The Role of Glucocorticoids in Breast Cancer Therapy
Abstract
:1. Introduction
2. Genomic Effects of Glucocorticoids
3. Non-Genomic Effects of Glucocorticoids
4. Glucocorticoids and the Circadian Cycle
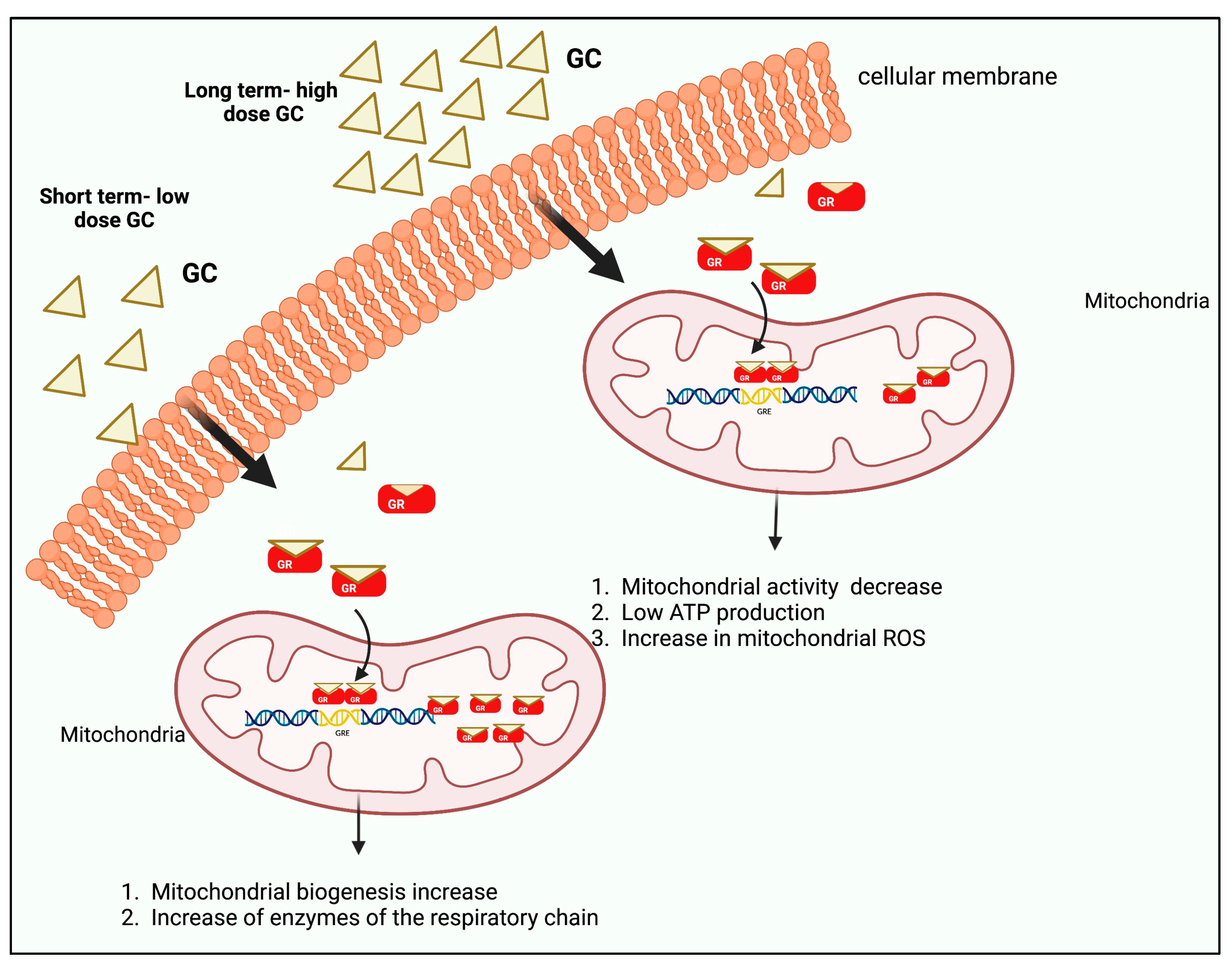
5. Glucocorticoid Effects on Mitochondria
6. Glucocorticoids and Cancer
7. Glucocorticoids and Their Receptors in Breast Cancer
Glucocorticoid Receptors in Triple Negative Breast Cancer
8. Glucocorticoids, Glucocorticoid Receptors, and the Treatment of Triple Negative Breast Cancer
9. Glucocorticoids and Drug resistance in Triple Negative Breast Cancer
10. The Role of the GR as a Biomarker in Breast Cancer Progression
11. Future Perspectives
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunt, H.J.; Belanoff, J.K.; Walters, I.; Gourdet, B.; Thomas, J.; Barton, N.; Unitt, J.; Phillips, T.; Swift, D.; Eaton, E. Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexah ydro-1H-pyrazolo [3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist. J. Med. Chem. 2017, 60, 3405–3421. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, Y. Glucocorticoids are double-edged sword in the treatment of COVID-19 and cancers. Int. J. Biol. Sci. 2021, 17, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volden, P.A.; Conzen, S.D. The influence of glucocorticoid signaling on tumor progression. Brain Behav. Immun. 2013, 30, S26–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunleavy, K.; Pittaluga, S.; Maeda, L.S.; Advani, R.; Chen, C.C.; Hessler, J.; Steinberg, S.M.; Grant, C.; Wright, G.; Varma, G.; et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med. 2013, 368, 1408–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noureddine, L.M.; Tredan, O.; Hussein, N.; Badran, B.; Le Romancer, M.; Poulard, C. Glucocorticoid Receptor: A Multifaceted Actor in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 4446. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [Green Version]
- Hammond, G.L. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef] [Green Version]
- Scherholz, M.L.; Schlesinger, N.; Androulakis, I.P. Chronopharmacology of glucocorticoids. Adv. Drug Deliv. Rev. 2019, 151–152, 245–261. [Google Scholar] [CrossRef]
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. Semin. Fetal Neonatal. Med. 2019, 24, 170–175. [Google Scholar] [CrossRef]
- Serra, H.A.; Roganovich, J.M.; Rizzo, L.F. [Glucocorticoids: Examples of translational medicine; from molecular aspects to bedside]. Medicina (B Aires) 2012, 72, 158–170. [Google Scholar] [PubMed]
- Draper, N.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charmandari, E.; Kino, T.; Chrousos, G.P. Glucocorticoid Receptor. Encyclopedia of Endocrine Diseases; NIH/National Institute of Child Health and Human Development: Bethesda, MD, USA, 2004; pp. 229–234. [Google Scholar] [CrossRef]
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, K.R. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 2017, 18, 159–174. [Google Scholar] [CrossRef]
- Pratt, W.B.; Silverstein, A.M.; Galigniana, M.D. A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37. Cell Signal. 1999, 11, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Croxtall, J.D.; van Hal, P.T.; Choudhury, Q.; Gilroy, D.W.; Flower, R.J. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br. J. Pharmacol. 2002, 135, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, K.M.; Kannai, A.; Sasano, H. Possible roles for glucocorticoid signalling in breast cancer. Mol. Cell. Endocrinol. 2018, 466, 38–50. [Google Scholar] [CrossRef]
- Louw, A. GR Dimerization and the Impact of GR Dimerization on GR Protein Stability and Half-Life. Front. Immunol. 2019, 10, 1693. [Google Scholar] [CrossRef] [Green Version]
- Hudson, W.H.; Youn, C.; Ortlund, E.A. The structural basis of direct glucocorticoid-mediated transrepression. Nat. Struct. Mol. Biol. 2013, 20, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Herr, I.; Gassler, N.; Friess, H.; Buchler, M.W. Regulation of differential pro- and anti-apoptotic signaling by glucocorticoids. Apoptosis 2007, 12, 271–291. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr. Dev. 2013, 24, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Scheschowitsch, K.; Leite, J.A.; Assreuy, J. New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Front. Endocrinol. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, C.S.; de Castro, M. Generalized and tissue specific glucocorticoid resistance. Mol. Cell. Endocrinol. 2021, 530, 111277. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, W.M., 3rd; Honeycutt, J.L.; Deck, C.A.; Borski, R.J. Nongenomic glucocorticoid effects and their mechanisms of action in vertebrates. Int. Rev. Cell. Mol. Biol. 2019, 346, 51–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Watson, C.S.; Gametchu, B. Association of the glucocorticoid receptor alternatively-spliced transcript 1A with the presence of the high molecular weight membrane glucocorticoid receptor in mouse lymphoma cells. J. Cell. Biochem. 1999, 74, 430–446. [Google Scholar] [CrossRef]
- Vernocchi, S.; Battello, N.; Schmitz, S.; Revets, D.; Billing, A.M.; Turner, J.D.; Muller, C.P. Membrane glucocorticoid receptor activation induces proteomic changes aligning with classical glucocorticoid effects. Mol. Cell. Proteom. 2013, 12, 1764–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, L.; Berry, A.; Ohanian, V.; Ohanian, J.; Garside, H.; Ray, D. Caveolin mediates rapid glucocorticoid effects and couples glucocorticoid action to the antiproliferative program. Mol. Endocrinol. 2008, 22, 1320–1330. [Google Scholar] [CrossRef] [Green Version]
- Spies, C.M.; Schaumann, D.H.; Berki, T.; Mayer, K.; Jakstadt, M.; Huscher, D.; Wunder, C.; Burmester, G.R.; Radbruch, A.; Lauster, R.; et al. Membrane glucocorticoid receptors are down regulated by glucocorticoids in patients with systemic lupus erythematosus and use a caveolin-1-independent expression pathway. Ann. Rheum. Dis. 2006, 65, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B.; Hirschelmann, R. Dexamethasone megadoses stabilize rat liver lysosomal membranes by non-genomic and genomic effects. Pharm. Res. 2000, 17, 1489–1493. [Google Scholar] [CrossRef]
- Bartholome, B.; Spies, C.M.; Gaber, T.; Schuchmann, S.; Berki, T.; Kunkel, D.; Bienert, M.; Radbruch, A.; Burmester, G.R.; Lauster, R.; et al. Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB J. 2004, 18, 70–80. [Google Scholar] [CrossRef]
- Hafezi-Moghadam, A.; Simoncini, T.; Yang, Z.; Limbourg, F.P.; Plumier, J.C.; Rebsamen, M.C.; Hsieh, C.M.; Chui, D.S.; Thomas, K.L.; Prorock, A.J.; et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 2002, 8, 473–479. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Aoki, Y.; Katahira, M.; Oiso, Y.; Saito, H. Non-genomic mechanisms of glucocorticoid inhibition of adrenocorticotropin secretion: Possible involvement of GTP-binding protein. Biochem. Biophys. Res. Commun. 1997, 235, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, I.; Moutsatsou, P. Mitochondrial Glucocorticoid Receptors and Their Actions. Int. J. Mol. Sci. 2021, 22, 6054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Sheng, C.Q.; Liu, L.; Li, Z.; Wang, Y.; Zhou, J.R.; Jing, Z.P.; Chen, Y.Z.; Jiang, C.L. A novel strategy for development of glucocorticoids through non-genomic mechanism. Cell. Mol. Life Sci. 2011, 68, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Strehl, C.; Buttgereit, F. Optimized glucocorticoid therapy: Teaching old drugs new tricks. Mol. Cell. Endocrinol. 2013, 380, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mitre-Aguilar, I.B.; Cabrera-Quintero, A.J.; Zentella-Dehesa, A. Genomic and non-genomic effects of glucocorticoids: Implications for breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 1–10. [Google Scholar] [PubMed]
- Thiebaut, C.; Vlaeminck-Guillem, V.; Tredan, O.; Poulard, C.; Le Romancer, M. Non-genomic signaling of steroid receptors in cancer. Mol. Cell. Endocrinol. 2021, 538, 111453. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanoglu, B.; Aysal, A.; Kececi, S.D.; Ekmekci, S.; Erdogdu, I.H.; Ertunc, O.; Gundogdu, B.; Talu, C.K.; Sahin, Y.; Toper, M.H. A Nobel-Winning Scientist: Aziz Sancar and the Impact of his Work on the Molecular Pathology of Neoplastic Diseases. Turk Patoloji Derg. 2021, 37, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Olejniczak, I.; Oster, H.; Ray, D.W. Glucocorticoid circadian rhythms in immune function. Semin. Immunopathol. 2022, 44, 153–163. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef]
- Walker, J.J.; Spiga, F.; Gupta, R.; Zhao, Z.; Lightman, S.L.; Terry, J.R. Rapid intra-adrenal feedback regulation of glucocorticoid synthesis. J. R. Soc. Interface 2015, 12, 20140875. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Bever, S.R.; McKim, D.B.; Godbout, J.P.; Sheridan, J.F.; Obrietan, K.; Pyter, L.M. Mammary tumors compromise time-of-day differences in hypothalamic gene expression and circadian behavior and physiology in mice. Brain Behav. Immun. 2019, 80, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Mitre-Aguilar, I.B.; Barrios-Garcia, T.; Ruiz-Lopez, V.M.; Cabrera-Quintero, A.J.; Mejia-Dominguez, N.R.; Ventura-Gallegos, J.L.; Moreno-Mitre, D.; Aranda-Gutierrez, A.; Mejia-Rangel, J.; Escalona-Guzman, A.R.; et al. Glucocorticoid-dependent expression of IAP participates in the protection against TNF-mediated cytotoxicity in MCF7 cells. BMC Cancer 2019, 19, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- López, J.A.V. Ritmo circadiano: El reloj maestro. Alteraciones que comprometen el estado de sueño y vigilia en el área de la salud. Morfolia 2013, 5, 16–35. [Google Scholar]
- Butz, H.; Patocs, A. Mechanisms behind context-dependent role of glucocorticoids in breast cancer progression. Cancer Metastasis Rev. 2022, 41, 803–832. [Google Scholar] [CrossRef]
- Annett, S.; Fox, O.W.; Vareslija, D.; Robson, T. Dexamethasone promotes breast cancer stem cells in obese and not lean mice. Pharmacol. Res. Perspect. 2022, 10, e00923. [Google Scholar] [CrossRef]
- Hadadi, E.; Acloque, H. Role of circadian rhythm disorders on EMT and tumour-immune interactions in endocrine-related cancers. Endocr. Relat. Cancer 2021, 28, R67–R80. [Google Scholar] [CrossRef]
- Jensen, L.D.; Oliva, D.; Andersson, B.A.; Lewin, F. A multidisciplinary perspective on the complex interactions between sleep, circadian, and metabolic disruption in cancer patients. Cancer Metastasis Rev. 2021, 40, 1055–1071. [Google Scholar] [CrossRef]
- Allende, S.; Medina, J.L.; Spiegel, D.; Zeitzer, J.M. Evening salivary cortisol as a single stress marker in women with metastatic breast cancer. Psychoneuroendocrinology 2020, 115, 104648. [Google Scholar] [CrossRef]
- Abercrombie, H.C.; Giese-Davis, J.; Sephton, S.; Epel, E.S.; Turner-Cobb, J.M.; Spiegel, D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology 2004, 29, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benna, C.; Helfrich-Forster, C.; Rajendran, S.; Monticelli, H.; Pilati, P.; Nitti, D.; Mocellin, S. Genetic variation of clock genes and cancer risk: A field synopsis and meta-analysis. Oncotarget 2017, 8, 23978–23995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Santana, S.; Morell, S.; Leon, J.; Carazo-Gallego, A.; Jimenez-Lopez, J.C.; Morell, M. An Overview of the Polymorphisms of Circadian Genes Associated With Endocrine Cancer. Front. Endocrinol. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, G.; Li, G.; Ma, X.; Jin, Y.; Hu, N.; Li, J.; Wang, Z.; Wang, H. Dexamethasone-Induced Mitochondrial Dysfunction and Insulin Resistance-Study in 3T3-L1 Adipocytes and Mitochondria Isolated from Mouse Liver. Molecules 2019, 24, 1982. [Google Scholar] [CrossRef] [Green Version]
- Scheller, K.; Sekeris, C.E.; Krohne, G.; Hock, R.; Hansen, I.A.; Scheer, U. Localization of glucocorticoid hormone receptors in mitochondria of human cells. Eur. J. Cell Biol. 2000, 79, 299–307. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Psarra, A.M.; Tsiapara, A.; Paraskevakou, H.; Davaris, P.; Sekeris, C.E. Localization of the glucocorticoid receptor in rat brain mitochondria. Arch. Biochem. Biophys. 2001, 386, 69–78. [Google Scholar] [CrossRef]
- Psarra, A.M.; Solakidi, S.; Sekeris, C.E. The mitochondrion as a primary site of action of regulatory agents involved in neuroimmunomodulation. Ann. N. Y. Acad. Sci. 2006, 1088, 12–22. [Google Scholar] [CrossRef]
- Tsiriyotis, C.; Spandidos, D.A.; Sekeris, C.E. The mitochondrion as a primary site of action of glucocorticoids: Mitochondrial nucleotide sequences, showing similarity to hormone response elements, confer dexamethasone inducibility to chimaeric genes transfected in LATK- cells. Biochem. Biophys. Res. Commun. 1997, 235, 349–354. [Google Scholar] [CrossRef]
- Foo, S.L.; Sachaphibulkij, K.; Lee, C.L.Y.; Yap, G.L.R.; Cui, J.; Arumugam, T.; Lim, L.H.K. Breast cancer metastasis to brain results in recruitment and activation of microglia through annexin-A1/formyl peptide receptor signaling. Breast Cancer Res. 2022, 24, 25. [Google Scholar] [CrossRef]
- Mojica, C.A.R.; Ybanez, W.S.; Olarte, K.C.V.; Poblete, A.B.C.; Bagamasbad, P.D. Differential Glucocorticoid-Dependent Regulation and Function of the ERRFI1 Gene in Triple-Negative Breast Cancer. Endocrinology 2020, 161, bqaa082. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.A.; Deshpande, K.; Martirosian, V.; Saatian, B.; Julian, A.; Eisenbarth, R.; Das, D.; Iyer, M.; Neman, J. Cortisol promotes breast-to-brain metastasis through the blood-cerebrospinal fluid barrier. Cancer Rep. 2022, 5, e1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Rayburn, E.R.; Hill, D.L.; Rinehart, J.J.; Zhang, R. Dexamethasone as a chemosensitizer for breast cancer chemotherapy: Potentiation of the antitumor activity of adriamycin, modulation of cytokine expression, and pharmacokinetics. Int. J. Oncol. 2007, 30, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yang, G.; Cao, Z.; Shen, K.; Zheng, L.; Xiao, J.; You, L.; Zhang, T. The prospect of serum and glucocorticoid-inducible kinase 1 (SGK1) in cancer therapy: A rising star. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940946. [Google Scholar] [CrossRef]
- Bakour, N.; Moriarty, F.; Moore, G.; Robson, T.; Annett, S.L. Prognostic Significance of Glucocorticoid Receptor Expression in Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1649. [Google Scholar] [CrossRef]
- Paakinaho, V.; Palvimo, J.J. Genome-wide crosstalk between steroid receptors in breast and prostate cancers. Endocr Relat Cancer 2021, 28, R231–R250. [Google Scholar] [CrossRef]
- Kumar, S.; Freelander, A.; Lim, E. Type 1 Nuclear Receptor Activity in Breast Cancer: Translating Preclinical Insights to the Clinic. Cancers 2021, 13, 4972. [Google Scholar] [CrossRef]
- Moran, T.J.; Gray, S.; Mikosz, C.A.; Conzen, S.D. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000, 60, 867–872. [Google Scholar]
- Giudice, A.; Barbieri, A.; Bimonte, S.; Cascella, M.; Cuomo, A.; Crispo, A.; D’Arena, G.; Galdiero, M.; Della Pepa, M.E.; Botti, G.; et al. Dissecting the prevention of estrogen-dependent breast carcinogenesis through Nrf2-dependent and independent mechanisms. Onco. Targets Ther. 2019, 12, 4937–4953. [Google Scholar] [CrossRef] [Green Version]
- Giudice, A.; Aliberti, S.M.; Barbieri, A.; Pentangelo, P.; Bisogno, I.; D’Arena, G.; Cianciola, E.; Caraglia, M.; Capunzo, M. Potential Mechanisms by which Glucocorticoids Induce Breast Carcinogenesis through Nrf2 Inhibition. Front. Biosci. 2022, 27, 223. [Google Scholar] [CrossRef]
- Diab, T.; AlKafaas, S.S.; Shalaby, T.I.; Hessien, M. Dexamethasone simulates the anticancer effect of nano-formulated paclitaxel in breast cancer cells. Bioorg. Chem. 2020, 99, 103792. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.M.; Huang, Y.C.; Sun, S.P.; Pan, Y.R.; Shen, C.Y.; Kao, M.C.; Wang, R.H.; Wang, L.H.; Lin, K.T. Effects of synthetic glucocorticoids on breast cancer progression. Steroids 2020, 164, 108738. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chuang, P.Y.; You, S.L.; Chiang, C.J.; Huang, C.S.; Wang, M.Y.; Chao, M.; Lu, Y.S.; Cheng, A.L.; Tang, C.H. Effect of glucocorticoid use on survival in patients with stage I-III breast cancer. Breast. Cancer Res. Treat. 2018, 171, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Abola, M.V.; Prasad, V.; Jena, A.B. Association between treatment toxicity and outcomes in oncology clinical trials. Ann. Oncol. 2014, 25, 2284–2289. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Provenzano, E.; Ulaner, G.A.; Chin, S.F. Molecular Classification of Breast Cancer. PET Clin. 2018, 13, 325–338. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Delgado-Magallon, A.; Montes-Alvarado, J.B.; Ramirez-Ramirez, D.; Flores-Alonso, J.C.; Cortes-Hernandez, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Eleonora, H. Molecular subtypes of breast cancer—What breast imaging radiologists need to know. Rev. Chil. Radiol. 2021, 27, 17–26. [Google Scholar]
- Conway, M.E.; McDaniel, J.M.; Graham, J.M.; Guillen, K.P.; Oliver, P.G.; Parker, S.L.; Yue, P.; Turkson, J.; Buchsbaum, D.J.; Welm, B.E.; et al. STAT3 and GR Cooperate to Drive Gene Expression and Growth of Basal-Like Triple-Negative Breast Cancer. Cancer Res. 2020, 80, 4355–4370. [Google Scholar] [CrossRef]
- Lucena, M.A.; González, R.C.J.; de Reyes Lartategui, S.; Aragón, G.T.; Barrón, M.T.S.; Rubio, G.J.; Poyatos, P.T. Clasificación actual del cáncer de mama. Implicación en el tratamiento y pronóstico de la enfermedad. Cir. Andal. 2021, 32, 155–159. [Google Scholar]
- Sorkhy, M.A.; Alfahl, Z.; Ritchie, J. Cortisol and Breast Cancer: A review of clinical and molecular evidence. Ann. Cancer Res. Ther. 2018, 26, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Iftikhar, A.; Islam, M.; Shepherd, S.; Jones, S.; Ellis, I. Cancer and Stress: Does It Make a Difference to the Patient When These Two Challenges Collide? Cancers 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R. Anti-inflammatory functions of glucocorticoid-induced genes. Mol. Cell. Endocrinol. 2007, 275, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic glucocorticoids: Mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaides, N.C.; Chrousos, G.; Kino, T. Glucocorticoid Receptor. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011, 71, 6360–6370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, H.D.; Antonova, L.; Mueller, C.R. The unliganded glucocorticoid receptor positively regulates the tumor suppressor gene BRCA1 through GABP beta. Mol Cancer Res. 2012, 10, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Hapgood, J.P.; Avenant, C.; Moliki, J.M. Glucocorticoid-independent modulation of GR activity: Implications for immunotherapy. Pharmacol. Ther. 2016, 165, 93–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, N.; Hoffman, J.A.; Archer, T.K. BAF Complexes and the Glucocorticoid Receptor in Breast Cancers. Curr. Opin. Endocr. Metab. Res. 2020, 15, 8–14. [Google Scholar] [CrossRef]
- Buoso, E.; Ronfani, M.; Galasso, M.; Ventura, D.; Corsini, E.; Racchi, M. Cortisol-induced SRSF3 expression promotes GR splicing, RACK1 expression and breast cancer cells migration. Pharmacol. Res. 2019, 143, 17–26. [Google Scholar] [CrossRef]
- Obradovic, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.M.; Munst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids promote breast cancer metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, J.S.; Patil, S.; Rajarajan, S.; Ce, A.; Nair, M.; Alexander, A.; Ramesh, R.; Bs, S.; Sridhar, T. Triple-negative breast cancers with expression of glucocorticoid receptor in immune cells show better prognosis. Ann. Oncol. 2021, 32 (Suppl. 2), S35. [Google Scholar] [CrossRef] [PubMed]
- Regan Anderson, T.M.; Ma, S.H.; Raj, G.V.; Cidlowski, J.A.; Helle, T.M.; Knutson, T.P.; Krutilina, R.I.; Seagroves, T.N.; Lange, C.A. Breast Tumor Kinase (Brk/PTK6) Is Induced by HIF, Glucocorticoid Receptor, and PELP1-Mediated Stress Signaling in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Hwang, S.E.; Yoon, S.P. Dexamethasone reduces side population fraction through downregulation of ABCG2 transporter in MCF-7 breast cancer cells. Mol. Med. Rep. 2017, 16, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.L.; O’Malley, B.W. Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004, 25, 45–71. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Luchtenborg, M.; Davies, E.A.; Jack, R.H. The treatment and survival of patients with triple negative breast cancer in a London population. Springerplus 2014, 3, 553. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, D.; Gonzalez, R.; Kirchner, B.; Mazzone, C.; Pfaffl, M.W.; Schelling, G.; Steinlein, O.; Reithmair, M. Glucocorticoid receptor overexpression slightly shifts microRNA expression patterns in triple-negative breast cancer. Int. J. Oncol. 2018, 52, 1765–1776. [Google Scholar] [CrossRef]
- Kerkvliet, C.P.; Truong, T.H.; Ostrander, J.H.; Lange, C.A. Stress sensing within the breast tumor microenvironment: How glucocorticoid receptors live in the moment. Essays Biochem. 2021, 65, 971–983. [Google Scholar] [CrossRef]
- Leehy, K.A.; Regan Anderson, T.M.; Daniel, A.R.; Lange, C.A.; Ostrander, J.H. Modifications to glucocorticoid and progesterone receptors alter cell fate in breast cancer. J. Mol. Endocrinol. 2016, 56, R99–R114. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Chaudhuri, S.; Brickley, D.R.; Pang, D.; Karrison, T.; Conzen, S.D. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004, 64, 1757–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkashif, A.; Bingham, V.; Haddock, P.; Humphries, M.P.; McQuaid, S.; Mullan, P.B.; McCarthy, H.O.; Buckley, N.E. Glucocorticoid Receptor Expression Predicts Good Outcome in response to Taxane-Free, Anthracycline-Based Therapy in Triple Negative Breast Cancer. J. Oncol. 2020, 2020, 3712825. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Kocherginsky, M.; Krausz, T.; Kim, S.Y.; Conzen, S.D. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol. Ther. 2006, 5, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Wang, L.H. New dimension of glucocorticoids in cancer treatment. Steroids 2016, 111, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goya, L.; Maiyar, A.C.; Ge, Y.; Firestone, G.L. Glucocorticoids induce a G1/G0 cell cycle arrest of Con8 rat mammary tumor cells that is synchronously reversed by steroid withdrawal or addition of transforming growth factor-alpha. Mol. Endocrinol. 1993, 7, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Mikosz, C.A.; Brickley, D.R.; Sharkey, M.S.; Moran, T.W.; Conzen, S.D. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J. Biol. Chem. 2001, 276, 16649–16654. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, S.; Jin, Y.; Nagaich, A.K. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) alpha and activator protein 1 (AP1) in dexamethasone-mediated interference of ERalpha activity. J. Biol. Chem. 2013, 288, 24020–24034. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Wang, D.; Yuan, X.; Liu, Y.; Guo, X.; Li, J.; Song, J. Glucocorticoid receptor-IRS-1 axis controls EMT and the metastasis of breast cancers. J. Mol. Cell Biol. 2019, 11, 1042–1055. [Google Scholar] [CrossRef] [Green Version]
- Yiding, C.; Zhongyi, H. Differences between the quality aspects of various generic and branded docetaxel formulations. Curr. Med. Res. Opin. 2021, 37, 1421–1433. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Zou, T.; Du, C.; Li, S.; Chen, C.; Liu, R.; Wang, K. Dexamethasone induces docetaxel and cisplatin resistance partially through up-regulating Kruppel-like factor 5 in triple-negative breast cancer. Oncotarget 2017, 8, 11555–11565. [Google Scholar] [CrossRef] [Green Version]
- Skor, M.N.; Wonder, E.L.; Kocherginsky, M.; Goyal, A.; Hall, B.A.; Cai, Y.; Conzen, S.D. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin. Cancer Res. 2013, 19, 6163–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Lan, X.; Wu, D.; Sunkel, B.; Ye, Z.; Huang, J.; Liu, Z.; Clinton, S.K.; Jin, V.X.; Wang, Q. Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer. Nat. Commun. 2015, 6, 8323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buoso, E.; Galasso, M.; Ronfani, M.; Serafini, M.M.; Lanni, C.; Corsini, E.; Racchi, M. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol. Res. 2017, 120, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Koc, E.C.; Koc, F.C.; Kartal, F.; Tirona, M.; Koc, H. Role of mitochondrial translation in remodeling of energy metabolism in ER/PR(+) breast cancer. Front. Oncol. 2022, 12, 897207. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, A.; Ali, R.; Alhallaj, A.; Alzahrani, H.; Samman, N.; Mashhour, A.; Baz, O.; Barhoumi, T.; Alghanem, B.; Khan, A.; et al. Nuclear Receptors Are Differentially Expressed and Activated in KAIMRC1 Compared to MCF7 and MDA-MB231 Breast Cancer Cells. Molecules 2019, 24, 2028. [Google Scholar] [CrossRef] [PubMed]
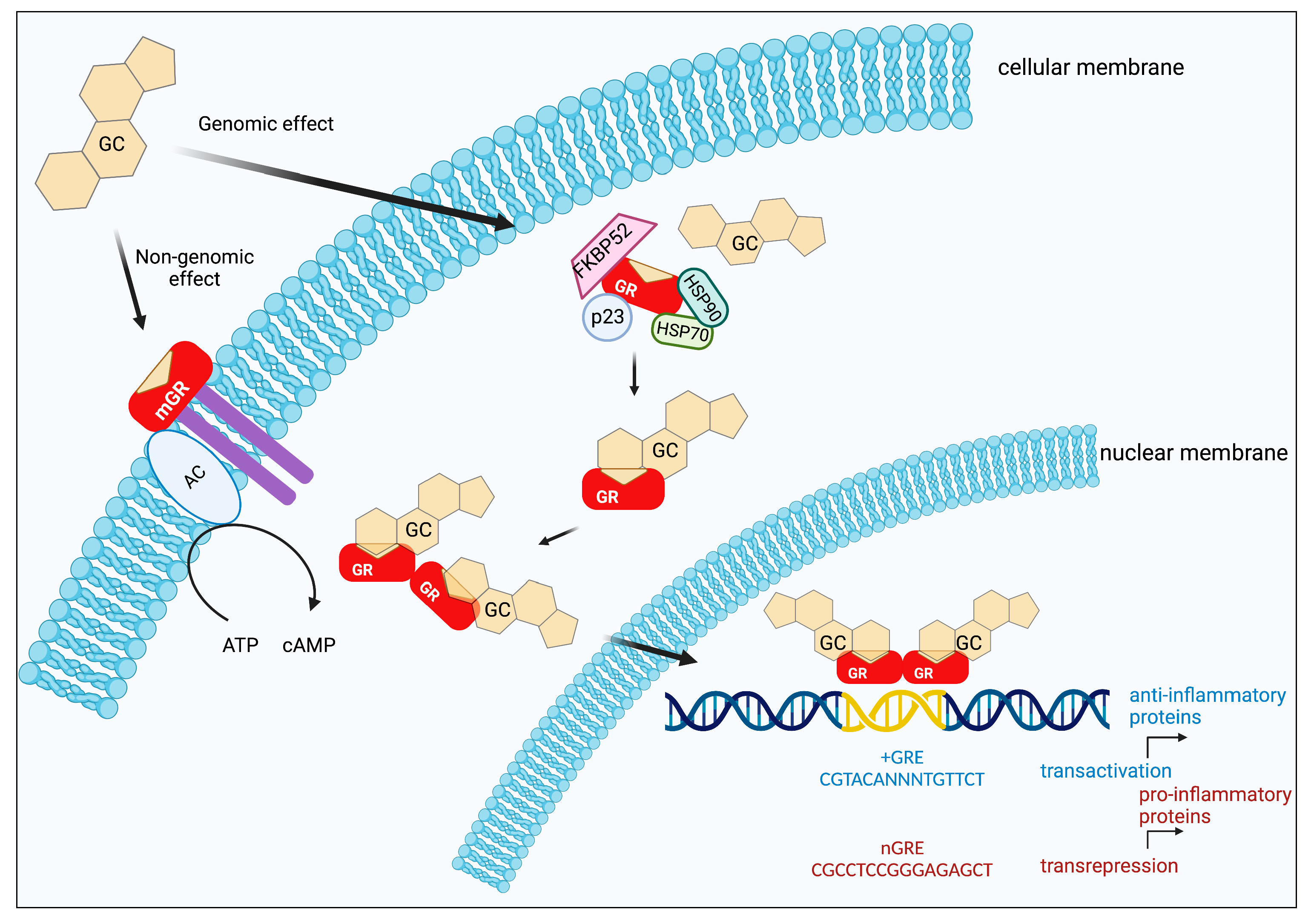
| Region | Modification | Residues | Aftermath | Ref. |
|---|---|---|---|---|
| Amino-terminal domain | Phosphorylation in 11 serine sites | T8, S45, S113, S134, S141, S203, S211, S226, S234, S267, S404 | Affect target gene expression, nuclear export, binding with other proteins, conformational changes, and degradation | [4] |
| SUMOylation in two residues | K277, K293 | |||
| Ubiquitination in one site | K419 | |||
| Ligand-binding domain | Oxidation | C481 | General activity | [21] |
| Hinge region | Four sites to acetylation | K480, K492, K494, K495 | Binding to DNA | [22] |
| Ligand-binding domain | One site for SUMOylation | L703 | Binding to other molecules | [23] |
| Modality | Effects | Examples | Ref. |
|---|---|---|---|
| GC interacts with the cellular membrane | Influences membrane fluidity and composition and leads to regulation of signal-transducer/effector systems. | Membrane stabilizing effects of GCs in rat liver. | [29] |
| GC interacts with GR in the cellular membrane | GC binds a GR localized in the plasma membrane, leading to the regulation of signal-transducer/effector systems. | Membrane glucocorticoid receptors are expressed in T lymphocytes. | [30] |
| GC binds cytoplasmic GR | GC binds cytoplasmatic GR but does not travel to the nucleus. | Dexamethasone enhances the activation of endothelial NO synthase in the heart. | [31] |
| GC binds to other membrane receptors | GC binds a putative pertussis toxin-sensitive inhibitory G-protein coupled receptor. | These non-genomic mechanisms are involved in the glucocorticoid-negative regulation of ACTH expression, and a pertussis toxin-sensitive GTP-binding protein participates. | [32] |
| Mitochondrial GC | GC is introduced into the mitochondria and binds to mitochondrial GRE elements, or binds to mitochondrial proteins, affecting their functions. | In lung and during hepatic inflammation. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitre-Aguilar, I.B.; Moreno-Mitre, D.; Melendez-Zajgla, J.; Maldonado, V.; Jacobo-Herrera, N.J.; Ramirez-Gonzalez, V.; Mendoza-Almanza, G. The Role of Glucocorticoids in Breast Cancer Therapy. Curr. Oncol. 2023, 30, 298-314. https://doi.org/10.3390/curroncol30010024
Mitre-Aguilar IB, Moreno-Mitre D, Melendez-Zajgla J, Maldonado V, Jacobo-Herrera NJ, Ramirez-Gonzalez V, Mendoza-Almanza G. The Role of Glucocorticoids in Breast Cancer Therapy. Current Oncology. 2023; 30(1):298-314. https://doi.org/10.3390/curroncol30010024
Chicago/Turabian StyleMitre-Aguilar, Irma B., Daniel Moreno-Mitre, Jorge Melendez-Zajgla, Vilma Maldonado, Nadia J. Jacobo-Herrera, Victoria Ramirez-Gonzalez, and Gretel Mendoza-Almanza. 2023. "The Role of Glucocorticoids in Breast Cancer Therapy" Current Oncology 30, no. 1: 298-314. https://doi.org/10.3390/curroncol30010024
APA StyleMitre-Aguilar, I. B., Moreno-Mitre, D., Melendez-Zajgla, J., Maldonado, V., Jacobo-Herrera, N. J., Ramirez-Gonzalez, V., & Mendoza-Almanza, G. (2023). The Role of Glucocorticoids in Breast Cancer Therapy. Current Oncology, 30(1), 298-314. https://doi.org/10.3390/curroncol30010024





