Patient-Reported Experiences of Breast Cancer Screening, Diagnosis, and Treatment Delay, and Telemedicine Adoption during COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Development and Distribution
2.2. Study Measures
2.2.1. Personal History of Breast Cancer
2.2.2. Sociodemographic Characteristics
2.2.3. Delays in Care
2.2.4. Telemedicine Use
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Impact of COVID-19 on Cancer Screening, Care, and Telemedicine Use
3.3. Factors Associated with COVID-19-Related Delays in Care and Telemedicine Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef]
- Indini, A.; Aschele, C.; Cavanna, L.; Clerico, M.; Daniele, B.; Fiorentini, G.; Fioretto, L.; Giordano, M.; Montesarchio, V.; Ortega, C.; et al. Reorganisation of medical oncology departments during the novel coronavirus disease-19 pandemic: A nationwide Italian survey. Eur. J. Cancer 2020, 132, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Chang, Y.L.; Shen, C.T.; Chung, W.S.; Tsai, H.J.; Chen, F.M. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. Breast 2020, 54, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Filipe, M.D.; van Deukeren, D.; Kip, M.; Doeksen, A.; Pronk, A.; Verheijen, P.M.; Heikens, J.T.; Witkamp, A.J.; Richir, M.C. Effect of the COVID-19 Pandemic on Surgical Breast Cancer Care in the Netherlands: A Multicenter Retrospective Cohort Study. Clin. Breast Cancer 2020, 20, 454–461. [Google Scholar] [CrossRef]
- Epic Health Research Network. Delayed Cancer Screenings. Available online: https://ehrn.org/articles/delays-in-preventive-cancer-screenings-during-covid-19-pandemic/ (accessed on 4 February 2022).
- Epic Health Research Network. Delayed Cancer Screenings—A Second Look. Available online: https://ehrn.org/articles/delayed-cancer-screenings-a-second-look/ (accessed on 4 February 2022).
- Bassett, M.T.; Chen, J.T.; Krieger, N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: A cross-sectional study. PLoS Med. 2020, 17, e1003402. [Google Scholar] [CrossRef]
- Mueller, J.T.; McConnell, K.; Burow, P.B.; Pofahl, K.; Merdjanoff, A.A.; Farrell, J. Impacts of the COVID-19 pandemic on rural America. Proc. Natl. Acad. Sci. USA 2021, 118, 2019378118. [Google Scholar] [CrossRef]
- Cortiula, F.; Pettke, A.; Bartoletti, M.; Puglisi, F.; Helleday, T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann. Oncol. 2020, 31, 553–555. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Burki, T.K. Cancer care in the time of COVID-19. Lancet Oncol. 2020, 21, 628. [Google Scholar] [CrossRef] [Green Version]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Dauri, M.; D’angelillo, R.M.; Buonomo, C.; De Majo, A.; Pistolese, C.; Portarena, I.; Mauriello, A.; et al. Awake breast cancer surgery: Strategy in the beginning of COVID-19 emergency. Breast Cancer 2021, 28, 137–144. [Google Scholar] [CrossRef] [PubMed]
- de Joode, K.; Dumoulin, D.; Engelen, V.; Bloemendal, H.; Verheij, M.; van Laarhoven, H.; Dingemans, I.; Dingemans, A.; van der Veldt, A. Impact of the coronavirus disease 2019 pandemic on cancer treatment: The patients’ perspective. Eur. J. Cancer 2020, 136, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Tagliamento, M.; Di Maio, M.; Martelli, V.; De Maria, A.; Barisione, E.; Grosso, M.; Boccardo, F.; Pronzato, P.; Del Mastro, L. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol. Pract. 2020, 16, e1304–e1314. [Google Scholar] [CrossRef] [PubMed]
- Tsamakis, K.; Gavriatopoulou, M.; Schizas, D.; Stravodimou, A.; Mougkou, A.; Tsiptsios, D.; Sioulas, V.; Spartalis, E.; Sioulas, A.D.; Tsamakis, C.; et al. Oncology during the COVID-19 pandemic: Challenges, dilemmas and the psychosocial impact on cancer patients. Oncol. Lett. 2020, 20, 441–447. [Google Scholar] [CrossRef]
- Balogun, O.D.; Bea, V.J.; Phillips, E. Disparities in Cancer Outcomes Due to COVID-19-A Tale of 2 Cities. JAMA Oncol. 2020, 6, 1531–1532. [Google Scholar] [CrossRef]
- Mayor, S. COVID-19: Impact on cancer workforce and delivery of care. Lancet Oncol. 2020, 21, 633. [Google Scholar] [CrossRef]
- Schrag, D.; Hershman, D.L.; Basch, E. Oncology Practice During the COVID-19 Pandemic. JAMA 2020, 323, 2005–2006. [Google Scholar] [CrossRef] [Green Version]
- Papautsky, E.L.; Hamlish, T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res. Treat. 2020, 184, 249–254. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Geng, C.; Liu, Z.; Lin, Y.; Nie, J.; Sun, G.; Ouyang, Q.; Wang, X.; Li, X.; et al. Suboptimal declines and delays in early breast cancer treatment after COVID-19 quarantine restrictions in China: A national survey of 8397 patients in the first quarter of 2020. EClinicalMedicine 2020, 26, 100503. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardoso, M.J.; Poortmans, P.; Gentilini, O.; Pravettoni, G.; Mazzocco, K.; Houssami, N.; Pagani, O.; Senkus, E.; Cardoso, F.; et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast 2020, 52, 8–16. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Kidney Cancer Coalition. The Impact of COVID-19 on Kidney Cancer Patients. Available online: https://ikcc.org/wp-content/uploads/2021/07/IKCC_2021_Covid-Report_A4_RZ_Web.pdf (accessed on 1 August 2022).
- Cancer Health. Cancer Care in the COVID-19 Pandemic. Available online: https://www.cancerhealth.com/survey/cancer-care-covid19-pandemic-us-provider-survey (accessed on 1 August 2022).
- American Society of Clinical Oncology. COVID-19 Patient Care Information; American Society of Clinical Oncology: Alexandria, VA, USA, 2020. [Google Scholar]
- London, J.W.; Fazio-Eynullayeva, E.; Palchuk, M.B.; Sankey, P.; McNair, C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin. Cancer Inform. 2020, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.A.; Kallan, M.J.; Julien, H.M.; Haynes, N.; Khatana, S.A.M.; Nathan, A.S.; Snider, C.; Chokshi, N.P.; Eneanya, N.D.; Takvorian, S.U.; et al. Patient Characteristics Associated With Telemedicine Access for Primary and Specialty Ambulatory Care During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2031640. [Google Scholar] [CrossRef]
- Marlow, N.M.; Pavluck, A.L.; Bian, J.; Ward, E.M.; Halpern, M.T. The Relationship between Insurance Coverage and Cancer Care: A Literature Synthesis; NBK542737; RTI Press: Berkeley, CA, USA, 2009. [Google Scholar]
- Sharpless, N.E. COVID-19 and cancer. Science 2020, 368, 1290. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Joung, R.H.; Nelson, H.; Mullett, T.W.; Kurtzman, S.H.; Shafir, S.; Harris, J.B.; Yao, K.A.; Brajcich, B.C.; Bilimoria, K.Y.; Cance, W.G. A national quality improvement study identifying and addressing cancer screening deficits due to the COVID-19 pandemic. Cancer 2022, 128, 2119–2125. [Google Scholar] [CrossRef]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef]
- Hartman, H.E.; Sun, Y.; Devasia, T.P.; Chase, E.C.; Jairath, N.K.; Dess, R.T.; Jackson, W.C.; Morris, E.; Li, P.; Hochstedler, K.A.; et al. Integrated Survival Estimates for Cancer Treatment Delay Among Adults With Cancer During the COVID-19 Pandemic. JAMA Oncol. 2020, 6, 1881–1889. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef]
- Malagón, T.; Yong, J.H.E.; Tope, P.; Miller, W.H.; Franco, E.L.; McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int. J. Cancer 2022, 150, 1244–1254. [Google Scholar] [CrossRef]
- Marcondes, F.O.; Cheng, D.; Warner, E.T.; Kamran, S.C.; Haas, J.S. The trajectory of racial/ethnic disparities in the use of cancer screening before and during the COVID-19 pandemic: A large U.S. academic center analysis. Prev. Med. 2021, 151, 106640. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.T.; Tamimi, R.M.; Hughes, M.E.; Ottesen, R.A.; Wong, Y.N.; Edge, S.B.; Theriault, R.L.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; et al. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res. Treat. 2012, 136, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Tsapatsaris, A.; Babagbemi, K.; Reichman, M.B. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: A review. Clin. Imaging 2022, 82, 224–227. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Total N = 493 | No Personal History of Breast Cancer N = 245 | Personal History of Breast Cancer N = 248 |
|---|---|---|---|
| Age (years) | N (%) | N (%) | N (%) |
| 18–39 | 31 (6.3%) | 4 (1.6%) | 27 (10.9%) |
| 40–49 | 104 (21.1%) | 66 (26.9%) | 38 (15.3%) |
| 50–59 | 142 (28.8%) | 79 (32.2%) | 63 (25.4%) |
| 60–69 | 143 (29.0%) | 67 (27.3%) | 76 (30.6%) |
| ≥70 | 58 (11.8%) | 19 (7.8%) | 39 (15.7%) |
| Missing | 15 (3.0%) | 10 (4.1%) | 5 (2.0%) |
| Race and Ethnicity | |||
| White | 279 (56.6%) | 83 (33.9%) | 196 (79.0%) |
| Hispanic/Latinx | 28 (5.7%) | 23 (9.4%) | 5 (2.0%) |
| Black or African-American | 115 (23.3%) | 82 (33.5%) | 33 (13.3%) |
| Asian | 27 (5.5%) | 23 (9.4%) | 4 (1.6%) |
| More than one race | 17 (3.4%) | 16 (6.5%) | 1 (0.4%) |
| Missing | 27 (5.5%) | 18 (7.3%) | 9 (3.6%) |
| Family History | |||
| No | 333 (67.5%) | 161 (65.7%) | 172 (69.4%) |
| Yes | 134 (27.2%) | 70 (28.6%) | 64 (25.8%) |
| Missing | 26 (5.3%) | 14 (5.7%) | 12 (4.8%) |
| U.S. Region | |||
| Northeast | 146 (29.6%) | 69 (28.2%) | 77 (31.0%) |
| Midwest | 67 (13.6%) | 34 (13.9%) | 33 (13.3%) |
| South | 163 (33.1%) | 94 (38.4%) | 69 (27.8%) |
| West | 77 (15.6%) | 29 (11.8%) | 48 (19.4%) |
| Missing | 40 (8.1%) | 19 (7.8%) | 21 (8.5%) |
| Level of Urbanicity | |||
| Urban | 197 (40.0%) | 102 (41.6%) | 95 (38.3%) |
| Suburban | 236 (47.9%) | 112 (45.7%) | 124 (50.0%) |
| Rural | 46 (9.3%) | 22 (9.0%) | 24 (9.7%) |
| Missing | 14 (2.8%) | 9 (3.7%) | 5 (2.0%) |
| Healthcare Site | |||
| Academic center | 167 (33.9%) | 72 (29.4%) | 95 (38.3%) |
| Regional center | 117 (23.7%) | 48 (19.6%) | 69 (27.8%) |
| Community hospital | 82 (16.6%) | 52 (21.2%) | 30 (12.1%) |
| Private practice | 125 (25.4%) | 73 (29.8%) | 52 (21.0%) |
| Missing | 2 (0.4%) | 0 (0.0%) | 2 (0.8%) |
| College degree | |||
| No | 117 (23.7%) | 68 (27.8%) | 49 (19.8%) |
| Yes | 366 (74.2%) | 170 (69.4%) | 196 (79.0%) |
| Missing | 10 (2.0%) | 7 (2.9%) | 3 (1.2%) |
| Household income | |||
| <$50,000 | 107 (21.7%) | 62 (25.3%) | 45 (18.1%) |
| $50,000–$74,999 | 70 (14.2%) | 33 (13.5%) | 37 (14.9%) |
| $75,000–$99,999 | 74 (15.0%) | 40 (16.3%) | 34 (13.7%) |
| $100,000–$149,999 | 79 (16.0%) | 37 (15.1%) | 42 (16.9%) |
| ≥$150,000 | 78 (15.8%) | 29 (11.8%) | 49 (19.8%) |
| Missing | 85 (17.2%) | 44 (18.0%) | 41 (16.5%) |
| Insurance * | |||
| Private insurance | 300 (60.9%) | 152 (62.0%) | 148 (59.7%) |
| Self-insured | 36 (7.3%) | 13 (5.3%) | 23 (9.3%) |
| Medicare | 142 (28.8%) | 57 (23.3%) | 85 (34.3%) |
| Medicaid | 39 (7.9%) | 26 (10.6%) | 13 (5.2%) |
| Other insurance | 45 (9.1%) | 24 (9.8%) | 21 (8.5%) |
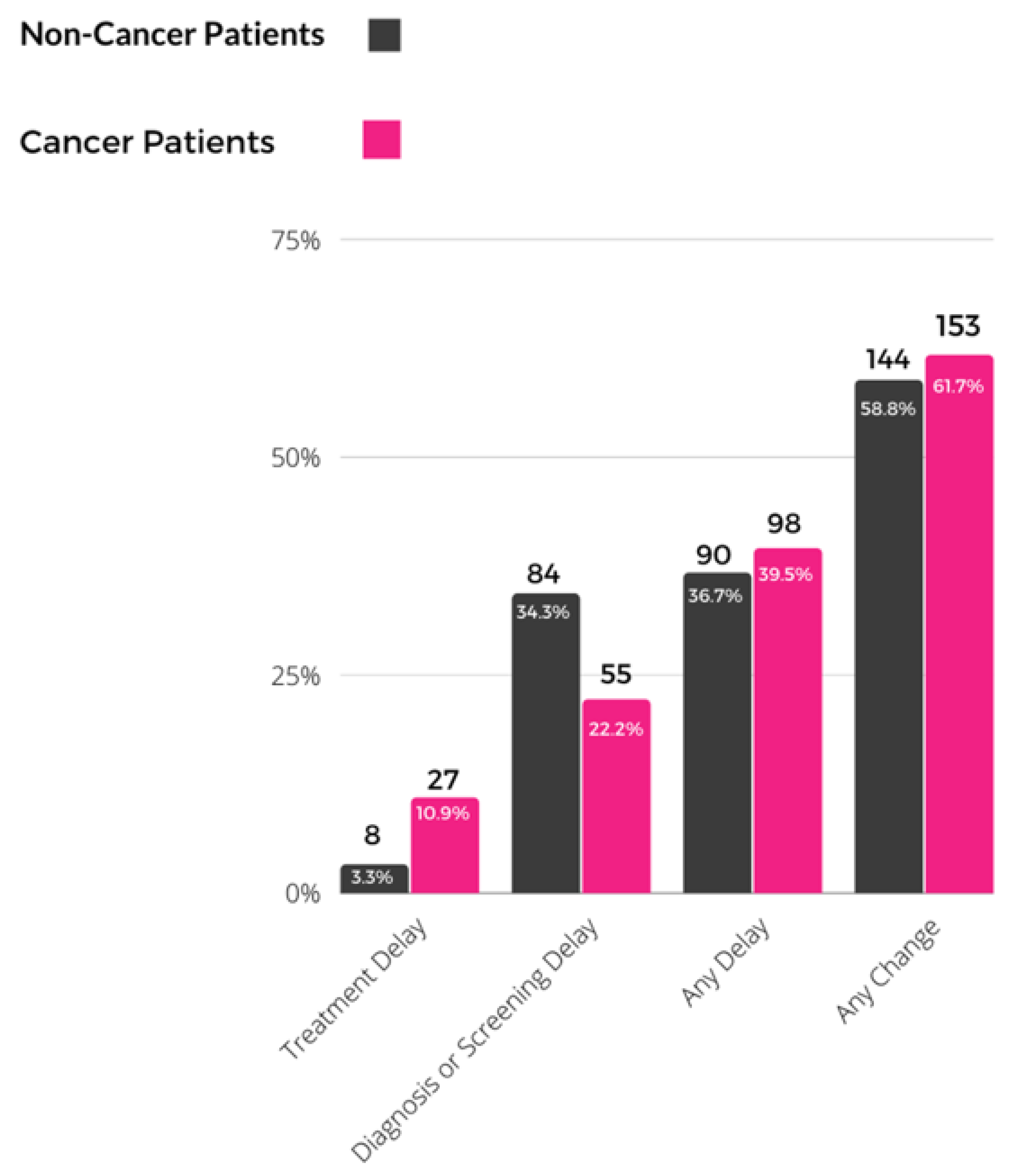
| N of Patients with Any Care Delay | % of Patients with Any Care Delay | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Personal history of breast cancer | |||||
| No | 90 | 36.7% | 1.00 | Reference | |
| Yes | 98 | 39.5% | 0.99 | 0.63, 1.55 | 0.968 |
| Age (years) | |||||
| 18–39 | 13 | 41.9% | 1.00 | Reference | |
| 40–49 | 38 | 36.5% | 0.68 | 0.28, 1.64 | 0.389 |
| 50–59 | 60 | 42.3% | 0.91 | 0.39, 2.10 | 0.821 |
| 60–69 | 49 | 34.3% | 0.60 | 0.24, 1.45 | 0.254 |
| ≥70 | 21 | 36.2% | 0.62 | 0.20, 1.90 | 0.402 |
| P trend | 0.867 | ||||
| Race and Ethnicity | |||||
| White | 112 | 40.1% | 1.00 | Reference | |
| Hispanic/Latinx | 9 | 32.1% | 0.62 | 0.25, 1.56 | 0.311 |
| Black or African American | 42 | 36.5% | 0.80 | 0.46, 1.41 | 0.445 |
| Asian | 6 | 22.2% | 0.42 | 0.15, 1.17 | 0.097 |
| More than one race | 8 | 47.1% | 1.13 | 0.37, 3.41 | 0.831 |
| U.S. Region | |||||
| Northeast | 55 | 37.7% | 1.00 | Reference | |
| Midwest | 27 | 40.3% | 1.11 | 0.58, 2.11 | 0.757 |
| South | 57 | 35.0% | 1.15 | 0.68, 1.94 | 0.597 |
| West | 30 | 39.0% | 1.23 | 0.65, 2.32 | 0.522 |
| Level of Urbanicity | |||||
| Urban | 78 | 39.6% | 1.00 | Reference | |
| Suburban | 80 | 33.9% | 0.74 | 0.48, 1.13 | 0.163 |
| Rural | 23 | 50.0% | 1.29 | 0.64, 2.57 | 0.474 |
| Healthcare Site | |||||
| Academic center | 66 | 39.5% | 1.00 | Reference | |
| Regional center | 53 | 45.3% | 1.14 | 0.67, 1.92 | 0.634 |
| Community hospital | 30 | 36.6% | 0.75 | 0.41, 1.36 | 0.344 |
| Private practice | 38 | 30.4% | 0.65 | 0.38, 1.12 | 0.123 |
| College degree | |||||
| No | 49 | 41.9% | 1.00 | Reference | |
| Yes | 134 | 36.6% | 0.89 | 0.53, 1.48 | 0.643 |
| Household income | |||||
| <$50,000 | 43 | 40.2% | 1.00 | Reference | |
| $50,000–$74,999 | 31 | 44.3% | 1.27 | 0.64, 2.51 | 0.494 |
| $75,000–$99,999 | 28 | 37.8% | 0.97 | 0.48, 1.97 | 0.938 |
| $100,000–$149,999 | 27 | 34.2% | 0.84 | 0.40, 1.77 | 0.655 |
| ≥$150,000 | 24 | 30.8% | 0.65 | 0.30, 1.39 | 0.268 |
| P trend | 0.562 | ||||
| Insurance * | |||||
| Private insurance | 75 | 38.9% | 1.00 | Reference | |
| 113 | 37.7% | 1.84 | 0.90, 3.75 | 0.096 | |
| Self-insured | 174 | 38.1% | 1.00 | Reference | |
| 14 | 38.9% | 1.94 | 0.76, 4.92 | 0.163 | |
| Medicare | 135 | 38.5% | 1.00 | Reference | |
| 53 | 37.3% | 1.23 | 0.65, 2.35 | 0.525 | |
| Medicaid | 168 | 37.0% | 1.00 | Reference | |
| 20 | 51.3% | 2.58 | 1.05, 6.32 | 0.039 | |
| Other government insurance | 170 | 37.9% | 1.00 | Reference | |
| 18 | 40.0% | 1.72 | 0.77, 3.83 | 0.188 |
| Characteristic | N | % | OR | 95% CI | p |
|---|---|---|---|---|---|
| Personal history of breast cancer | |||||
| No | 166 | 67.7% | 1.00 | Reference | |
| Yes | 173 | 69.8% | 1.11 | 0.68, 1.80 | 0.678 |
| Age (years) | |||||
| 18–39 | 23 | 74.2% | 1.00 | Reference | |
| 40–49 | 70 | 67.3% | 0.84 | 0.32, 2.24 | 0.734 |
| 50–59 | 99 | 69.7% | 0.93 | 0.36, 2.38 | 0.880 |
| 60–69 | 103 | 72.0% | 1.13 | 0.42, 3.02 | 0.813 |
| ≥70 | 33 | 56.9% | 0.53 | 0.16, 1.77 | 0.305 |
| P trend | 0.885 | ||||
| Race and Ethnicity | |||||
| White | 192 | 68.8% | 1.00 | Reference | |
| Hispanic/Latinx | 17 | 60.7% | 0.72 | 0.29, 1.76 | 0.473 |
| Black or African-American | 80 | 69.6% | 0.84 | 0.46, 1.51 | 0.553 |
| Asian | 19 | 70.4% | 1.10 | 0.41, 2.92 | 0.855 |
| More than one race | 10 | 58.8% | 0.68 | 0.22, 2.13 | 0.509 |
| U.S. Region | |||||
| Northeast | 102 | 69.9% | 1.00 | Reference | |
| Midwest | 43 | 64.2% | 0.94 | 0.48, 1.85 | 0.854 |
| South | 121 | 74.2% | 1.27 | 0.72, 2.24 | 0.405 |
| West | 47 | 61.0% | 0.74 | 0.38, 1.42 | 0.361 |
| Area | |||||
| Urban | 128 | 65.0% | 1.00 | Reference | |
| Suburban | 173 | 73.3% | 1.31 | 0.83, 2.05 | 0.247 |
| Rural | 30 | 65.2% | 1.01 | 0.48, 2.09 | 0.988 |
| Healthcare Location | |||||
| Academic center | 118 | 70.7% | 1.00 | Reference | |
| Regional center | 79 | 67.5% | 1.06 | 0.60, 1.86 | 0.846 |
| Community hospital | 50 | 61.0% | 0.85 | 0.46, 1.59 | 0.622 |
| Private practice | 92 | 73.6% | 1.50 | 0.84, 2.68 | 0.169 |
| College degree | |||||
| No | 79 | 67.5% | 1.00 | Reference | |
| Yes | 252 | 68.9% | 1.02 | 0.99, 1.06 | 0.152 |
| Household income b | |||||
| <$50,000 | 62 | 57.9% | 1.00 | Reference | |
| $50,000–$74,999 | 48 | 68.6% | 1.53 | 0.75, 3.11 | 0.241 |
| $75,000–$99,999 | 51 | 68.9% | 1.52 | 0.74, 3.12 | 0.252 |
| $100,000–$149,999 | 60 | 75.9% | 2.15 * | 1.01, 4.55 | 0.047 |
| ≥$150,000 | 61 | 78.2% | 2.38 * | 1.09, 5.17 | 0.029 |
| P trend | 0.423 | ||||
| Insurance c | |||||
| Private insurance | 123 | 63.7% | 1.00 | Reference | |
| 216 | 72.0% | 0.87 | 0.41, 1.84 | 0.708 | |
| Self-insured | 324 | 70.9% | 1.00 | Reference | |
| 15 | 41.7% | 0.28 ** | 0.11, 0.73 | 0.009 | |
| Medicare | 245 | 69.8% | 1.00 | Reference | |
| 94 | 66.2% | 1.12 | 0.56, 2.22 | 0.754 | |
| Medicaid | 313 | 68.9% | 1.00 | Reference | |
| 26 | 66.7% | 1.09 | 0.43, 2.79 | 0.853 | |
| Other government insurance | 308 | 68.8% | 1.00 | Reference | |
| 31 | 68.9% | 0.97 | 0.41, 2.26 | 0.938 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Carfang, L.; Restrepo, E.; Benjamin, C.; Epstein, M.M.; Fairley, R.; Roudebush, L.; Hertz, C.; Eshraghi, L.; Warner, E.T. Patient-Reported Experiences of Breast Cancer Screening, Diagnosis, and Treatment Delay, and Telemedicine Adoption during COVID-19. Curr. Oncol. 2022, 29, 5919-5932. https://doi.org/10.3390/curroncol29080467
Du S, Carfang L, Restrepo E, Benjamin C, Epstein MM, Fairley R, Roudebush L, Hertz C, Eshraghi L, Warner ET. Patient-Reported Experiences of Breast Cancer Screening, Diagnosis, and Treatment Delay, and Telemedicine Adoption during COVID-19. Current Oncology. 2022; 29(8):5919-5932. https://doi.org/10.3390/curroncol29080467
Chicago/Turabian StyleDu, Simo, Laura Carfang, Emily Restrepo, Christine Benjamin, Mara M. Epstein, Ricki Fairley, Laura Roudebush, Crystal Hertz, Leah Eshraghi, and Erica T. Warner. 2022. "Patient-Reported Experiences of Breast Cancer Screening, Diagnosis, and Treatment Delay, and Telemedicine Adoption during COVID-19" Current Oncology 29, no. 8: 5919-5932. https://doi.org/10.3390/curroncol29080467
APA StyleDu, S., Carfang, L., Restrepo, E., Benjamin, C., Epstein, M. M., Fairley, R., Roudebush, L., Hertz, C., Eshraghi, L., & Warner, E. T. (2022). Patient-Reported Experiences of Breast Cancer Screening, Diagnosis, and Treatment Delay, and Telemedicine Adoption during COVID-19. Current Oncology, 29(8), 5919-5932. https://doi.org/10.3390/curroncol29080467





