Abstract
This study aimed to examine the prognostic value of the neutrophil-to-lymphocyte ratio (NLR) and other clinicopathological features in HER2+ MBC patients who received first-line anti-HER2 therapy. A total of 129 patients were assigned to NLR-low and NLR-high groups based on a cutoff value of 3.0 at baseline. Peripheral blood lymphocyte subsets and gene mutations in circulating tumor DNA were analyzed by flow cytometry and Next-generation sequencing, respectively. Survival was evaluated by the Kaplan–Meier method and Cox regression analysis. Of the 129 patients, 77 and 52 were assigned to the NLR-low (≤3) and NLR-high (>3) groups, respectively. Compared with NLR-high patients, the NLR-low patients had significantly longer median progression-free survival (PFS) (11.7 vs. 7.7 months) (p = 0.001, HR = 2.703 95% CI 1.543–4.736 and overall survival (OS) (37.4 vs. 28.7 months) (p = 0.044, HR = 2.254 95% CI 1.024–4.924). Furthermore, this association was independent of metastatic sites or estrogen receptor status. Peripheral blood CD3+ (p = 0.034) and CD4+ (p = 0.010) T cell numbers were significantly higher in the NLR-low group than the NLR-high group. The mutational profile of MBC was generally similar between the two groups. Baseline NLR was a prognostic factor of PFS and OS for patients with HER2+ MBC in the first-line setting. These results may facilitate the selection of patients who will benefit most from anti-HER2 treatment.
1. Introduction
Breast cancer has become the most common cancer in the world. In 2020, there were an estimated 2.3 million newly diagnosed cases, accounting for 11.7% of all cancers. Despite recent improvements in treatment strategies, breast cancer causes approximately 685,000 deaths annually, making it the fifth leading cause of cancer mortality [1]. Breast cancer is a heterogeneous disease with multiple subtypes that differ markedly in their biology and prognosis [2,3]. The proportion of human epidermal growth factor receptor 2 positive (HER2+) breast cancer is about 20–30%. Before anti-HER2 targeted therapy, HER2+ was considered a poor prognostic factor [4].
Age, tumor stage, grade, and hormone receptor status are among the most important prognostic factors for patients with metastatic breast cancer (MBC), which is a multifactorial disease with complex biological behavior. A consensus-validated life expectancy model for MBC has not yet been developed [5]. Previous studies have shown that the immune system plays an important role in anti-HER2 targeted therapy [6]. Among them, antibody-dependent cytotoxicity (ADCC) mediated by natural killer (NK) cells and granulocytes is the main mechanism of trastuzumab (anti-HER2 monoclonal antibody) [7]. Additionally, the abundance of tumor-infiltrating lymphocytes (TILs) significantly correlates with the prognosis of trastuzumab-treated patients with MBC [8], further supporting an important interaction between host immunity and anti-HER2 therapy. The North Central Cancer Treatment Group trial N9831 demonstrated that enriched immune function gene expression in the tumor was a predictor of relapse-free survival in MBC patients receiving chemotherapy with trastuzumab but not in those receiving chemotherapy alone [9]. Preclinical studies have indicated that ADCC contributes to the anti-HER2+ tumor effects of trastuzumab [10]; However, there are few data on the prognostic role of immune cells for survival in HER2-positive breast cancer patients [11].
Neutrophils and lymphocytes are important types of anti-tumor immunity. More and more studies have investigated the prognostic value of neutrophils and lymphocytes in various cancer types, including breast cancer, especially the ratio of neutrophils to lymphocytes (NLR) [12,13]. However, few studies have examined the prognostic significance of NLR in patients, specifically with HER2+ breast cancer [14]. Ulas et al. reported that there was no significant association between disease-free survival (DFS) or overall survival (OS) with NLR in patients with early breast cancer who received adjuvant trastuzumab [15]. While Yao et al. found that NLR has a significant prognostic value regardless of molecular subtype [16]. Thus, additional studies are required before the prognostic value of NLR in HER2+ breast cancer can be clarified.
In the present study, we investigated the relationship between baseline NLR and survival in patients with HER2+ MBC who received trastuzumab as first-line therapy. We also examined the relationship between NLR and various clinicopathologic factors, including the abundance of circulating lymphocyte subsets and gene mutations present in circulating tumor DNA.
2. Methods
2.1. Study Design
We retrospectively identified 129 consecutive female patients who were diagnosed with HER2+ MBC and received first-line anti-HER2-based treatment at Peking University Cancer Hospital between January 2015 and June 2020. All patients had histologically confirmed invasive HER2+ breast cancer. HER2 positivity was centrally confirmed by using an immunohistochemical (IHC) score of 3+ or, for those with an IHC score of 2+, a positive fluorescence in situ hybridization test, in conformity with the American Society of Clinical Oncology/College of American Pathologists HER2 testing in breast cancer guidelines [17].
Exclusion criteria included concurrent infectious disease, autoimmune disease, hematological disorder, or other malignancies; insufficient clinical data; or withdrawal after the first cycle. Treatment efficacy was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [18]. Overall response rate (ORR) was defined as the proportion of patients with a complete response (CR) or partial response (PR) to therapy. Clinical benefit rate (CBR) was defined as the percentage of patients with CR, PR, or stable disease (SD). Progression-free survival (PFS) was defined as the time from treatment initiation to disease progression, death, or loss to follow-up. OS was defined as the time from treatment initiation to death or loss to follow-up.
This study was approved by the Peking University Cancer Hospital Ethical Committee (No. 2016KT47) and was conducted in accordance with the Declaration of Helsinki. Informed consent was waived by the committee because of the retrospective nature of the study.
2.2. Measurement of NLR
Baseline NLR was calculated as the neutrophil count divided by the lymphocyte count in blood samples taken within 7 days before initiation of trastuzumab treatment. Previous work indicated that the NLR range can be large (2.5–4.0), and a standard cutoff value has not yet been agreed upon [19]. In this study, the median value of NLR is 2.87 (range 0.02–14.33), and the mean value was 3.08 ± 2.15. We adopted 3.0 as the cutoff value for the assignment of patients to NLR-low and NLR-high groups based on the value reported by previous studies [20].
2.3. Detection of Peripheral Blood T Lymphocyte Subsets
Peripheral blood samples (200 μL) were incubated with anti-human antibody cocktails in the dark for 10 min at room temperature, and samples were then subjected to hemolysis for an additional 10 min. The cells were centrifuged for 5 min at 1300× g at room temperature and resuspended in 500 μL phosphate-buffered saline for flow cytometry. Antibody cocktails included CD3-PC5/CD4-FITC/CD8-PE (IM1650), CD3-FITC/CD16/CD56-PE (A07735), CD14/16-FITC/CD85k (ILT3)-PE/CD33-PC5 (A23413), CD4-FITC (A007750), CD8-FITC (A07756), CD19-PC5 (A07771), and CD25-PE (A07774) (all from Beckman Coulter, Brea, CA, USA). Flow cytometry was performed using an FC500 and CXP analysis software (both Beckman Coulter). Each analysis included 10,000 gated events.
2.4. Extraction of ctDNA
Cell-free DNA (cfDNA), which includes ctDNA, was extracted from cell-free plasma samples, and gDNA was extracted from purified peripheral blood mononuclear cells (PBMCs). Of the 129 patients, 57 had baseline peripheral blood samples available for cfDNA extraction, and 57 matched PBMC samples were available for gDNA extraction. Briefly, blood samples were centrifuged at 820× g for 10 min, the supernatants were transferred to fresh tubes and re-centrifuged at 16,000× g for 10 min, and the supernatants were stored at −80 °C. cfDNA was extracted using a QIAamp circulating nucleic acid kit (Qiagen, Germantown, MD), and the quantity and quality were checked using a Qubit fluorimeter and Bioanalyzer 2100. Samples with severe gDNA contamination were further processed using bead-based size selection to remove large gDNA fragments. gDNA was extracted from matched PBMC samples. Up to 250 ng gDNA was enzymatically fragmented and stored at −20 °C. Both cfDNA and gDNA were quantified using a LINE1 qPCR assay [21].
2.5. Library Preparation, Capture, and Sequencing
Extracted cfDNA (5–30 ng) or fragmented gDNA (40 ng) were subjected to library construction, including end-repair dA-tailing and adapter ligation. Ligated library fragments with appropriate adapters were amplified by PCR. The amplified DNA libraries were further evaluated using a Bioanalyzer 2100, and samples with sufficient material proceeded to hybrid capture. Library capture was conducted using biotin-labeled DNA probes. In brief, the library was hybridized overnight with the PredicineCARETM 152-gene panel reagents (Huidu Shanghai Medical Sciences Ltd., Shanghai, China) (Supplementary Table S1) and captured with beads. The unbound fragments were washed away, and the enriched fragments were amplified by PCR. Purified products were checked on a Bioanalyzer 2100 and sequenced using a HiSeq X Ten (Illumina, San Diego, CA, USA) and a paired-end 2 × 150 bp sequencing kit (Illumina).
2.6. Statistical Analysis
Statistical tests were selected based on the distribution of variables. Student’s t-tests were used to compare normally distributed variables, and Mann–Whitney U tests were used for non-normally distributed variables. The chi-square test or Fisher’s exact test, as appropriate, was performed to compare clinical and pathological characteristics between two groups stratified by the NLR value (NLR ≤ 3 vs. NLR > 3). Survival curves were obtained using the Kaplan–Meier method and compared using a log-rank test. Univariable and multivariable Cox regression analysis was performed to identify factors that independently influenced survival. The patients’ age, primary breast cancer stage, histological grade, hormone receptor status, disease-free survival, visceral metastasis, number of metastatic sites, and NLR were included in the multivariable analysis. All tests were two-tailed, and p < 0.05 was considered statistically significant. SPSS® software version 22 (IBM Inc., Broadway, NY, USA) was used for the statistical analysis.
3. Results
3.1. Patient Characteristics
A total of 129 patients with HER2+ MBC who received at least two cycles of anti-HER2 targeted treatment as first-line therapy were enrolled in this study. All the patients could evaluate the response rate. Of those, 16 were lost to follow-up, and 50 died. The median follow-up time was 21.0 months (range 2.0–46.0 months). The patients’ clinicopathological characteristics are presented in Table 1. The median age at diagnosis was 51.0 years (range 25–82 years), 63 (48.8%) patients were hormone-receptor-positive, and 89 (69%) patients had visceral metastasis. The median value of NLR is 2.87 (range 0.02–14.33). In total, 77 and 52 patients were assigned to the NLR-low (≤3) and NLR-high (>3) groups, respectively. The two NLR groups had similar clinicopathological features (Table 1).

Table 1.
Clinicopathological characteristics of 129 patients with HER2+ MBC.
3.2. Association between NLR and Response to First-Line Anti-HER2 Treatment
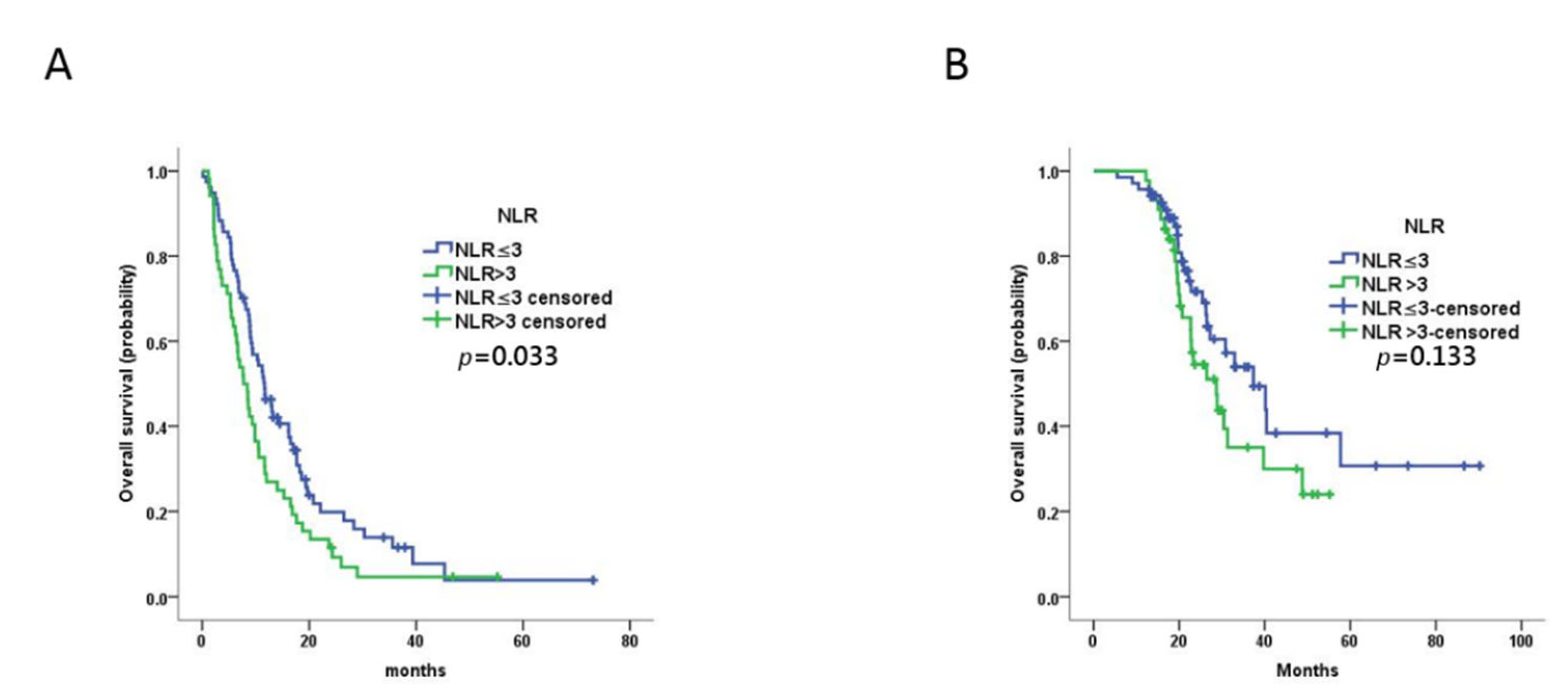
The ORR was 48.8% (63/129), CBR was 82.9% (107/129), PR was 48.8% (63/129), SD was 34.1% (44/129), and progressive disease (PD) was 17.1% (22/129). The ORR and CBR were not significantly different between the NLR-low and NLR-high groups (p = 0.828 and p = 0.309, respectively; Table 2). The median PFS of all patients was 9.9 months (95% confidence interval (CI) 8.0–11.7 months). The median PFS for the NLR-low group (11.7 months, 95% CI 8.9–14.4 months) was significantly longer than that for the NLR-high group (7.7 months, 95% CI 5.5–10.0 months; p = 0.033) (Figure 1). Univariable analysis revealed that PFS was not associated with any of the other variables evaluated (age at cancer diagnosis, primary breast cancer stage, grade, hormone receptor expression, DFS, visceral metastasis, number of metastatic sites). Multivariable analysis confirmed the prognostic value of NLR (p = 0.001, hazard ratio (HR) = 2.703 95% CI 1.543–4.736) (Table 3).

Table 2.
Correlations between baseline NLR and treatment outcomes in patients with MBC.
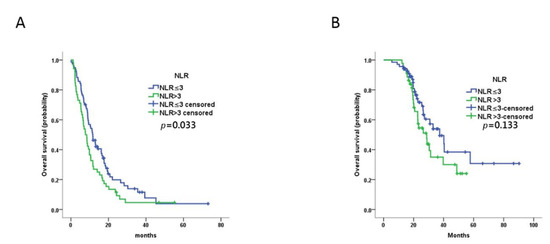
Figure 1.
Prognostic value of baseline NLR in MBC patients treated with trastuzumab as first-line therapy. (A,B) Kaplan–Meier plots of progression-free survival (A) and overall survival (B) for patients assigned to NLR-high (n = 52) and NLR-low (n = 77) groups at baseline.

Table 3.
Univariable and multivariable analysis of progression-free survival.
The median OS for all patients was 30.8 months (95% CI 22.0–39.6 months). In contrast to PFS, there was no significant difference between the OS of the NLR-low group (37.4 months, 95% CI 25.7–49.0 months) and the NLR-high group (28.7 months, 95% CI 21.4–36.1 months) in univariable analysis (p = 0.133) (Figure 1). However, NLR (p = 0.044, HR = 2.254 95% CI 1.024–4.924) and pathologic grade (p = 0.023, HR = 2.712 95% CI 1.149–6.402) were prognostic factors for OS in multivariable analysis (Table 4).

Table 4.
Univariable and multivariable analysis of overall survival.
3.3. Association between NLR and Peripheral Blood T Lymphocyte Subsets
We previously showed that the abundance of peripheral blood CD8 + CD28+ cytotoxic T lymphocytes is predictive of PFS in breast cancer, particularly in patients with HER2+ breast cancer who received anti-HER2 therapy [22]. Therefore, we compared the distribution of several peripheral blood lymphocyte subsets between the NLR-low and NLR-high groups. Patients in the NLR-low had a significantly higher percentage of CD3+ T cells (p = 0.034) and CD4+ T cells (p = 0.010) than patients in the NLR-high group. However, the abundance of the remaining lymphocyte subsets, including CD8+ CD28+ cells, did not differ significantly between the groups (Table 5).

Table 5.
Peripheral lymphocyte subsets percentage in MBC patients with low and high baseline NLR.
3.4. Association between NLR and Gene Mutations
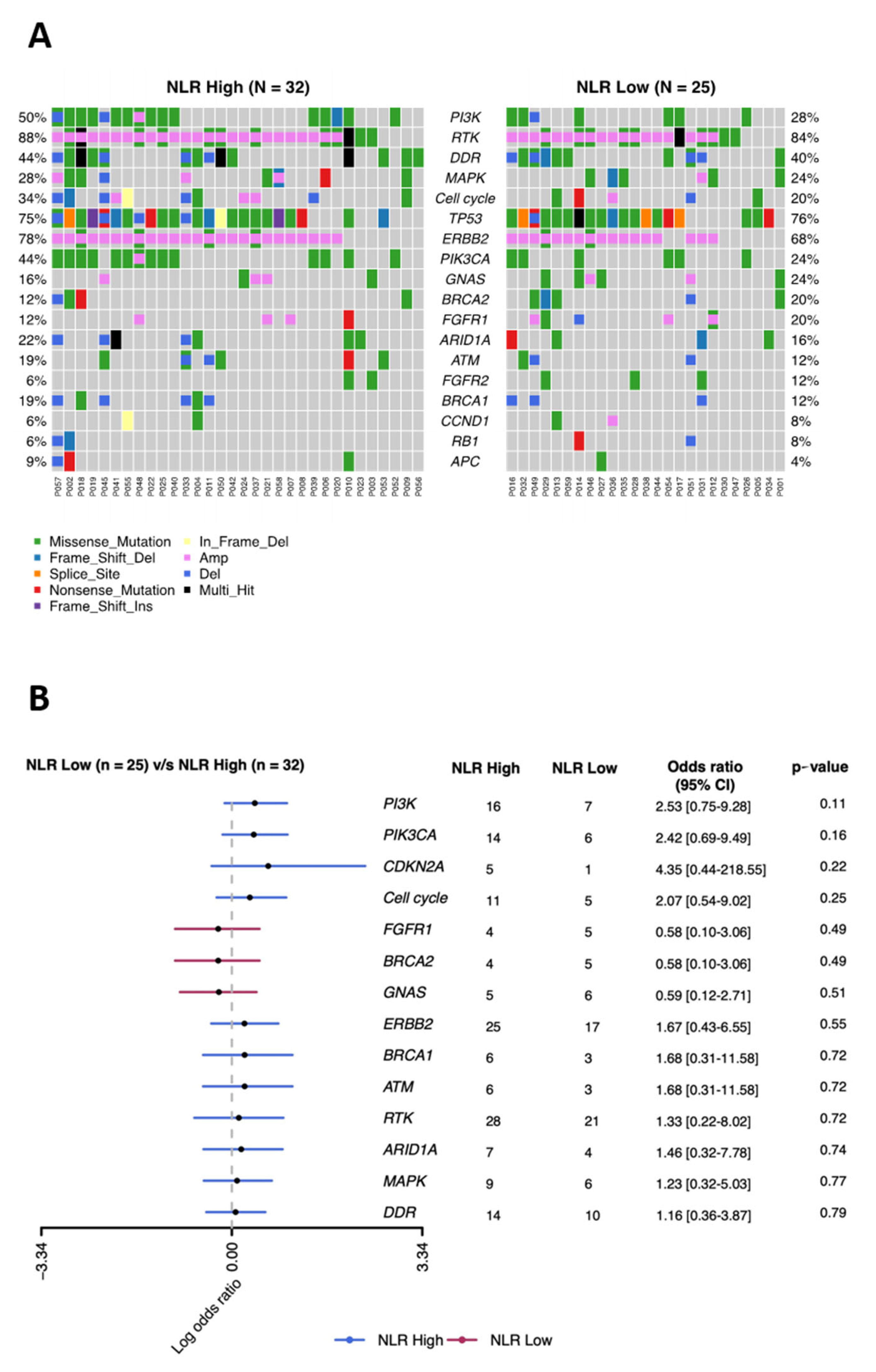
To determine whether genomic alterations in MBC tumors correlated with patient NLR and to identify potential genomic markers related to NLR, we performed a PredicineCARE screen of plasma cfDNA from 59 patients. This screen analyzes 152 cancer-related genes by next-generation sequencing and has broad genomic coverage. Overall, the genomic profile of cfDNA from the NLR-low and NLR-high groups were similar, with frequent mutations identified in components of the phosphoinositide 3-kinase (PI3K) signaling pathway (AKT1, AKT2, MTOR, PIK3CA, PIK3CB, PIK3CD, PIK3R1, PTEN, RHEB, STK11, TSC1, and TSC2) (50% in the NLR-low group vs. 28% in the NLR-high group), receptor tyrosine kinases (ABL1, EGFR, ERBB2, ERBB3, ERBB4, PDGFRA, PDGFRB, MET, FGFR1, FGFR2, FGFR3, FGFR4, FLT3, ALK, RET, ROS1, KIT, IGF1R, NTRK1, NTRK2, and NTRK3) (88% vs. 84%), DNA damage repair pathway (ATM, ATR, BRCA1, BRCA2, CHEK2, MLH1, MSH2, MSH6, MUTYH, PALB2, PMS2, POLD1, POLE, RAD51B, RAD51C, RAD51D, XPC, and XRCC1) (44% vs. 40%), mitogen activated protein kinase (MAPK) signaling pathway (PTPN11, KRAS, HRAS, NRAS, RIT1, ARAF, BRAF, RAF1, MAP2K1, MAP2K2, MAPK1, and NF1) (28% vs. 24%), and cell cycle pathway (CDKN2A, CDKN2B, CCND1, CCND2, CCND3, CCNE1, CDK4, CDK6, RB1, and TOP2A) (34% vs. 20%) (Figure 2A,B). The pathway mutation was defined as mutated if any of the genes in the given pathway was mutated. Although none of the mutation frequencies differed significantly between the NLR-low and NLR-high groups, several mutations were slightly more common in the NLR-high group; these included genes in the PI3K pathway (odds ratio (OR) = 2.53) and cell cycle pathway (OR = 2.07), as well as the PIK3CA, which encodes PI3K catalytic subunit alpha (OR = 2.42), and CDKN2A, which encodes cyclin-dependent kinase inhibitor 2A (OR = 4.35) (Figure 2B).

Figure 2.
Comparison of the mutational landscape of 152 tumor-related genes in MBC patients with low and high baseline NLR. (A) Distribution of single nucleotide variants and copy number variants in the indicated genes with mutation rates of genes ≥5%. (B) Relative enrichment of key genes in the NLR-high versus the NLR-low group.
4. Discussion
This study demonstrated that high pretreatment NLR was associated with significantly worse prognosis in HER2+ MBC patients receiving first-line anti-HER2 therapy compared with patients with low NLR. Patients with high baseline NLR also had lower baseline CD3+ T cell and CD4+ T cell levels; however, there were no significant differences between the two groups with respect to variation in sequence or copy number of 152 genes with relevance to cancer.
4.1. NLR and Survival
NLR has a predictive prognostic value in some solid malignant tumors [23], and baseline NLR measured prior to treatment initiation can predict the survival of patients with early breast cancer [13,20,24]. A meta-analysis of eight studies showed that elevated NLR was associated with a significantly lower overall survival in patients with breast cancer [19]. Previous studies have also shown a significant correlation between NLR and survival in patients with MBC [25,26,27,28,29,30]. However, we consider that the results of those studies should be interpreted with caution because not all molecular subtypes of breast cancer were represented, and the heterogeneity of the study populations was very large.
4.2. NLR and HER2+ Breast Cancer Subtypes
The predictive value of NLR in breast cancer of different molecular subtypes is controversial. Some studies have shown that high NLR is significantly correlated with poor prognosis in triple-negative breast cancer (TNBC) but not in luminal A-like, luminal B-like, or HER2-enriched subtypes [8]. The predictive prognostic value of TILs has been established for TNBC [31], but whether peripheral blood neutrophils or lymphocytes have similar value in TNBC is not known. Noh et al. [13] found a significant prognostic value of NLR in luminal subtype breast cancer (ER+ or PR+, HER2−), whereas Yao et al. [16] found that NLR has a significant prognostic value regardless of luminal type or TNBC. Therefore, the prognostic significance of NLR in HER2+ breast cancers remains unclear.
Different subtypes of breast cancer are associated with immune heterogeneity. TNBC and HER2+ breast cancers with aggressive biological behavior have high genomic instability and tumor mutation burden, both of which promote the production of tumor neoantigens and increase antitumor immune activity. In addition, HER2 itself acts as a tumor-associated neoantigen in HER2+ breast cancer. Cytotoxic therapy and anti-HER2 targeted therapy can further activate the immune system through immunogenic cell death and ADCC, respectively [6]. The antitumor effects of anti-HER2 antibodies are mediated, at least in part, through ADCC by innate immune cells [18]. The binding of anti-HER2 antibodies to HER2 induces NK cell-mediated ADCC, which is followed by induction of tumor-specific cytotoxicity mediated by cells of the adaptive immune system [32].
Of the two previous studies examining NLR and HER2+ breast cancer [30,33], one found that NLR correlated with PFS and OS in patients who received T-DM1 [30], and the second study found that improved PFS was not significantly associated with NLR but absolute lymphocyte count ≥1500/μL. In the present study, we also found that NLR was an independent predictor of PFS and OS in patients with HER2+ MBC undergoing first-line anti-HER2 therapy. Therefore, further study of NLR in breast cancer patients is warranted, especially studies of different treatment lines and drugs.
4.3. Optimal Cutoff Point of the NLR
The optimal NLR for determining associations with survival in patients with HER2-positive MBC is still unclear. Azab et al. [34] found no significant difference between the three lower quartiles of NLR and mortality in patients with breast cancer, suggesting that there may be a threshold value at which NLR correlates with mortality. In the past, receiver operating characteristic curve analysis has been used to determine the optimal NLR value for predicting survival, but no consensus value has yet been identified [13,16]. In the present study, with a limited number of subjects, we selected 3.0 as the cutoff value based on a previous study [20].
4.4. NLR and Lymphocyte Subsets
We previously showed that circulating peripheral CD8+ CD28+ T cell ratio could predict PFS in HER2+ MBC patients receiving anti-HER2 therapy (13.1 vs. 5.6 months, p = 0.001) [22], prompting us to further explore the relationship between NLR and the abundance of additional peripheral blood lymphocyte subsets in the present study. We found that NLR-low patients had higher levels of peripheral CD3+ T cells and CD4+ T cells but not of any other lymphocyte subsets examined compared with NLR-high patients.
T cells play a crucial role in the antitumor immune response, and the level of tumor-infiltrating T cells has been correlated with patient survival in some cancers [35], and CD4+ T cells are generally mediated antitumor effects by regulating the activity of other cells by cytokine production, and by establishing long-term antitumor memory [36]. CD4+ T cells can activate monocytes/macrophages, NK cells, and specific CD8+ cytotoxic T cells and are thus pivotal to the antitumor response, suggesting an explanation for the significant association between circulating CD4+ cells and OS previously demonstrated in some cancers [37]. In addition, the HER2-specific response of circulating CD4+ T cells after anti-HER2 treatment has been correlated with HER2+ breast cancer recurrence. An anti-HER2 response could be detected in vitro by measuring the production of interferon-γ (IFN–γ), interleukin (IL)-4, and IL-10 after stimulation of PBMCs with HER2 peptides (REFS?). Datta J et al. found that patients with an anti-HER2 CD4+ T cell response had a higher DFS rate than those lacking a CD4+ T cell response [38]. Our finding of elevated CD4+ T cells in NLR-low patients, who also had a longer median PFS compared with NLR-high patients, is thus consistent with the possibility that NLR-low patients may have mounted a more robust antitumor response than NLR-high patients.
4.5. NLR and ctDNA
Analysis of ctDNA has proven to be useful not only for examining the genomic status of tumors but also for shedding light on other aspects of the disease, including the immune status. Pedersen et al. [39] reported that the baseline ctDNA level was closely related to the therapeutic effect of immune checkpoint inhibitors in patients with metastatic melanoma. DNA can stimulate the immune response via regulation of IFN and other proinflammatory mediators in immune cells in a manner dependent on structure and sequence (REFS), and immune cell activation can be observed with DNA alone or in complex with other molecules [40]. The large number of nucleic acid receptors expressed by immune cells attests to the important role of DNA in the innate immune system [41]. In our study, however, we found no significant genomic differences between ctDNA from the NLR-high and -low patient groups. However, several signaling and cell cycle pathway genes were slightly more frequently mutated in the NLR-high group, suggesting that additional investigations with a larger sample size will be of interest.
4.6. Limitations
First, this was a retrospective study, and the main limitations are retrospective data collection and the single-center design. Second, the sample size was relatively small for survival analysis. Third, the critical cutoff value for the NLR was based on findings from a previous study. Larger-scale confirmatory studies will be needed to validate the predictive role of NLR in HER2+ MBC patients undergoing first-line anti-HER2 therapy. Finally, we analyzed the data employing a single baseline NLR value, and it is possible that dynamic monitoring of NLR throughout treatment may be helpful in determining its predictive value.
5. Conclusions
Our results suggest that baseline NLR, an easily measured biomarker, is an independent predictor of PFS and OS for patients with HER2+ MBC undergoing first-line anti-HER2 treatment. NLR-low status in this patient population at baseline may reflect an enhanced activity of the immune system. These findings are a useful reminder that NLR should be taken into consideration when making treatment decisions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29090483/s1, Table S1: Gene list of PredicineCARETM 152-gene panel.
Author Contributions
B.S., X.L. and H.L.: concept and design of the article; interpretation of data; drafted and revised the article; B.S., X.L., H.L., G.S., L.D., H.J., Y.Y., R.Z., R.R., J.Z., Y.L., H.W. and J.W.: acquisition and analysis of data; revised the article critically for important intellectual content; B.S., X.L., H.L., G.S., L.D., H.J., Y.Y., R.Z., R.R., J.Z., Y.L., H.W. and J.W.: approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Peking University Cancer Hospital Ethical Committee (No. 2016KT47) and was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was waived by the committee because of the retrospective nature of the study.
Data Availability Statement
The data set supporting the results of this article are included within the article and Supplementary Materials. Other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors wish to thank the patients who gave their willingness to get enrolled in the present study. The authors also want to thank Huidu Shanghai Medical Sciences Ltd. for the processing of row data regarding NGS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Arciero, C.A.; Guo, Y.; Jiang, R.; Behera, M.; O’Regan, R.; Peng, L.; Li, X. ER(+)/HER2(+) Breast Cancer Has Different Metastatic Patterns and Better Survival Than ER(-)/HER2(+) Breast Cancer. Clin. Breast Cancer 2019, 19, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Meisel, J.; Jiang, R.; Behera, M.; Peng, L. Validation of the newly proposed American Joint Committee on Cancer (AJCC) breast cancer prognostic staging group and proposing a new staging system using the National Cancer Da-tabase. Breast Cancer Res. Treat. 2018, 171, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Pegram, M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin. Oncol. 2001, 28, 13–19. [Google Scholar] [CrossRef]
- Masood, S. Prognostic/Predictive Factors in Breast Cancer. Clin. Lab. Med. 2005, 25, 809–825. [Google Scholar] [CrossRef]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef]
- Denkert, C.; Darb-Esfahani, S.; Loibl, S.; Anagnostopoulos, I.; Johrens, K. Anti-cancer immune response mechanisms in neo-adjuvant and targeted therapy. Semin. Immunopathol. 2011, 33, 341–351. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Perez, E.A.; Thompson, E.A.; Ballman, K.V.; Anderson, S.K.; Asmann, Y.W.; Kalari, K.; Eckel-Passow, J.E.; Dueck, A.C.; Tenner, K.S.; Jen, J.; et al. Genomic Analysis Reveals That Immune Function Genes Are Strongly Linked to Clinical Outcome in the North Central Cancer Treatment Group N9831 Adjuvant Trastuzumab Trial. J. Clin. Oncol. 2015, 33, 701–708. [Google Scholar] [CrossRef]
- Kute, T.E.; Savage, L.; Stehle, J.R.; Kim-Shapiro, J.W.; Blanks, M.J.; Wood, J.; Vaughn, J.P. Breast tumor cells isolated from in vitro resistance to trastuzumab remain sensitive to trastuzumab anti-tumor effects in vivo and to ADCC killing. Cancer Immunol. Immunother. 2009, 58, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; De Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Machiels, J.P.; Coulie, P.G.; Berliere, M.; Poncelet, A.J.; Tombal, B.; De Kock, M. Neutrophil:lymphocyte ratio and in-traoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann. Surg. Oncol. 2013, 20 (Suppl. S3), S650–S660. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Eomm, M.; Han, A. Usefulness of Pretreatment Neutrophil to Lymphocyte Ratio in Predicting Disease-Specific Survival in Breast Cancer Patients. J. Breast Cancer 2013, 16, 55–59. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, E.Y.; Yun, J.S.; Park, Y.L.; Do, S.-I.; Chae, S.W.; Park, C.H. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer 2018, 18, 938. [Google Scholar] [CrossRef]
- Ulas, A.; Avci, N.; Kos, T.; Cubukcu, E.; Olmez, O.F.; Bulut, N.; Degirmenci, M. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J. BUON 2015, 20, 714–722. [Google Scholar]
- Fu, P.; Yao, M.; Liu, Y.; Jin, H.; Liu, X.; Lv, K.; Wei, H.; Du, C.; Wang, S.; Wei, B. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014, 7, 1743–1752. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Q.; Pan, Y.; He, B.; Ying, H.; Sun, H.; Wang, S. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio 2015, 5, 502–507. [Google Scholar] [CrossRef]
- Pistelli, M.; De Lisa, M.; Ballatore, Z.; Caramanti, M.; Pagliacci, A.; Battelli, N.; Ridolfi, F.; Santoni, M.; Maccaroni, E.; Bracci, R.; et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer 2015, 15, 195. [Google Scholar] [CrossRef]
- Kohli, M.; Tan, W.; Zheng, T.; Wang, A.; Montesinos, C.; Wong, C.; Du, P.; Jia, S.; Yadav, S.; Horvath, L.G.; et al. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. EBioMedicine 2020, 54, 102728. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-R.; Yu, J.-J.; Song, G.-H.; Di, L.-J.; Jiang, H.-F.; Yan, Y.; Liang, X.; Zhang, R.-Y.; Ran, R.; Wang, J.; et al. Peripheral cytotoxic T lymphocyte predicts first-line progression free survival in HER2-positive advanced breast cancer. Breast 2020, 55, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Amir, E. Prognostic role of neutro-phil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Shah, N.; Radbel, J.; Tan, P.; Bhatt, V.; Vonfrolio, S.; Habeshy, A.; Picon, A.; Bloom, S. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med. Oncol. 2013, 30, 432. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Sangai, T.; Sakakibara, M.; Sakakibara, J.; Ishigami, E.; Hayama, S.; Nakagawa, A.; Masuda, T.; Tabe, S.; Nagashima, T. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol. Clin. Oncol. 2016, 6, 266–270. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Araki, K.; Bun, A.; Ozawa, H.; Fujimoto, Y.; Higuchi, T.; Nishimukai, A.; Kira, A.; Imamura, M.; Takatsuka, Y.; et al. Significant Association Between Low Baseline Neutrophil-to-Lymphocyte Ratio and Improved Progression-free Survival of Patients With Locally Advanced or Metastatic Breast Cancer Treated With Eribulin But Not With Nab-Paclitaxel. Clin. Breast Cancer 2018, 18, 400–409. [Google Scholar] [CrossRef]
- Takuwa, H.; Tsuji, W.; Yamamoto, Y.; Shintaku, M.; Yotsumoto, F. Low neutrophil-lymphocyte ratio correlates with extended survival in patients with metastatic breast cancer who achieved clinically complete response following multidisciplinary therapy: A retrospective study. Oncol. Lett. 2018, 15, 6681–6687. [Google Scholar] [CrossRef]
- Iimori, N.; Kashiwagi, S.; Asano, Y.; Goto, W.; Takada, K.; Takahashi, K.; Ohira, M. Clinical Significance of the Neutro-phil-to-Lymphocyte Ratio in Endocrine Therapy for Stage IV Breast Cancer. In Vivo 2018, 32, 669–675. [Google Scholar]
- Vernieri, C.; Mennitto, A.; Prisciandaro, M.; Huber, V.; Milano, M.; Rinaldi, L.; de Braud, F. The neutrophil-to-lymphocyte and plate-let-to-lymphocyte ratios predict efficacy of platinum-based chemotherapy in patients with metastatic triple negative breast cancer. Sci. Rep. 2018, 8, 8703. [Google Scholar] [CrossRef]
- Imamura, M.; Morimoto, T.; Egawa, C.; Fukui, R.; Bun, A.; Ozawa, H.; Miyagawa, Y.; Fujimoto, Y.; Higuchi, T.; Miyoshi, Y. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci. Rep. 2019, 9, 1811. [Google Scholar] [CrossRef]
- Balkenhol, M.C.; Ciompi, F.; Swiderska-Chadaj, Z.; van de Loo, R.; Intezar, M.; Otte-Holler, I.; Geijs, D.; Lotz, J.; Weiss, N.; de Bel, T.; et al. Optimized tumour infiltrating lymphocyte assessment for triple negative breast cancer prognostics. Breast 2021, 56, 78–87. [Google Scholar] [CrossRef]
- Kroemer, G.; Senovilla, L.; Galluzzi, L.; Andre, F.; Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 2015, 21, 1128–1138. [Google Scholar] [CrossRef]
- Araki, K.; Ito, Y.; Fukada, I.; Kobayashi, K.; Miyagawa, Y.; Imamura, M.; Kira, A.; Takatsuka, Y.; Egawa, C.; Suwa, H.; et al. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer 2018, 18, 982. [Google Scholar] [CrossRef]
- Azab, B.; Bhatt, V.; Phookan, J.; Murukutla, S.; Kohn, N.; Terjanian, T.; Widmann, W.D. Usefulness of the Neutrophil-to-Lymphocyte Ratio in Predicting Short- and Long-Term Mortality in Breast Cancer Patients. Ann. Surg. Oncol. 2011, 19, 217–224. [Google Scholar] [CrossRef]
- Martinet, O.; Divino, C.; Zang, Y.; Gan, Y.; Mandeli, J.; Thung, S.; Pan, P.-Y.; Chen, S.-H. T cell activation with systemic agonistic antibody versus local 4-1BB ligand gene delivery combined with interleukin-12 eradicate liver metastases of breast cancer. Gene Ther. 2002, 9, 786–792. [Google Scholar] [CrossRef][Green Version]
- Cintolo, J.A.; Datta, J.; Mathew, S.J.; Czerniecki, B.J. Dendritic cell-based vaccines: Barriers and opportunities. Futur. Oncol. 2012, 8, 1273–1299. [Google Scholar] [CrossRef]
- Ossendorp, F.; Toes, R.; Offringa, R.; van der Burg, S.; Melief, C. Importance of CD4+ T helper cell responses in tumor immunity. Immunol. Lett. 2000, 74, 75–79. [Google Scholar] [CrossRef]
- Datta, J.; Fracol, M.; McMillan, M.T.; Berk, E.; Xu, S.; Goodman, N.; Czerniecki, B.J. Association of Depressed Anti-HER2 T-Helper Type 1 Response With Recurrence in Patients With Completely Treated HER2-Positive Breast Cancer: Role for Immune Monitoring. JAMA Oncol. 2016, 2, 242–246. [Google Scholar] [CrossRef]
- Pedersen, J.G.; Madsen, A.T.; Gammelgaard, K.R.; Aggerholm-Pedersen, N.; Sørensen, B.S.; Øllegaard, T.H.; Jakobsen, K.R. Inflammatory Cytokines and ctDNA Are Biomarkers for Progression in Advanced-Stage Melanoma Patients Receiving Checkpoint Inhibitors. Cancers 2020, 12, 1414. [Google Scholar] [CrossRef]
- Pisetsky, D.S. The origin and properties of extracellular DNA: From PAMP to DAMP. Clin. Immunol. 2012, 144, 32–40. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T.; Akira, S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoim-munity. Immunol. Rev. 2011, 243, 61–73. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).