The Efficacy and Safety of Celecoxib in Addition to Standard Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction and Assessment of Study Quality
2.4. Statistical Analysis
3. Results
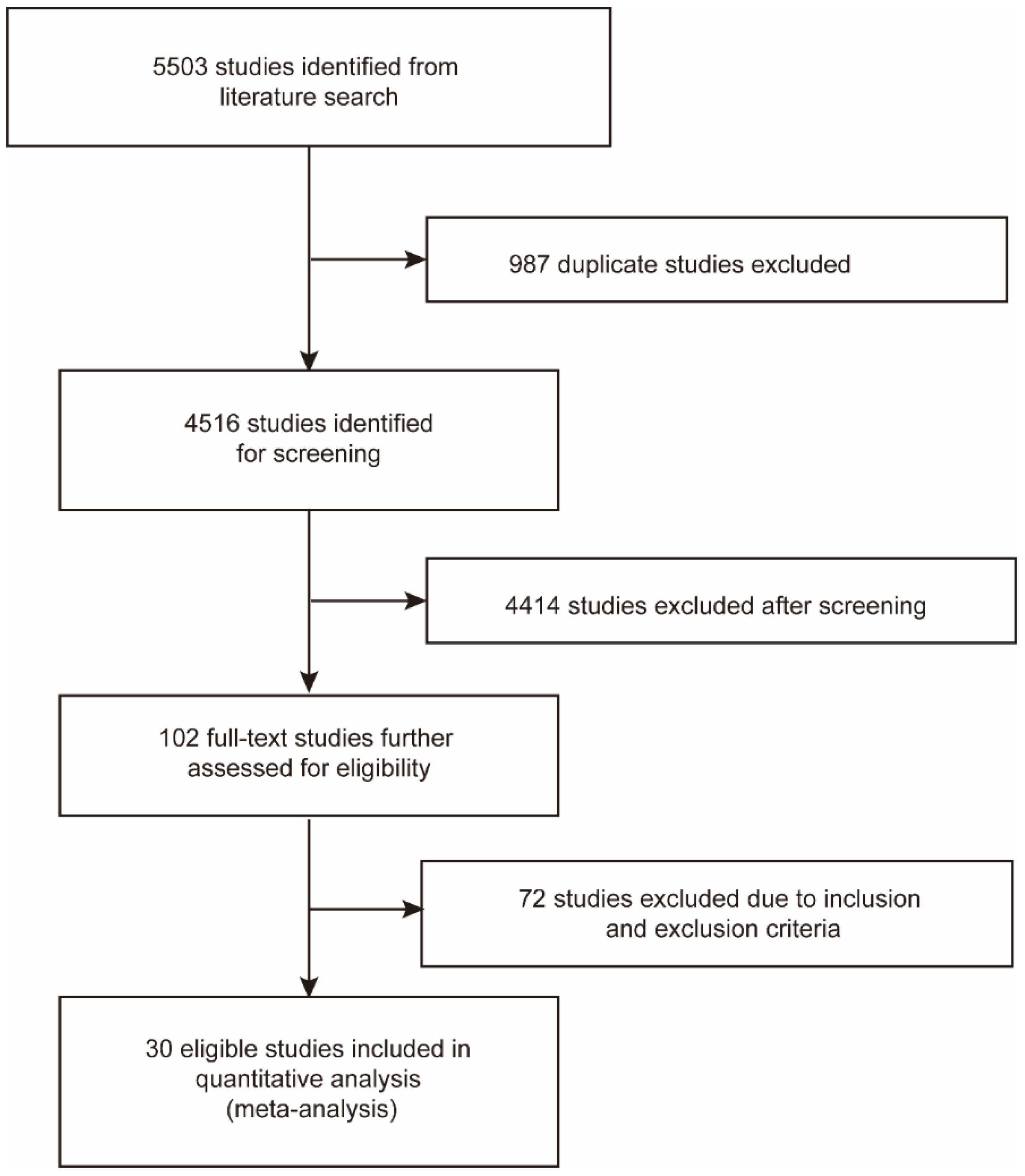
3.1. Study Selection and Associated Characteristics
3.2. Overall Efficacy of Celecoxib in Standard Anticancer Therapy
3.3. Celecoxib and Overall Survival (OS) in Palliative Therapy
3.4. Celecoxib and Local Control in Palliative Therapy
3.5. Celecoxib and Adjuvant Therapy
3.6. Celecoxib and Neoadjuvant Therapy
3.7. Safety of Celecoxib
3.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Krysan, K.; Cui, X.; Gardner, B.K.; Reckamp, K.L.; Wang, X.; Hong, L.; Walser, T.C.; Rodriguez, N.L.; Pagano, P.C.; Garon, E.B.; et al. Elevated neutrophil gelatinase-associated lipocalin contributes to erlotinib resistance in non-small cell lung cancer. Am. J. Transl. Res. 2013, 5, 481–496. [Google Scholar] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, A.J.; Lippman, S.M.; Mann, J.R.; Subbaramaiah, K.; DuBois, R.N. Cyclooxygenase-2 and epidermal growth factor receptor: Pharmacologic targets for chemoprevention. J. Clin. Oncol. 2005, 23, 254–266. [Google Scholar] [CrossRef]
- Smith, W.L.; Langenbach, R. Why there are two cyclooxygenase isozymes. J. Clin. Investig. 2001, 107, 1491–1495. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.F. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am. J. Gastroenterol. 2002, 97, 1037–1041. [Google Scholar] [CrossRef]
- Ristimäki, A.; Sivula, A.; Lundin, J.; Lundin, M.; Salminen, T.; Haglund, C.; Joensuu, H.; Isola, J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002, 62, 632–635. [Google Scholar]
- Saba, N.F.; Choi, M.; Muller, S.; Shin, H.J.; Tighiouart, M.; Papadimitrakopoulou, V.A.; El-Naggar, A.K.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Role of cyclooxygenase-2 in tumor progression and survival of head and neck squamous cell carcinoma. Cancer Prev. Res. 2009, 2, 823–829. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar]
- Belton, O.; Byrne, D.; Kearney, D.; Leahy, A.; Fitzgerald, D.J. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation 2000, 102, 840–845. [Google Scholar] [CrossRef]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001, 286, 954–959. [Google Scholar] [CrossRef] [PubMed]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Ashbeck, E.L.; Roe, D.J.; Fales, L.; Buckmeier, J.; Wang, F.; Bhattacharyya, A.; Hsu, C.H.; Chow, S.H.; Ahnen, D.J.; et al. Celecoxib for the Prevention of Colorectal Adenomas: Results of a Suspended Randomized Controlled Trial. J. Natl. Cancer Inst. 2016, 108, djw151. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Solomon, S.D.; Kim, K.; Tang, J.; Rosenstein, R.B.; Wittes, J.; Corle, D.; et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 2006, 355, 873–884. [Google Scholar] [CrossRef]
- Flamiatos, J.F.; Beer, T.M.; Graff, J.N.; Eilers, K.M.; Tian, W.; Sekhon, H.S.; Garzotto, M. Cyclooxygenase-2 (COX-2) inhibition for prostate cancer chemoprevention: Double-blind randomised study of pre-prostatectomy celecoxib or placebo. BJU Int. 2017, 119, 709–716. [Google Scholar] [CrossRef]
- Thompson, P.A.; Martinez, J.A. The Importance of Drug Concentration at the Site of Action: Celecoxib and Colon Polyp Prevention as a Case Study. Cancer Prev. Res. 2022, 15, 205–208. [Google Scholar] [CrossRef]
- Mao, J.T.; Roth, M.D.; Fishbein, M.C.; Aberle, D.R.; Zhang, Z.F.; Rao, J.Y.; Tashkin, D.P.; Goodglick, L.; Holmes, E.C.; Cameron, R.B.; et al. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev. Res. 2011, 4, 984–993. [Google Scholar] [CrossRef]
- Tudor, D.V.; Baldea, I.; Olteanu, D.E.; Fischer-Fodor, E.; Piroska, V.; Lupu, M.; Calinici, T.; Decea, R.M.; Filip, G.A. Celecoxib as a Valuable Adjuvant in Cutaneous Melanoma Treated with Trametinib. Int. J. Mol. Sci. 2021, 22, 4387. [Google Scholar] [CrossRef]
- Toloczko-Iwaniuk, N.; Dziemianczyk-Pakiela, D.; Nowaszewska, B.K.; Celinska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention—Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef]
- Hsu, A.L.; Ching, T.T.; Wang, D.S.; Song, X.; Rangnekar, V.M.; Chen, C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 2000, 275, 11397–11403. [Google Scholar] [CrossRef]
- Lin, E.; Morris, J.S.; Ayers, G.D. Effect of celecoxib on capecitabine-induced hand-foot syndrome and antitumor activity. Oncology 2002, 16, 31–37. [Google Scholar] [PubMed]
- Shan, B.Z.; Guo, B.; Li, Y.S.; Sun, X.F. Effect of celecoxib on protein expression of FAK and Cx43 in DMBA induced rat tongue carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9454–9463. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.V.; D’Agostino, A.; Ma, Q.; Eroles, P.; Cavallin, L.; Chiozzini, C.; Sapochnik, D.; Cymeryng, C.; Hyjek, E.; Cesarman, E.; et al. KSHV G-protein coupled receptor vGPCR oncogenic signaling upregulation of Cyclooxygenase-2 expression mediates angiogenesis and tumorigenesis in Kaposi’s sarcoma. PLoS Pathog. 2020, 16, e1009006. [Google Scholar] [CrossRef]
- Yoshida, H.; Yoshimura, H.; Matsuda, S.; Yamamoto, S.; Ohmori, M.; Ohta, K.; Ryoke, T.; Itoi, H.; Kiyoshima, T.; Kobayashi, M.; et al. Celecoxib suppresses lipopolysaccharide-stimulated oral squamous cell carcinoma proliferation in vitro and in vivo. Oncol. Lett. 2019, 18, 5793–5800. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, K.; Deng, L.; Liang, J.; Liang, H.; Feng, D.; Ling, B. Cyclooxygenase 2 Promotes Proliferation and Invasion in Ovarian Cancer Cells via the PGE2/NF-kappaB Pathway. Cell Transplant. 2019, 28, 1S–13S. [Google Scholar] [CrossRef]
- Tai, Y.; Zhang, L.H.; Gao, J.H.; Zhao, C.; Tong, H.; Ye, C.; Huang, Z.Y.; Liu, R.; Tang, C.W. Suppressing growth and invasion of human hepatocellular carcinoma cells by celecoxib through inhibition of cyclooxygenase-2. Cancer Manag. Res. 2019, 11, 2831–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Wu, X.J.; Wan, D.S.; Lu, Z.H.; Kong, L.H.; Pan, Z.Z.; Chen, G. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: Result of a single-center, prospective randomized phase III trial. Ann. Oncol. 2012, 23, 1348–1353. [Google Scholar] [CrossRef]
- Suddek, G.M.; El-Kenawi, A.E.; Abdel-Aziz, A.; El-Kashef, H.A. Celecoxib, a selective cyclooxygenase-2 inhibitor, attenuates renal injury in a rat model of Cisplatin-induced nephrotoxicity. Chemotherapy 2011, 57, 321–326. [Google Scholar] [CrossRef]
- Falandry, C.; Debled, M.; Bachelot, T.; Delozier, T.; Crétin, J.; Romestaing, P.; Mille, D.; You, B.; Mauriac, L.; Pujade-Lauraine, E.; et al. Celecoxib and exemestane versus placebo and exemestane in postmenopausal metastatic breast cancer patients: A double-blind phase III GINECO study. Breast Cancer Res. Treat. 2009, 116, 501–508. [Google Scholar] [CrossRef]
- Debucquoy, A.; Roels, S.; Goethals, L.; Libbrecht, L.; Cutsem, E.V.; Geboes, K.; Penninckx, F.; D’Hoore, A.; McBride, W.H.; Haustermans, K. Double blind randomized phase II study with radiation + 5-fluorouracil ± celecoxib for resectable rectal cancer. Radiother. Oncol. 2009, 93, 273–278. [Google Scholar] [CrossRef]
- Koch, A.; Bergman, B.; Holmberg, E.; Sederholm, C.; Ek, L.; Kosieradzki, J.; Lamberg, K.; Thaning, L.; Ydreborg, S.O.; Sörenson, S. Effect of celecoxib on survival in patients with advanced non-small cell lung cancer: A double blind randomised clinical phase III trial (CYCLUS study) by the Swedish Lung Cancer Study Group. Eur. J. Cancer 2011, 47, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Strasser-Weippl, K.; Higgins, M.J.; Chapman, J.W.; Ingle, J.N.; Sledge, G.W.; Budd, G.T.; Ellis, M.J.; Pritchard, K.I.; Clemons, M.J.; Badovinac-Crnjevic, T.; et al. Effects of Celecoxib and Low-dose Aspirin on Outcomes in Adjuvant Aromatase Inhibitor-Treated Patients: CCTG MA.27. J. Natl. Cancer Inst. 2018, 110, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Maiello, E.; Giuliani, F.; Gebbia, V.; Di Renzo, N.; Pezzella, G.; Romito, S.; Mallamaci, R.; Lopez, M.; Colucci, G. FOLFIRI with or without celecoxib in advanced colorectal cancer: A randomized phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Ann. Oncol. 2006, 17 (Suppl. 7), 55–59. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Mason, M.D.; Clarke, N.W.; James, N.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Attard, G.; Cross, W.; Jones, R.J.; Parker, C.C.; et al. Adding Celecoxib With or Without Zoledronic Acid for Hormone-Naïve Prostate Cancer: Long-Term Survival Results From an Adaptive, Multiarm, Multistage, Platform, Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Tan, W.S.; Porta, N.; Mostafid, H.; Huddart, R.; Protheroe, A.; Bogle, R.; Blazeby, J.; Palmer, A.; Cresswell, J.; et al. BOXIT—A Randomised Phase III Placebo-controlled Trial Evaluating the Addition of Celecoxib to Standard Treatment of Transitional Cell Carcinoma of the Bladder (CRUK/07/004). Eur. Urol. 2019, 75, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, L.W.; Yip, A.Y.; Loo, W.T.; Lam, C.K.; Toi, M. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J. Steroid Biochem. Mol. Biol. 2008, 111, 13–17. [Google Scholar] [CrossRef]
- Hamy, A.S.; Tury, S.; Wang, X.; Gao, J.; Pierga, J.Y.; Giacchetti, S.; Brain, E.; Pistilli, B.; Marty, M.; Espié, M.; et al. Celecoxib With Neoadjuvant Chemotherapy for Breast Cancer Might Worsen Outcomes Differentially by COX-2 Expression and ER Status: Exploratory Analysis of the REMAGUS02 Trial. J. Clin. Oncol. 2019, 37, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Ahmadloo, N.; Nazer Mozaffari, M.A.; Mohammadianpanah, M.; Omidvari, S.H.; Mosalaei, A.; Mosleh Shirazi, M.A. Combined neoadjuvant chemotherapy and celecoxib in locally advanced breast cancer. Iran. Red Crescent Med. J. 2009, 11, 419–424. [Google Scholar]
- Guo, Q.; Li, Q.; Wang, J.; Liu, M.; Wang, Y.; Chen, Z.; Ye, Y.; Guan, Q.; Zhou, Y. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study. Medicine 2019, 98, e16234. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Shi, Q.; Fuchs, C.S.; Meyer, J.; Niedzwiecki, D.; Zemla, T.; Kumthekar, P.; Guthrie, K.A.; Couture, F.; Kuebler, P.; et al. Effect of Celecoxib vs Placebo Added to Standard Adjuvant Therapy on Disease-Free Survival among Patients with Stage III Colon Cancer: The CALGB/SWOG 80702 (Alliance) Randomized Clinical Trial. JAMA 2021, 325, 1277–1286. [Google Scholar] [CrossRef]
- Bi, N.; Liang, J.; Zhou, Z.; Chen, D.; Fu, Z.; Yang, X.; Feng, Q.; Hui, Z.; Xiao, Z.; Lv, J.; et al. Effect of Concurrent Chemoradiation With Celecoxib vs Concurrent Chemoradiation Alone on Survival Among Patients With Non-Small Cell Lung Cancer With and Without Cyclooxygenase 2 Genetic Variants: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1918070. [Google Scholar] [CrossRef]
- Mohammadianpanah, M.; Razmjou-Ghalaei, S.; Shafizad, A.; Ashouri-Taziani, Y.; Khademi, B.; Ahmadloo, N.; Ansari, M.; Omidvari, S.; Mosalaei, A.; Mosleh-Shirazi, M.A. Efficacy and safety of concurrent chemoradiation with weekly cisplatin ± low-dose celecoxib in locally advanced undifferentiated nasopharyngeal carcinoma: A phase II-III clinical trial. J. Cancer Res. Ther. 2011, 7, 442–447. [Google Scholar] [CrossRef]
- Aghili, M.; Ghalehtaki, R.; Rayzan, E.; Farzin, M.; Mojahed, M.M.; Izadi, S.; Kazemian, A. The efficacy of celecoxib during chemoradiation in locally advanced head and neck carcinoma; a phase 2 randomized placebo control clinical trial. Int. J. Cancer Manag. 2021, 14, e103653. [Google Scholar] [CrossRef]
- Edelman, M.J.; Watson, D.; Wang, X.; Morrison, C.; Kratzke, R.A.; Jewell, S.; Hodgson, L.; Mauer, A.M.; Gajra, A.; Masters, G.A.; et al. Eicosanoid modulation in advanced lung cancer: Cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy—Cancer and leukemia group B trial 30203. J. Clin. Oncol. 2008, 26, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Köhne, C.H.; De Greve, J.; Hartmann, J.T.; Lang, I.; Vergauwe, P.; Becker, K.; Braumann, D.; Joosens, E.; Müller, L.; Janssens, J.; et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann. Oncol. 2008, 19, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Francis, A.; Poole, C.; Brookes, C.; Stein, R.; Bartlett, J.; Dunn, J.; Canney, P.; Sutton, R.; Daoud, R.; et al. NEO-EXCEL phase III neoadjuvant trial of pre-operative exemestane or letrozole +/− celecoxib in the treatment of ER positive postmenopausal early breast cancer. Cancer Res. 2016, 76, PD2–02. [Google Scholar] [CrossRef]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Chow, L.W.; Toi, M.; Takebayashi, Y. Neoadjuvant use of 5-fluorouracil, epirubicin and cyclophosphamide with and without celecoxib for locally advanced breast cancer: A phase II study correlating response to celecoxib with high levels of COX-2 gene expression in the tumor. J. Clin. Oncol. 2004, 22, 9603. [Google Scholar] [CrossRef]
- Jin, C.H.; Wang, A.H.; Chen, J.M.; Li, R.X.; Liu, X.M.; Wang, G.P.; Xing, L.Q. Observation of curative efficacy and prognosis following combination chemotherapy with celecoxib in the treatment of advanced colorectal cancer. J. Int. Med. Res. 2011, 39, 2129–2140. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, C.; Xu, J.; Lv, M. P2-327: First-line chemotherapy of vinorelbine/cisplatin (NP) combined with cyclooxygenase-2 inhibitor celecoxib in advanced non-small-cell lung cancer (NSCLC). J. Thorac. Oncol. 2007, 2, S702–S703. [Google Scholar] [CrossRef]
- Edelman, M.J.; Wang, X.; Hodgson, L.; Cheney, R.T.; Baggstrom, M.Q.; Thomas, S.P.; Gajra, A.; Bertino, E.; Reckamp, K.L.; Molina, J.; et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance). J. Clin. Oncol. 2017, 35, 2184–2192. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Koczywas, M.; Cristea, M.C.; Dowell, J.E.; Wang, H.J.; Gardner, B.K.; Milne, G.L.; Figlin, R.A.; Fishbein, M.C.; Elashoff, R.M.; et al. Randomized phase 2 trial of erlotinib in combination with high-dose celecoxib or placebo in patients with advanced non-small cell lung cancer. Cancer 2015, 121, 3298–3306. [Google Scholar] [CrossRef]
- Reyners, A.K.; de Munck, L.; Erdkamp, F.L.; Smit, W.M.; Hoekman, K.; Lalisang, R.I.; de Graaf, H.; Wymenga, A.N.; Polee, M.; Hollema, H.; et al. A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, Fallopian tube or primary peritoneal carcinomas: The DoCaCel study. Ann. Oncol. 2012, 23, 2896–2902. [Google Scholar] [CrossRef]
- Lilenbaum, R.; Socinski, M.A.; Altorki, N.K.; Hart, L.L.; Keresztes, R.S.; Hariharan, S.; Morrison, M.E.; Fayyad, R.; Bonomi, P. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 4825–4832. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Cristea, M.; Frankel, P.; Ruel, C.; Chen, C.; Wang, Y.; Morgan, R.; Leong, L.; Chow, W.; Koczywas, M.; et al. Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer. Cancer Treat. Res. Commun. 2019, 21, 100155. [Google Scholar] [CrossRef] [PubMed]
- Groen, H.J.; Sietsma, H.; Vincent, A.; Hochstenbag, M.M.; van Putten, J.W.; van den Berg, A.; Dalesio, O.; Biesma, B.; Smit, H.J.; Termeer, A.; et al. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: The NVALT-4 study. J. Clin. Oncol. 2011, 29, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Gharib, F.; Zamzam, Y.; Sad, L.M. Role of COX-2 inhibitors as maintenance therapy in non-metastatic triple negative breast cancer Egyptian patients, single institution study. Oncol. Radiother. 2020, 1, 1–6. [Google Scholar]
- Dirix, L.Y.; Ignacio, J.; Nag, S.; Bapsy, P.; Gomez, H.; Raghunadharao, D.; Paridaens, R.; Jones, S.; Falcon, S.; Carpentieri, M.; et al. Treatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxib. J. Clin. Oncol. 2008, 26, 1253–1259. [Google Scholar] [CrossRef]
- Edelman, M.J.; Wang, X.; Hodgson, L.; Cheney, R.T.; Baggstrom, M.; Sachdev, T.; Gajra, A.; Bertino, E.; Reckamp, K.; Molina, J.; et al. Phase III randomized, placebo controlled trial of COX-2 inhibition in addition to standard chemotherapy for advanced non-small cell lung cancer (NSCLC):CALGB 30801 (Alliance). Cancer Res. 2014, 74, CT238. [Google Scholar] [CrossRef]
- Gulyas, M.; Mattsson, J.S.M.; Lindgren, A.; Ek, L.; Lamberg Lundström, K.; Behndig, A.; Holmberg, E.; Micke, P.; Bergman, B. COX-2 expression and effects of celecoxib in addition to standard chemotherapy in advanced non-small cell lung cancer. Acta Oncol. 2018, 57, 244–250. [Google Scholar] [CrossRef]
- Pierga, J.Y.; Delaloge, S.; Espié, M.; Brain, E.; Sigal-Zafrani, B.; Mathieu, M.C.; Bertheau, P.; Guinebretière, J.M.; Spielmann, M.; Savignoni, A.; et al. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res. Treat. 2010, 122, 429–437. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sporn, M.B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976, 36, 2699–2702. [Google Scholar] [PubMed]
- Sporn, M.B.; Suh, N. Chemoprevention: An essential approach to controlling cancer. Nat. Rev. Cancer 2002, 2, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, C.; Cerella, C.; Dicato, M.; Ghibelli, L.; Diederich, M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int. J. Cell Biol. 2010, 2010, 215158. [Google Scholar] [CrossRef] [PubMed]
- Grösch, S.; Maier, T.J.; Schiffmann, S.; Geisslinger, G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J. Natl. Cancer Inst. 2006, 98, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Antitumour Activities of Selected Pure Compounds Identified from the Serum of Crocodylus porosus, Malayopython reticulatus, Varanus salvator and Cuora kamaroma amboinensis. Asian Pac. J. Cancer Prev. 2021, 22, 97–106. [Google Scholar] [CrossRef]
- Gitlitz, B.J.; Bernstein, E.; Santos, E.S.; Otterson, G.A.; Milne, G.; Syto, M.; Burrows, F.; Zaknoen, S. A randomized, placebo-controlled, multicenter, biomarker-selected, phase 2 study of apricoxib in combination with erlotinib in patients with advanced non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 577–582. [Google Scholar] [CrossRef]
- Reddy, B.S.; Hirose, Y.; Lubet, R.; Steele, V.; Kelloff, G.; Paulson, S.; Seibert, K.; Rao, C.V. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000, 60, 293–297. [Google Scholar]
- Wu, Z.; Qu, B.; Huang, X.; Song, Y.; Gao, P.; Shi, J.; Zhou, C.; Wang, Z. The potential adjunctive benefit of adding metformin to standard treatment in inoperable cancer patients: A meta-analysis of randomized controlled trials. Ann. Transl. Med. 2020, 8, 1404. [Google Scholar] [CrossRef]
- Lorenz, M.; Slaughter, H.S.; Wescott, D.M.; Carter, S.I.; Schnyder, B.; Dinchuk, J.E.; Car, B.D. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp. Hematol. 1999, 27, 1494–1502. [Google Scholar] [CrossRef]
- Ono, K.; Akatsu, T.; Murakami, T.; Kitamura, R.; Yamamoto, M.; Shinomiya, N.; Rokutanda, M.; Sasaki, T.; Amizuka, N.; Ozawa, H.; et al. Involvement of cyclo-oxygenase-2 in osteoclast formation and bone destruction in bone metastasis of mammary carcinoma cell lines. J. Bone. Miner. Res. 2002, 17, 774–781. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, Y.; Yuan, H.; Wu, M.; Li, S.; Gong, W.; Yu, J.; Xia, W.; Zhang, Y.; Ding, G.; et al. Inhibition of COX-2/PGE2 cascade ameliorates cisplatin-induced mesangial cell apoptosis. Am. J. Transl. Res. 2017, 9, 1222–1229. [Google Scholar] [PubMed]
- Lucci, A.; Krishnamurthy, S.; Singh, B.; Bedrosian, I.; Meric-Bernstam, F.; Reuben, J.; Broglio, K.; Mosalpuria, K.; Lodhi, A.; Vincent, L.; et al. Cyclooxygenase-2 expression in primary breast cancers predicts dissemination of cancer cells to the bone marrow. Breast Cancer Res. Treat. 2009, 117, 61–68. [Google Scholar] [CrossRef] [Green Version]




| Index of Risk | 95%CI | p-Value | Heterogeneity (I2, p-Value) | |
|---|---|---|---|---|
| Overall survival (OS) | HR = 1.00 | 0.92–1.08 | 0.57 | 0.00%, 0.93 |
| Progression-free survival (PFS) | HR = 1.02 | 0.91–1.13 | 0.76 | 0.00%, 0.63 |
| Disease-free survival (DFS) | HR = 1.05 | 0.80–1.38 | 0.06 | 64.40%, 0.73 |
| Objective response rate (ORR) | RR = 1.13 | 1.03–1.23 | 0.33 | 10.20%, 0.01 |
| Disease control rate (DCR) | RR = 1.05 | 0.99–1.11 | 0.11 | 0.00%, 0.76 |
| Pathological complete response (pCR) | RR = 1.28 | 0.88–1.85 | 0.53 | 0.00%, 0.95 |
| HR | 95%CI | p-Value | Heterogeneity (I2, p-Value) | |
|---|---|---|---|---|
| Overall survival | ||||
| Concomitant therapy methods | ||||
| Chemotherapy | 1.08 | 0.96–1.21 | 0.23 | 0.00%, 0.79 |
| Chemoradiotherapy | 0.99 | 0.69–1.43 | 0.96 | 0.00%, 0.96 |
| Therapy stages | ||||
| First-line | 1.03 | 0.93–1.15 | 0.59 | 0.00%, 0.91 |
| ≥First-line | 0.93 | 0.64–1.34 | 0.68 | 0.00%, 0.86 |
| COX-2 status | ||||
| High COX-2 status | 0.94 | 0.67–1.31 | 0.70 | 62.60%,0.05 |
| Low COX-2 status | 1.13 | 0.78–1.64 | 0.53 | 51.50%, 0.13 |
| PGEM status | ||||
| High PGEM status | 0.79 | 0.47–1.34 | 0.39 | 0.00%, 0.49 |
| Low PGEM status | 1.27 | 0.89–1.81 | 0.19 | 0.00%, 0.90 |
| EGFR status | ||||
| EGFR wild-type | 1.03 | 0.62–1.70 | 0.92 | 0.00%, 0.99 |
| Use of NSAIDs | ||||
| No use of NSAIDs | 0.98 | 0.81–1.18 | 0.80 | 0.00%, 0.61 |
| Use of NSAIDs | 0.66 | 0.23–1.91 | 0.44 | 64.30%, 0.09 |
| Performance Status (PS, WHO) | ||||
| PS: 0 | 0.88 | 0.66–1.19 | 0.41 | 0.00%, 0.89 |
| PS: ≥1 | 1.02 | 0.83–1.25 | 0.86 | 0.00%, 0.40 |
| Sample size | ||||
| <200 | 1.12 | 0.92–1.36 | 0.27 | 0.00%, 0.71 |
| ≥200 | 1.02 | 0.91–1.15 | 0.69 | 0.00%, 0.87 |
| Progression-free survival | ||||
| Concomitant therapy strategies | ||||
| Chemotherapy | 1.02 | 0.91–1.15 | 0.75 | 0.00%, 0.47 |
| Therapy stages | ||||
| First-line | 1.01 | 0.89–1.15 | 0.83 | 0.00%, 0.44 |
| ≥First-line | 0.85 | 0.62–1.18 | 0.34 | 0.00%, 0.74 |
| COX-2status | ||||
| High COX-2 status | 1.03 | 0.82–1.30 | 0.79 | 0.00%, 0.86 |
| PGEM status | ||||
| High PGEM status | 0.71 | 0.48–1.07 | 0.10 | 0.00%, 0.74 |
| Low PGEM status | 1.05 | 0.78–1.42 | 0.73 | 0.00%, 0.33 |
| EGFR status | ||||
| EGFR wild-type | 0.57 | 0.35–0.94 | 0.03 | 0.00%, 0.72 |
| Sample size | ||||
| <200 | 0.99 | 0.81–1.20 | 0.90 | 0.00%, 0.63 |
| ≥200 | 1.03 | 0.90–1.18 | 0.65 | 9.70%, 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.-Y.; Li, J.-Y.; Li, T.-H.; Song, Y.-X.; Sun, J.-X.; Chen, X.-W.; Zhao, J.-H.; Li, Y.; Wu, Z.-H.; Gao, P.; et al. The Efficacy and Safety of Celecoxib in Addition to Standard Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Oncol. 2022, 29, 6137-6153. https://doi.org/10.3390/curroncol29090482
Ye S-Y, Li J-Y, Li T-H, Song Y-X, Sun J-X, Chen X-W, Zhao J-H, Li Y, Wu Z-H, Gao P, et al. The Efficacy and Safety of Celecoxib in Addition to Standard Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Current Oncology. 2022; 29(9):6137-6153. https://doi.org/10.3390/curroncol29090482
Chicago/Turabian StyleYe, Shi-Yu, Jia-Yi Li, Teng-Hui Li, Yong-Xi Song, Jing-Xu Sun, Xiao-Wan Chen, Jun-Hua Zhao, Yuan Li, Zhong-Hua Wu, Peng Gao, and et al. 2022. "The Efficacy and Safety of Celecoxib in Addition to Standard Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Current Oncology 29, no. 9: 6137-6153. https://doi.org/10.3390/curroncol29090482
APA StyleYe, S.-Y., Li, J.-Y., Li, T.-H., Song, Y.-X., Sun, J.-X., Chen, X.-W., Zhao, J.-H., Li, Y., Wu, Z.-H., Gao, P., & Huang, X.-Z. (2022). The Efficacy and Safety of Celecoxib in Addition to Standard Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Current Oncology, 29(9), 6137-6153. https://doi.org/10.3390/curroncol29090482




