Mahner, S.; et al. A Novel Two-Lipid Signature Is a Strong and Independent Prognostic Factor in Ovarian Cancer. Cancers 2021, 13, 1764. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Gentry-Maharaj, A.; Burnell, M.; Dilley, J.; Ryan, A.; Karpinskyj, C.; Gunu, R.; Mallett, S.; Deeks, J.; Campbell, S.; Jacobs, I.; et al. Serum HE4 and diagnosis of ovarian cancer in postmenopausal women with adnexal masses. Am. J. Obstet Gynecol. 2020, 222, 56.e1–56.e17. [Google Scholar] [CrossRef] [Green Version]
- Braicu, E.I.; Fotopoulou, C.; Van Gorp, T.; Richter, R.; Chekerov, R.; Hall, C.; Butz, H.; Castillo-Tong, D.C.; Mahner, S.; Zeillinger, R.; et al. Preoperative HE4 expression in plasma predicts surgical outcome in primary ovarian cancer patients: Results from the OVCAD study. Gynecol. Oncol. 2013, 128, 245–251. [Google Scholar] [CrossRef]
- Nakamoto, S.; Ikeda, M.; Kubo, S.; Yamamoto, M.; Yamashita, T.; Notsu, A. Systemic immunity markers associated with lymphocytes predict the survival benefit from paclitaxel plus bevacizumab in HER2 negative advanced breast cancer. Sci. Rep. 2021, 11, 6328. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. Preoperative Neutrophil-to-Lymphocyte Ratio as a Predictive and Prognostic Factor for High-Grade Serous Ovarian Cancer. PLoS ONE 2016, 11, e0156101. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, T.; Ouyang, L.; Li, X.; He, W.; Zhang, Z.; Shen, H.; You, Z.; Yang, G.; Lai, H. A novel diagnostic nomogram based on serological and ultrasound findings for preoperative prediction of malignancy in patients with ovarian masses. Gynecol. Oncol. 2021, 160, 704–712. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, S.; Shan, B.; Zhou, S.; Wu, X.; Yang, H.; Ye, S. Serum D-dimer, albumin and systemic inflammatory response markers in ovarian clear cell carcinoma and their prognostic implications. J. Ovarian Res. 2020, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Araki, K.; Harimoto, N.; Kubo, N.; Igarashi, T.; Ishii, N.; Yamanaka, T.; Hagiwara, K.; Kuwano, H.; Shirabe, K. D-dimer predicts postoperative recurrence and prognosis in patients with liver metastasis of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, Z.; Qiu, Y.; Zhang, J.; Wu, H.; Liang, R.; Chen, G.; Qin, G.; Li, Y.; Zou, D. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 1494–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ge, H.; Miao, Z.; Shao, S.; Shi, H.; Dong, C. Dynamic Changes in the Systemic Inflammation Response Index Predict the Outcome of Resectable Gastric Cancer Patients. Front. Oncol. 2021, 11, 577043. [Google Scholar] [CrossRef]
- Hu, W.; Yu, J.; Huang, Y.; Hu, F.; Zhang, X.; Wang, Y. Lymphocyte-Related Inflammation and Immune-Based Scores Predict Prognosis of Chordoma Patients After Radical Resection. Transl. Oncol. 2018, 11, 444–449. [Google Scholar] [CrossRef]
- Xu, M.; Wu, Q.; Cai, L.; Sun, X.; Xie, X.; Sun, P. Systemic Inflammatory Score predicts Overall Survival in patients with Cervical Cancer. J. Cancer 2021, 12, 3671–3677. [Google Scholar] [CrossRef]
- Michels, N.; van Aart, C.; Morisse, J.; Mullee, A.; Huybrechts, I. Chronic inflammation towards cancer incidence: A systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol./Hematol. 2021, 157, 103177. [Google Scholar] [CrossRef]
- Furrer, D.; Gregoire, J.; Turcotte, S.; Plante, M.; Bachvarov, D.; Trudel, D.; Tetu, B.; Douville, P.; Bairati, I. Performance of preoperative plasma tumor markers HE4 and CA125 in predicting ovarian cancer mortality in women with epithelial ovarian cancer. PLoS ONE 2019, 14, e0218621. [Google Scholar] [CrossRef]
- Starzer, A.M.; Steindl, A.; Mair, M.J.; Deischinger, C.; Simonovska, A.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Heller, G.; Preusser, M.; et al. Systemic inflammation scores correlate with survival prognosis in patients with newly diagnosed brain metastases. Br. J. Cancer 2021, 124, 1294–1300. [Google Scholar] [CrossRef]
- Polterauer, S.; Grimm, C.; Seebacher, V.; Concin, N.; Marth, C.; Tomovski, C.; Husslein, H.; Leipold, H.; Hefler-Frischmuth, K.; Tempfer, C.; et al. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: A multicenter study. Oncologist 2009, 14, 979–985. [Google Scholar] [CrossRef]
- Ethier, J.L.; Desautels, D.N.; Templeton, A.J.; Oza, A.; Amir, E.; Lheureux, S. Is the neutrophil-to-lymphocyte ratio prognostic of survival outcomes in gynecologic cancers? A systematic review and meta-analysis. Gynecol. Oncol. 2017, 145, 584–594. [Google Scholar] [CrossRef]
- Badora-Rybicka, A.; Nowara, E.; Starzyczny-Słota, D. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio before chemotherapy as potential prognostic factors in patients with newly diagnosed epithelial ovarian cancer. ESMO Open 2016, 1, e000039. [Google Scholar] [CrossRef]
- Yu, W.; Ye, Z.; Fang, X.; Jiang, X.; Jiang, Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J. Ovarian Res. 2019, 12, 88. [Google Scholar] [CrossRef]
- Calura, E.; Paracchini, L.; Fruscio, R.; DiFeo, A.; Ravaggi, A.; Peronne, J.; Martini, P.; Sales, G.; Beltrame, L.; Bignotti, E.; et al. A prognostic regulatory pathway in stage I epithelial ovarian cancer: New hints for the poor prognosis assessment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1511–1519. [Google Scholar] [CrossRef]

| Variables | Number of Patients |
|---|---|
| Age (years, median, IQR) | 54 (48.00–62.00) |
| BMI (kg/m2, median, IQR) | 23 (20.70–24.50) |
| Menopause (%) | |
| No | 225 (35.40) |
| Yes | 410 (64.60) |
| FIGO stage (%) | |
| Early | 138 (21.70) |
| Advanced | 497 (78.30) |
| Grade (%) | |
| G3 | 575 (90.60) |
| G1/G2 | 60 (9.40) |
| Histology (%) | |
| Serous | 544 (85.70) |
| Non-serous | 91 (14.30) |
| Lymphatic metastasis (%) | |
| No | 460 (72.40) |
| Yes | 175 (27.60) |
| Ascites (mL, %) | |
| <1000 | 427 (67.20) |
| ≥1000 | 208 (32.80) |
| CA125 (U/mL, median, IQR) | 521.00 (161.50–1478.00) |
| HE4 (pmol/L, median, IQR) | 285.80 (133.40–683.98) |
| NLR (median, IQR) | 2.90 (2.00–4.15) |
| PLR (median, IQR) | 193.50 (137.06–278.89) |
| MLR (median, IQR) | 0.27 (0.20–0.38) |
| FAR (median, IQR) | 0.09 (0.07–0.12) |
| D-dimer (μg/mL, median, IQR) | 3.16 (1.02–6.92) |
| Follow-up time (months, median, IQR) | 34.90 (24.83–50.53) |
| OS time (months, median, IQR) | 31.40 (21.30–47.63) |
| Variables | Risk Model Score (RMS) | ||
|---|---|---|---|
| <3 (n = 232) | ≥3 (n = 403) | p Value | |
| Age (years) | |||
| <50 | 80(34.5) | 124(30.8) | |
| ≥50 | 152(65.5) | 279(69.2) | 0.335 |
| BMI (kg/m2) | |||
| <23 | 117(50.4) | 182(45.2) | |
| ≥23 | 115(49.6) | 221(54.8) | 0.200 |
| Menopause | |||
| No | 85(36.6) | 140(34.7) | |
| Yes | 147(63.4) | 263(65.3) | 0.630 |
| FIGO stage | |||
| Early | 85(36.6) | 53(13.2) | |
| Advanced | 147(63.4) | 350(86.8) | <0.0001 |
| Grade | |||
| G3 | 191(82.3) | 384(95.3) | |
| G1/G2 | 41(17.7) | 19(4.7) | <0.0001 |
| Histology | |||
| Serous | 176(75.9) | 368(91.3) | |
| Non-serous | 56(24.1) | 35(9.7) | <0.0001 |
| Lymphatic metastasis | |||
| No | 187(80.6) | 273(67.7) | |
| Yes | 45(19.4) | 130(32.3) | <0.0001 |
| Ascites (mL) | |||
| <1000 | 201(86.6) | 226(56.1) | |
| ≥1000 | 31(13.4) | 177(43.9) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Ma, X.; Hu, H.; Gong, Z.; Zheng, H.; Xie, S.; Guo, L.; Lu, R. Serology-Based Model for Personalized Epithelial Ovarian Cancer Risk Evaluation. Curr. Oncol. 2022, 29, 2695-2705. https://doi.org/10.3390/curroncol29040220
Yan T, Ma X, Hu H, Gong Z, Zheng H, Xie S, Guo L, Lu R. Serology-Based Model for Personalized Epithelial Ovarian Cancer Risk Evaluation. Current Oncology. 2022; 29(4):2695-2705. https://doi.org/10.3390/curroncol29040220
Chicago/Turabian StyleYan, Tianqing, Xiaolu Ma, Haoyun Hu, Zhiyun Gong, Hui Zheng, Suhong Xie, Lin Guo, and Renquan Lu. 2022. "Serology-Based Model for Personalized Epithelial Ovarian Cancer Risk Evaluation" Current Oncology 29, no. 4: 2695-2705. https://doi.org/10.3390/curroncol29040220





